Endophytic Bacteria with Potential Antimicrobial Activity Isolated from Theobroma cacao in Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Site
2.2. Isolation of Bacteria
2.3. Molecular Identification
2.4. Antimicrobial Evaluation
3. Results and Discussion
3.1. Characteristics of Isolated Strains
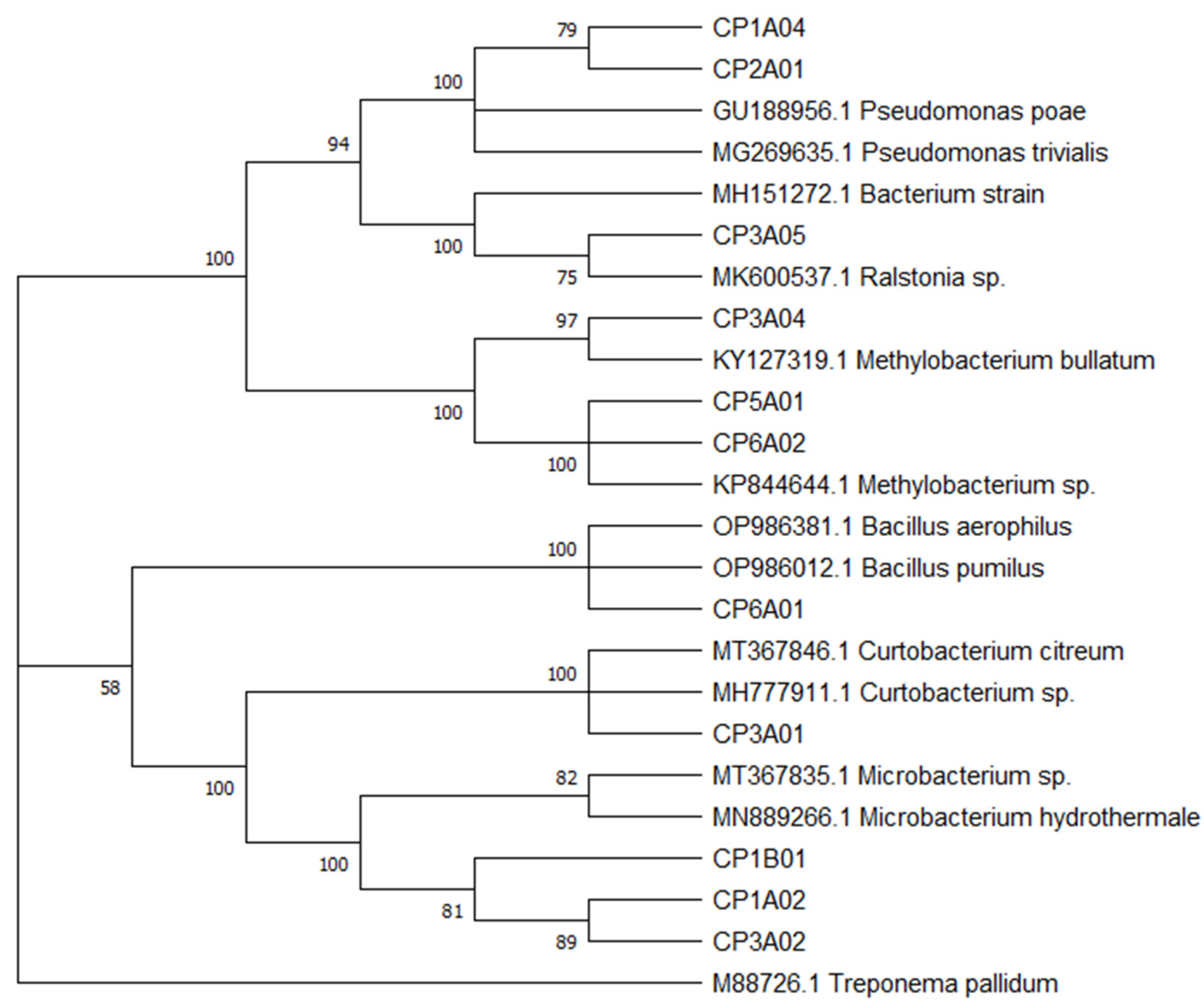
3.2. 16S rRNA Analysis
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villas Boas, D.F.; Luiz, J.H.H.; Malpass, G.R.P.; Okura, M.H.; De Souza, C.P.; Granato, A.C. Microrganismos endofíticos como fonte de compostos de interesse medicinal—Uma breve revisão. Rev. Bras. Ciência Tecnol. Inovação 2021, 5, 70–86. [Google Scholar] [CrossRef]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced from Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef]
- Beraldo-Borrazzo, J.; Mangolin, C.A.; Machado, M.D.F.P.D.S. Biotechnological prospection of endophytic bacteria associated with cactaceae. Rev. Ibero-Am. Ciências Ambient. 2021, 12, 567–579. [Google Scholar] [CrossRef]
- Alves-Júnior, M.; De Sousa, F.O.; Silva, T.F.; Albino, U.B.; Garcia, M.G.; Moreira, S.M.C.D.O.; Vieira, M.R.D.S. Functional and Morphological Analysis of Isolates of Phylloplane and Rhizoplane Endophytic Bacteria Interacting in Different Cocoa Production Systems in the Amazon. Curr. Res. Microb. Sci. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Rutkowska, N.; Drożdżyński, P.; Ryngajłło, M.; Marchut-Mikołajczyk, O. Plants as the Extended Phenotype of Endophytes—The Actual Source of Bioactive Compounds. Int. J. Mol. Sci. 2023, 24, 10096. [Google Scholar] [CrossRef]
- Sahu, K.P.; Kumar, A.; Patel, A.; Kumar, M.; Gopalakrishnan, S.; Prakash, G.; Rathour, R.; Gogoi, R. Rice Blast Lesions: An Unexplored Phyllosphere Microhabitat for Novel Antagonistic Bacterial Species against Magnaporthe oryzae. Microb. Ecol. 2021, 81, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial Relationships Between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Agrawal, S.; Bhatt, A. Microbial Endophytes: Emerging Trends and Biotechnological Applications. Curr. Microbiol. 2023, 80, 249–263. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A Review on Mechanisms and Prospects of Endophytic Bacteria in Biocontrol of Plant Pathogenic Fungi and Their Plant Growth-Promoting Activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial Endophytes: The Hidden Actor in Plant Immune Responses against Biotic Stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, C.; Kaur, G.; Kaur, J.; Rath, S.K.; Dwibedi, V. From Microscopy to Omics: A Comprehensive Review of Tools and Techniques in Studying Endophytic Adaptation under Abiotic and Biotic Stress. J. Plant Growth Regul. 2024, 1–19. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Palombo, E.A.; Jaimes Castillo, A.; Zaferanloo, B. Endophytes in Agriculture: Potential to Improve Yields and Tolerances of Agricultural Crops. Microorganisms 2023, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.E.; Sibanda, T.; Louw, S.; Uzabakiriho, J.D. Antimicrobial Potential of the Endophytic Actinobacteria Isolated from Harpagophytum procumbens: A Southern African Medicinal Plant. S. Afr. J. Bot. 2023, 156, 268–277. [Google Scholar] [CrossRef]
- Nalini, M.S.; Prakash, H.S. Diversity and Bioprospecting of Actinomycete Endophytes from the Medicinal Plants. Lett. Appl. Microbiol. 2017, 64, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Abdelshafy Mohamad, O.A.; Abaydulla, G.; Liu, Y.; Li, W.-J.; Li, L. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus roseus. Front. Microbiol. 2020, 11, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, V. Molecular and Biotechnological Aspects of Secondary Metabolites in Actinobacteria. Microbiol. Res. 2020, 231, 126374. [Google Scholar] [CrossRef]
- Eshboev, F.; Karakozova, M.; Abdurakhmanov, J.; Bobakulov, K.; Dolimov, K.; Abdurashidov, A.; Baymirzaev, A.; Makhnyov, A.; Terenteva, E.; Sasmakov, S.; et al. Antimicrobial and Cytotoxic Activities of the Secondary Metabolites of Endophytic Fungi Isolated from the Medicinal Plant Hyssopus Officinalis. Antibiotics 2023, 12, 1201. [Google Scholar] [CrossRef]
- Chóez-Guaranda, I.; Espinoza-Lozano, F.; Reyes-Araujo, D.; Romero, C.; Manzano, P.; Galarza, L.; Sosa, D. Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens. Molecules 2023, 28, 3208. [Google Scholar] [CrossRef]
- Khaeruni, A.; Hiqmawati, A.; Satrah, V.N.; Sutariati, G.A.K.; Wijayanto, T.; Rahni, N.M. Effectiveness and Synergistic of Endophytic Bacteria in Inhibiting the Development of Cocoa Black Pod Rot Disease (Phytophthora palmivora Bult.). AIP Conf. Proc. 2023, 2628, 120016. [Google Scholar] [CrossRef]
- Wijaya, G.; Wardani, A.K.; Eris, D. Biocontrol Activity of Endophytic Bacteria from Cocoa against Phytophthora sp. and Colletotrichum sp. Menara Perkeb. 2023, 91, 72–86. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic Actinobacteria of Medicinal Plants: Diversity and Bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Annapurna, K.; Fayzullaeva, M.; Sulaymonov, K.; Kadirova, D.; Jabbarov, Z.; Sayyed, R.Z. Isolation and Characterization of Endophytic Bacteria from Ginger (Zingiber officinale Rosc.). Ann. Phytomed. 2020, 9, 116–121. [Google Scholar] [CrossRef]
- Fitri, L.; Bessania, M.A.; Septi, N.; Suhartono, S. Isolation and Characterization of Soil Actinobacteria as Cellulolytic Enzyme Producer from Aceh Besar, Indonesia. Biodiversitas 2021, 22, 5169–5180. [Google Scholar] [CrossRef]
- Wilson, K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001, 2, 755–757. [Google Scholar] [CrossRef]
- Alves, H.D.O.; Miranda, M.D.; Polanczyk, R.A.; Nascimento, J.D.; Desiderio, J.A.; Souza, J.A.M.D. Integral Characterization of the 16S rRNA Gene of Non-Sporulating Bacteria and Its Action against Anticarsia gemmatalis Hübner (Lepidoptera: Erebidae). J. Agric. Sci. 2020, 12, 61–74. [Google Scholar] [CrossRef]
- Sofi, M.Y.; Shafi, A.; Masoodi, K. BioEdit in bioinformatics. Bioinform. Everyone 2022, 23, 231–236. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Dey, M.; Singh, D. A New Species of Cololejeunea (Hepaticae: Lejeuneaceae) from Eastern Himalaya, India. Taiwania 2008, 53, 258–263. [Google Scholar] [CrossRef]
- Arumugam, T.; Senthil Kumar, P.; Kameshwar, R.; Prapanchana, K. Screening of Novel Actinobacteria and Characterization of the Potential Isolates from Mangrove Sediment of South Coastal India. Microb. Pathog. 2017, 107, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zothanpuia; Passari, A.K.; Leo, V.V.; Chandra, P.; Kumar, B.; Nayak, C.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Singh, B.P. Bioprospection of Actinobacteria Derived from Freshwater Sediments for Their Potential to Produce Antimicrobial Compounds. Microb. Cell Fact. 2018, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.V.S.; Rodrigues, F.A.; Nadal, M.C.; Dambroz, C.M.d.S.; Martins, A.D.; Rodrigues, V.A.; dos Reis Ferreira, G.M.; Pasqual, M.; Buttros, V.H.; Dória, J. Plant-endophytic bacteria interactions associated with root and leaf microbiomes of Cattleya walkeriana and their effect on plant growth. Sci. Hortic. 2023, 309, 656–670. [Google Scholar] [CrossRef]
- Alsultan, W.; Vadamalai, G.; Khairulmazmi, A.; Saud, H.M.; Al-Sadi, A.M.; Rashed, O.; Jaaffar, A.K.M.; Nasehi, A. Isolation, Identification and Characterization of Endophytic Bacteria Antagonistic to Phytophthora Palmivora Causing Black Pod of Cocoa in Malaysia. Eur. J. Plant Pathol. 2019, 155, 1077–1091. [Google Scholar] [CrossRef]
- Vera-Loor, M.; Bernal-Cabrera, A.; Vera-Coello, D.; Leiva-Mora, M.; Rivero-Aragón, A.; Morales-Díaz De Villegas, L. Árbol filogenético y características de bacterias endófitas asociadas a Theobroma cacao L. en una zona de la provincia de Esmeraldas, Ecuador. Bioagro 2021, 33, 223–228. [Google Scholar] [CrossRef]
- Xia, Y.; He, R.; Xu, W.; Zhang, J. The Zoige Pioneer Plant Leymus secalinus Has Different Endophytic Bacterial Community Structures to Adapt to Environmental Conditions. PeerJ 2023, 11, e15363. [Google Scholar] [CrossRef]
- Mardiah, I. Identification of Endophytic Bacterial Isolated from Oil Palm Plants with Anti-Fungal Activity against Ganoderma boninense. Pharmacol. Clin. Pharm. Res. 2018, 3, 41–49. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Abdul-Raouf, U.M. Production of Bioactive β-Carotene by the Endophytic Bacterium Citricoccus parietis AUCs with Multiple in Vitro Biological Potentials. Microb. Cell Fact. 2023, 22, 90–98. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; El-Shall, F.N.; Arafa, A.A.; Shalabi, A.; El Awady, M. Bio-Production and Characterization of Carotenoid Yellow Pigment from Kocuria sp. GMA and Exploring Its Sustainable Antioxidant, Antimicrobial and Antibiofilm Properties. Egypt. J. Chem. 2024, 67, 57–68. [Google Scholar] [CrossRef]
- Kandasamy, G.D.; Kathirvel, P. Production, Characterization and in Vitro Biological Activities of Crude Pigment from Endophytic Micrococcus luteus Associated with Avicennia marina. Arch. Microbiol. 2024, 206, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Basit, A.; Majeed, M.; Shah, A.A.; Ullah, I.; Mohamed, H.I.; Khan, A.; Ghanaim, A.M. Chapter 16—Bacterial pigments and their applications. In Bacterial Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 277–298. [Google Scholar] [CrossRef]
- Ali, A.R.; Bahrami, Y.; Kakaei, E.; Mohammadzadeh, S.; Bouk, S.; Jalilian, N. Isolation and Identification of Endophytic Actinobacteria from Citrullus colocynthis (L.) Schrad and Their Antibacterial Properties. Microb. Cell Fact. 2022, 21, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Linelejan, Y.T.; Umboh, S.D.; Adam, A.A.; Muslem; Idroes, R. Endophytic Bacteria Isolated from the Leaf of Langusei (Ficus minahassae Tesym. & De Vr.) and Their Antibacterial Activities. IOP Conf. Ser. Mater. Sci. Eng. 2020, 796, 012047. [Google Scholar] [CrossRef]
- Murtado, A.; Mubarik, N.R.; Tjahjoleksono, A. Isolation and Characterization Endophytic Bacteria as Biological Control of Fungus Colletotrichum sp. on Onion Plants (Allium cepa L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012043. [Google Scholar] [CrossRef]
- Sansanwal, R.; Ahlawat2, U.; Batra, P.; Wati, L.; Kaushik, P. Biochemical and Molecular Characterization of Endophytic Bacteria from Root Nodules of Mungbean (Vigna radiata). Ann. Biol. 2023, 36, 7–10. [Google Scholar] [CrossRef]
- Aureen, L.G.; Saroj, B. Microbacterium Arborescens AGSB sp. nov., Isolated from the Rhizosphere of Sand Dune Plant, Ipomoea Pes Caprae. Afr. J. Microbiol. Res. 2013, 7, 5154–5158. [Google Scholar] [CrossRef][Green Version]
- Medison, R.G.; Tan, L.; Medison, M.B.; Chiwina, K.E. Use of Beneficial Bacterial Endophytes: A Practical Strategy to Achieve Sustainable Agriculture. AIMS Microbiol. 2022, 8, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Betancur, L.A.; Naranjo-Gaybor, S.J.; Vinchira-Villarraga, D.M.; Moreno-Sarmiento, N.C.; Maldonado, L.A.; Suarez-Moreno, Z.R.; Acosta-González, A.; Padilla-Gonzalez, G.F.; Puyana, M.; Castellanos, L.; et al. Marine Actinobacteria as a Source of Compounds for Phytopathogen Control: An Integrative Metabolic-Profiling/Bioactivity and Taxonomical Approach. PLoS ONE 2017, 12, e0170148. [Google Scholar] [CrossRef]
- Patel, A.; Sahu, K.P.; Mehta, S.; Javed, M.; Balamurugan, A.; Ashajyothi, M.; Sheoran, N.; Ganesan, P.; Kundu, A.; Gopalakrishnan, S.; et al. New Insights on Endophytic Microbacterium-Assisted Blast Disease Suppression and Growth Promotion in Rice: Revelation by Polyphasic Functional Characterization and Transcriptomics. Microorganisms 2023, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, M.-Y.; Khan, N.; Tan, L.-L.; Yang, S. Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Reyes-Peralta, J.; Mendoza-Herrera, A.; Rivera, G.; Bocanegra-García, V. Characterization of a Microbacterium sp. Strain isolated from soils contaminated with hydrocarbons in the Burgos Basin, Mexico. Rev. Int. Contam. Ambient. 2021, 37, 227–235. [Google Scholar] [CrossRef]
- Anavadiya, B.; Chouhan, S.; Saraf, M.; Goswami, D. Exploring Endophytic Actinomycetes: A Rich Reservoir of Diverse Antimicrobial Compounds for Combatting Global Antimicrobial Resistance. Microbe 2024, 4, 100110. [Google Scholar] [CrossRef]
- Almajali, I.; Al-Tarawneh, A.; Qaralleh, H.; Al-limoun, M.; Al-Sarayrah, M.; Alqaraleh, M.; Rayyan, W.; Khleifat, K.; Dmour, S. Biodegradation of Phenol by Curtobacteriumflaccumfaciens: Optimizationof Growth Conditions. Pol. J. Environ. Stud. 2021, 30, 5435–5442. [Google Scholar] [CrossRef]
- Khleifat, K.; Magharbeh, M.; Alqaraleh, M.; Al-Sarayrah, M.; Alfarrayeh, I.; Al Qaisi, Y.; Alsarayreh, A.; Al-kafaween, M.A. Biodegradation Modeling of Phenol Using Curtobacterium flaccumfaciens as Plant-Growth-Promoting Bacteria. Heliyon 2022, 8, e10490. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72–84. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Gao, W.; Li, J.; Zu, X.; Fu, D.; Feng, S.; Bai, X.; Zuo, Y.; Li, P. Actinobacteria–Derived Peptide Antibiotics since 2000. Peptides 2018, 103, 48–59. [Google Scholar] [CrossRef]
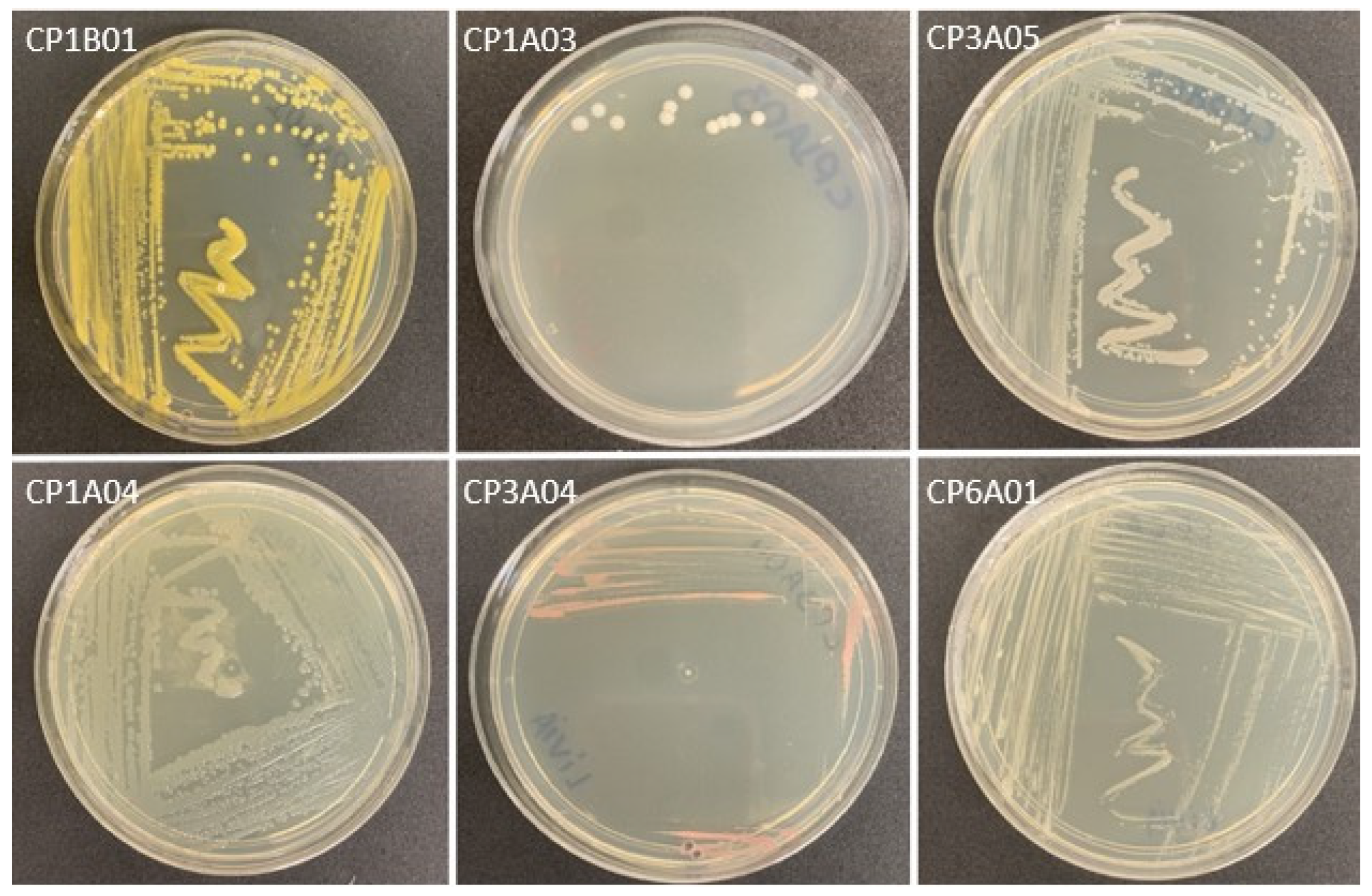
| Bacterial Code | Morphological Characterization | Biochemical Characterization | ||||||
|---|---|---|---|---|---|---|---|---|
| Macroscopy | Microscopy | Catalase | Citrate | Phenylalanine | Carbohydrates | CO2 | H2S | |
| CP1B01 | Circular and convex yellow colonies | Gram-positive rod | + | _ | _ | Glucose | + | _ |
| CP1A02 | Circular and convex yellow colonies | Gram-positive rod | + | _ | _ | Glucose | + | _ |
| CP1A04 | Irregular and flat non-pigmented colonies | Gram-negative rod | _ | + | _ | Glucose | + | _ |
| CP2A01 | Irregular and convex non-pigmented colonies | Gram-negative rod | + | _ | _ | Glucose | + | _ |
| CP3A01 | Circular and convex yellow colonies | Gram-positive rod | + | _ | _ | Glucose | _ | _ |
| CP3A02 | Circular and convex yellow colonies | Gram-positive rod | + | _ | _ | Glucose | _ | _ |
| CP3A04 | Irregular and umbilicated pink colonies | Gram-negative rod | + | _ | _ | Glucose | + | _ |
| CP3A05 | Circular and flat non-pigmented colonies | Gram-negative rod | + | + | _ | Absence | + | _ |
| CP5A01 | Irregular and flat pink colonies | Gram-negative rod | + | _ | _ | Glucose | _ | _ |
| CP6A01 | Irregular and umbilicated non-pigmented colonies | Gram-positive rod | + | + | _ | Glucose/Sucrose/Lactose | + | _ |
| CP6A02 | Circular and convex pink colonies | Gram-negative rod | + | _ | _ | Absence | + | _ |
| Bacterial Code | Genera | Bacteria Used in the Antagonism Assay | |||
|---|---|---|---|---|---|
| S. aureus ATCC | S. aureus Clinical Strain | ||||
| Inhibition Halos | Standard Deviation | Inhibition Halos | Standard Deviation | ||
| CP1B01 | Microbacterium | 26.53 mm | 1.66 | 26.2 mm | 2.3 |
| CP1A02 | Microbacterium | 27 mm | 4.35 | 28.53 mm | 1.07 |
| CP3A01 | Curtobacterium | 29.73 mm | 1.25 | 30.16 mm | 0.53 |
| CP3A02 | Microbacterium | 27.4 mm | 3.20 | 26.96 mm | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Pinto, L.F.; Tavares, T.C.S.; Cardenas-Alegria, O.V.; Lobato, E.M.S.G.; de Sousa, C.P.; Nunes, A.R.C. Endophytic Bacteria with Potential Antimicrobial Activity Isolated from Theobroma cacao in Brazilian Amazon. Microorganisms 2025, 13, 1686. https://doi.org/10.3390/microorganisms13071686
da Silva Pinto LF, Tavares TCS, Cardenas-Alegria OV, Lobato EMSG, de Sousa CP, Nunes ARC. Endophytic Bacteria with Potential Antimicrobial Activity Isolated from Theobroma cacao in Brazilian Amazon. Microorganisms. 2025; 13(7):1686. https://doi.org/10.3390/microorganisms13071686
Chicago/Turabian Styleda Silva Pinto, Lívia Freitas, Taynara Cristina Santos Tavares, Oscar Victor Cardenas-Alegria, Elaine Maria Silva Guedes Lobato, Cristina Paiva de Sousa, and Adriana Ribeiro Carneiro Nunes. 2025. "Endophytic Bacteria with Potential Antimicrobial Activity Isolated from Theobroma cacao in Brazilian Amazon" Microorganisms 13, no. 7: 1686. https://doi.org/10.3390/microorganisms13071686
APA Styleda Silva Pinto, L. F., Tavares, T. C. S., Cardenas-Alegria, O. V., Lobato, E. M. S. G., de Sousa, C. P., & Nunes, A. R. C. (2025). Endophytic Bacteria with Potential Antimicrobial Activity Isolated from Theobroma cacao in Brazilian Amazon. Microorganisms, 13(7), 1686. https://doi.org/10.3390/microorganisms13071686







