Application of Real-Time PCR Syndromic Panel on Lower Respiratory Tract Samples: Potential Use for Antimicrobial De-Escalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Molecular Assay
2.3. Cultural Assay
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Dominedò, C.; Torres, A. Multidrug Resistant Gram-Negative Bacteria in Community-Acquired Pneumonia. Crit. Care 2019, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yang, K.Y.; Peng, C.K.; Sheu, C.C.; Chan, M.C.; Feng, J.Y.; Wang, S.H.; Chen, C.M.; Zheng, Z.R.; Liang, S.J.; et al. Clinical outcome of nosocomial pneumonia caused by Carbapenem-resistant gram-negative bacteria in critically ill patients: A multicenter retrospective observational study. Sci. Rep. 2022, 12, 7501. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Gonçalves-Pereira, J.; Ribeiro, O.; Baptista, J.P.; Froes, F.; Paiva, J.A. Impact of antibiotic therapy in severe community-acquired pneumonia: Data from the Infauci study. J. Crit. Care 2018, 43, 183–189. [Google Scholar] [CrossRef]
- Candel, F.J.; Salavert, M.; Basaras, M.; Borges, M.; Cantón, R.; Cercenado, E.; Cilloniz, C.; Estella, Á.; García-Lechuz, J.M.; Garnacho Montero, J.; et al. Ten Issues for Updating in Community-Acquired Pneumonia: An Expert Review. J. Clin. Med. 2023, 12, 6864. [Google Scholar] [CrossRef]
- Barbier, F.; Dupuis, C.; Buetti, N.; Schwebel, C.; Azoulay, É.; Argaud, L.; Cohen, Y.; Hong Tuan Ha, V.; Gainnier, M.; Siami, S.; et al. Single-drug versus combination antimicrobial therapy in critically ill patients with hospital-acquired pneumonia and ventilator-associated pneumonia due to Gram-negative pathogens: A multicenter retrospective cohort study. Crit. Care 2024, 28, 10. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, K.; Lee, S.M.; Cho, Y.J.; Kim, Y.S.; Chong, Y.P.; Hong, S.B. The Distribution of Multidrug-resistant Microorganisms and Treatment Status of Hospital-acquired Pneumonia/Ventilator-associated Pneumonia in Adult Intensive Care Units: A Prospective Cohort Observational Study. J. Korean Med. Sci. 2021, 36, e251. [Google Scholar] [CrossRef]
- Patzke, C.L.; Armahizer, M.J.; Badjatia, N.; Motta, M. A Retrospective Analysis of Prolonged Empiric Antibiotic Therapy for Pneumonia Among Adult Neurocritical Care Patients. Neurohospitalist 2019, 9, 15–21. [Google Scholar] [CrossRef]
- Thomas, Z.; Bandali, F.; Sankaranarayanan, J.; Reardon, T.; Olsen, K.M.; Critical Care Pharmacotherapy Trials Network. A Multicenter Evaluation of Prolonged Empiric Antibiotic Therapy in Adult ICUs in the United States. Crit. Care Med. 2015, 43, 2527–2534. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Tanzarella, E.S.; Cutuli, S.L.; Lombardi, G.; Cammarota, F.; Caroli, A.; Franchini, E.; Sancho Ferrando, E.; Grieco, D.L.; Antonelli, M.; De Pascale, G. Antimicrobial De-Escalation in Critically Ill Patients. Antibiotics 2024, 13, 375. [Google Scholar] [CrossRef]
- Carroll, K.C.; Pfaller, M.A.; Karlowsky, J.A.; Landry, M.L.; Landry, A.J.; McAdam, R.P.; Bobbi, S.P. Manual of Clinical Microbiology, 13th ed.; ASM Press: Washington, DC, USA, 2024. [Google Scholar]
- Lee, S.H.; Ruan, S.Y.; Pan, S.C.; Lee, T.F.; Chien, J.Y.; Hsueh, P.R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef]
- Gastli, N.; Loubinoux, J.; Daragon, M.; Lavigne, J.P.; Saint-Sardos, P.; Pailhoriès, H.; Lemarié, C.; Benmansour, H.; d’Humières, C.; Broutin, L.; et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. 2021, 27, 1308–1314. [Google Scholar] [CrossRef]
- Verroken, A.; Favresse, J.; Anantharajah, A.; Rodriguez-Villalobos, H.; Wittebole, X.; Laterre, P.F. Optimized Antibiotic Management of Critically Ill Patients with Severe Pneumonia Following Multiplex Polymerase Chain Reaction Testing: A Prospective Clinical Exploratory Trial. Antibiotics 2024, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Buchan, B.W.; Windham, S.; Balada-Llasat, J.M.; Leber, A.; Harrington, A.; Relich, R.; Murphy, C.; Dien Bard, J.; Naccache, S.; Ronen, S.; et al. Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections. J. Clin. Microbiol. 2020, 58, e00135-20. [Google Scholar] [CrossRef] [PubMed]
- Plattner, A.S.; Lockowitz, C.R.; Dumm, R.; Banerjee, R.; Newland, J.G.; Same, R.G. Practice Versus Potential: The Impact of the BioFire FilmArray Pneumonia Panel on Antibiotic Use in Children. J. Pediatric Infect. Dis. Soc. 2024, 13, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Stafylaki, D.; Maraki, S.; Vaporidi, K.; Georgopoulos, D.; Kontoyiannis, D.P.; Kofteridis, D.P.; Chamilos, G. Impact of Molecular Syndromic Diagnosis of Severe Pneumonia in the Management of Critically Ill Patients. Microbiol. Spectr. 2022, 10, e0161622. [Google Scholar] [CrossRef]
- Monard, C.; Pehlivan, J.; Auger, G.; Alviset, S.; Tran Dinh, A.; Duquaire, P.; Gastli, N.; d’Humières, C.; Maamar, A.; Boibieux, A.; et al. Multicenter evaluation of a syndromic rapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit. Care 2020, 24, 434. [Google Scholar] [CrossRef]
- Crémet, L.; Gaborit, B.; Bouras, M.; Drumel, T.; Guillotin, F.; Poulain, C.; Persyn, E.; Lakhal, K.; Rozec, B.; Vibet, M.A.; et al. Evaluation of the FilmArray® Pneumonia Plus Panel for Rapid Diagnosis of Hospital-Acquired Pneumonia in Intensive Care Unit Patients. Front. Microbiol. 2020, 11, 2080. [Google Scholar] [CrossRef]
- Mitton, B.; Rule, R.; Said, M. Laboratory evaluation of the BioFire FilmArray Pneumonia plus panel compared to conventional methods for the identification of bacteria in lower respiratory tract specimens: A prospective cross-sectional study from South Africa. Diagn. Microbiol. Infect. Dis. 2021, 99, 115236. [Google Scholar] [CrossRef]
- Ferrer, J.; Clari, M.Á.; Giménez, E.; Carbonell, N.; Torres, I.; Blasco, M.L.; Albert, E.; Navarro, D. The Biofire® Filmarray® Pneumonia Plus panel for management of lower respiratory tract infection in mechanically-ventilated patients in the COVID-19 era: A diagnostic and cost-benefit evaluation. Diagn. Microbiol. Infect. Dis. 2023, 105, 115847. [Google Scholar] [CrossRef] [PubMed]
- Szymankiewicz, M.T.; Szczepanska, A.; Stefaniuk, E. Evaluation of the BioFire® FilmArray® Pneumonia plus Panel for Detecting Bacterial Etiological Agents of Lower Respiratory Tract Infections in an Oncologic Hospital. Comparison with Conventional Culture Method. Pol. J. Microbiol. 2023, 72, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Webber, D.M.; Wallace, M.A.; Burnham, C.A.; Anderson, N.W. Evaluation of the BioFire FilmArray Pneumonia Panel for Detection of Viral and Bacterial Pathogens in Lower Respiratory Tract Specimens in the Setting of a Tertiary Care Academic Medical Center. J. Clin. Microbiol. 2020, 58, e00343-20. [Google Scholar] [CrossRef] [PubMed]
- Van Der Westhuyzen, M.; Samodien, N.; Brink, A.J.; Moodley, C. Utility of the BioFire® FilmArray® Pneumonia Panel plus assay for syndromic testing of lower respiratory tract infections in a low/middle-income setting. JAC Antimicrob. Resist. 2023, 5, dlac139. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2020, 50, 1700582. [Google Scholar] [CrossRef]
- Zhu, M.; Pickens, C.I.; Markov, N.S.; Pawlowski, A.; Kang, M.; Rasmussen, L.V.; Walter, J.M.; Nadig, N.R.; Singer, B.D.; Wunderink, R.G.; et al. Antibiotic de-escalation patterns and outcomes in critically ill patients with suspected pneumonia as informed by bronchoalveolar lavage results. Eur. J. Clin. Microbiol. Infect. Dis. 2025. [Google Scholar] [CrossRef]
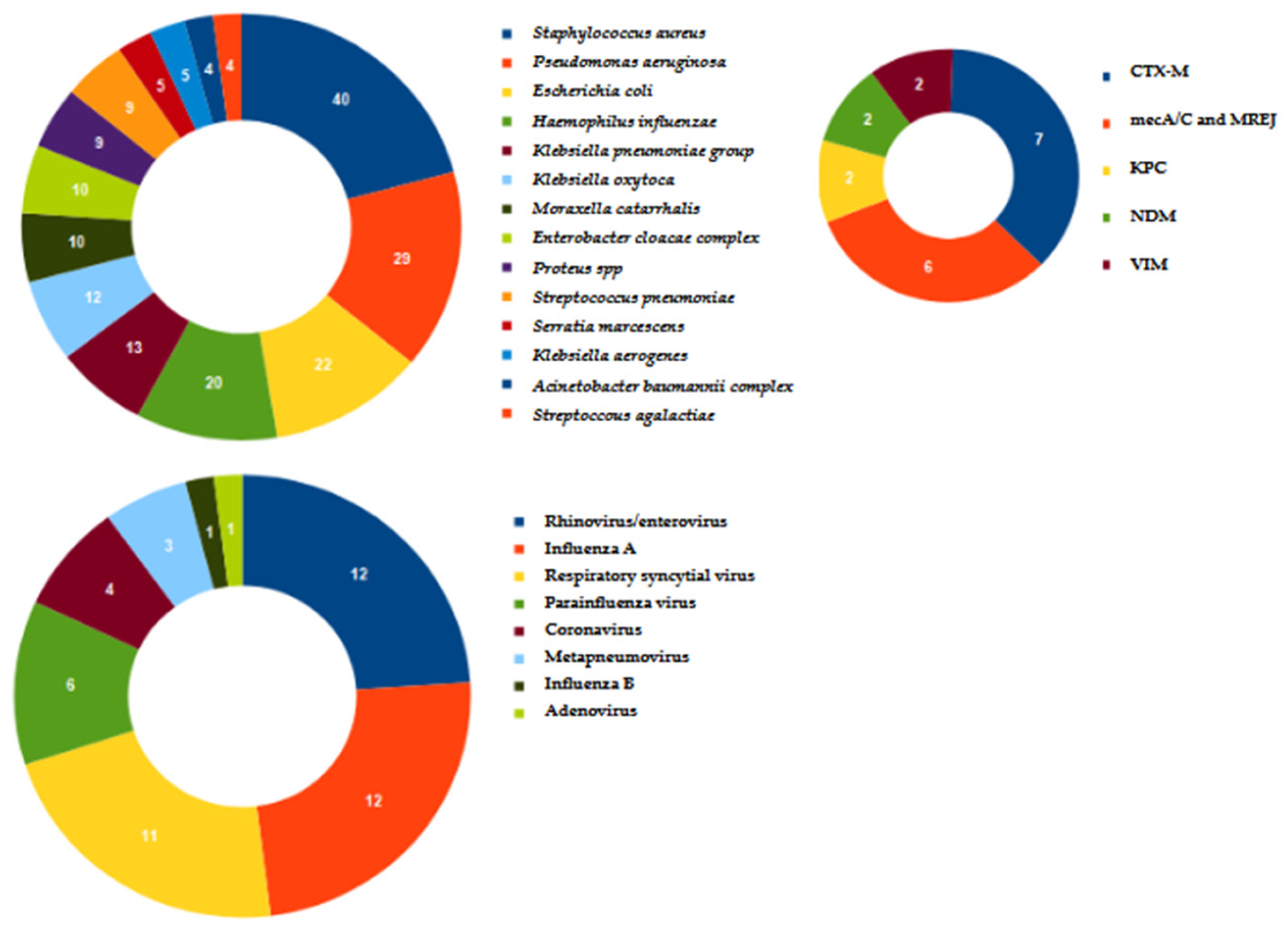
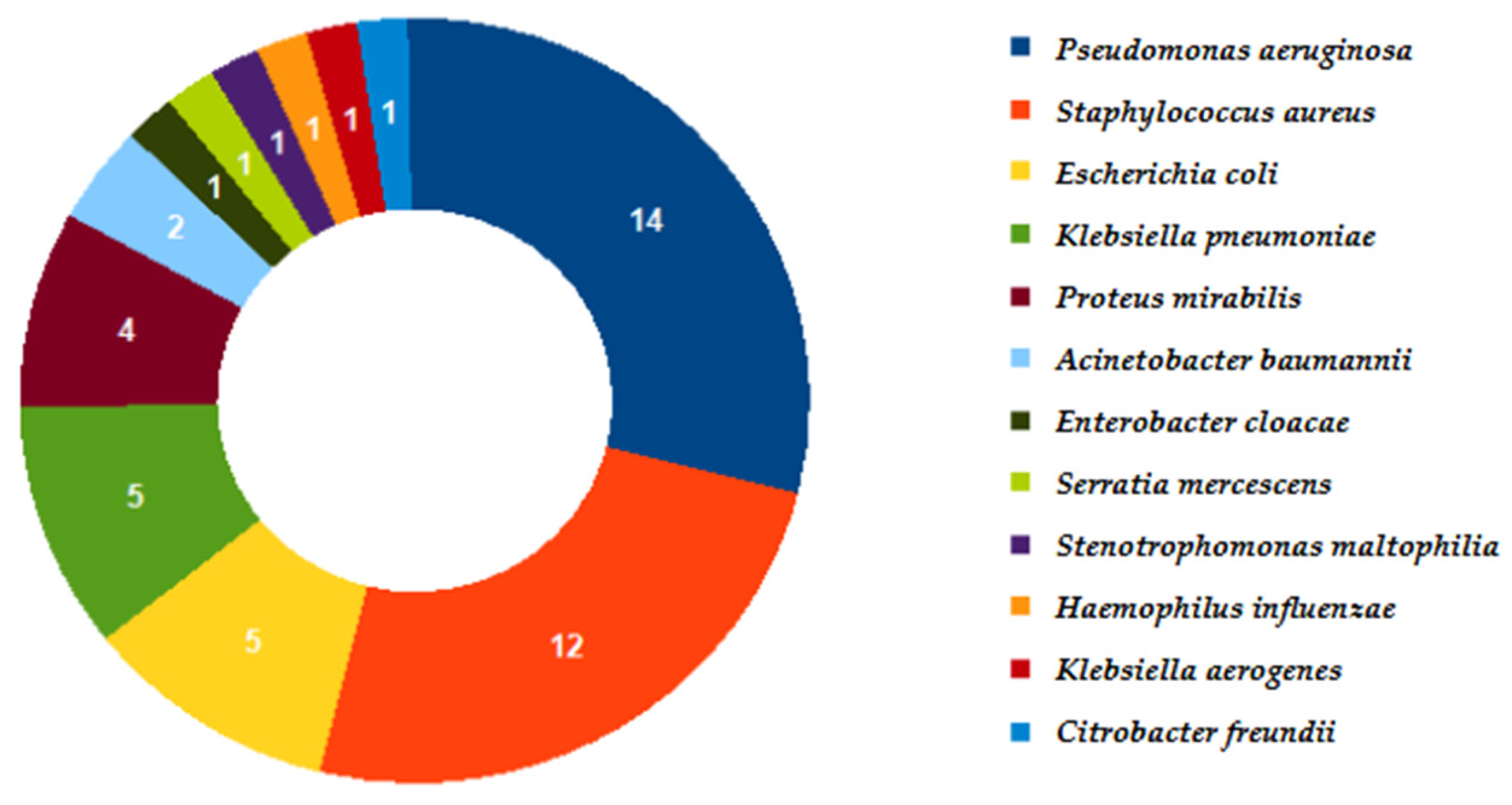
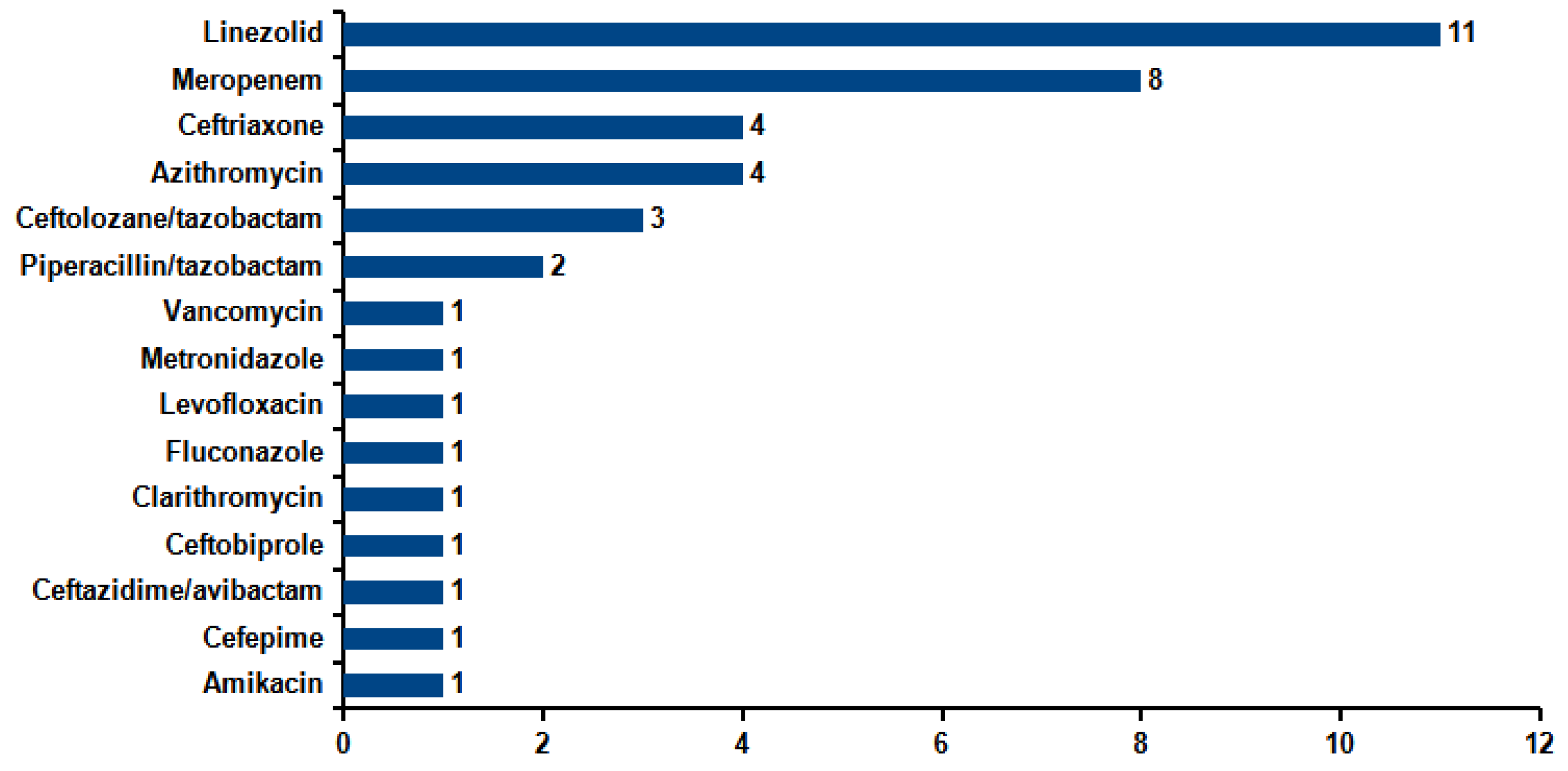
| Ward | N | % |
|---|---|---|
| Intensive Care | 61 | 35.1 |
| Infectious Diseases | 20 | 11.5 |
| Pulmonology | 28 | 16.1 |
| Hematology | 13 | 7.5 |
| Sub-Intensive Care | 13 | 7.5 |
| Pediatric Intensive Care | 10 | 5.7 |
| Emergency Medicine | 8 | 4.5 |
| Internal Medicine | 5 | 2.9 |
| Coronary Care Unit | 3 | 1.7 |
| General Surgery | 2 | 1.1 |
| Pediatrics | 2 | 1.1 |
| Oncology | 2 | 1.1 |
| Gastroenterology | 1 | 0.6 |
| Nephrology | 1 | 0.6 |
| Neurology | 1 | 0.6 |
| Pediatric Emergency Room | 1 | 0.6 |
| Respiratory Rehabilitation | 1 | 0.6 |
| Spinal Unit | 1 | 0.6 |
| Urology | 1 | 0.6 |
| Total | 174 | 100 |
| Cultural Method | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Molecular assay | Positive | 39 | 77 | 116 |
| Negative | 0 | 56 | 56 | |
| Total | 39 | 133 | 172 | |
| Microorganism | Identified only by Molecular Methods | Identified only by Culture | Identified by Both Methods |
|---|---|---|---|
| Acinetobacter baumannii | 2 | 0 | 2 |
| Klebsiella pneumoniae | 8 | 0 | 5 |
| Pseudomonas aeruginosa | 15 | 0 | 14 |
| Proteus spp. | 5 | 0 | 4 |
| Enterobacter cloacae complex | 9 | 0 | 1 |
| Escherichia coli | 17 | 0 | 5 |
| Haemophilus influenzae | 19 | 0 | 1 |
| Staphylococcus aureus | 28 | 0 | 12 |
| Stenotrophomonas maltophilia | 0 | 1 | 0 |
| Klebsiella oxytoca | 12 | 0 | 0 |
| Citrobacter freundii | 0 | 1 | 0 |
| Streptococcus agalactiae | 4 | 0 | 0 |
| Moraxella catarrhalis | 10 | 0 | 0 |
| Streptococcus pneumoniae | 9 | 0 | 0 |
| Klebsiella aerogenes | 4 | 0 | 1 |
| Serratia marcescens | 4 | 0 | 1 |
| Total | 146 | 2 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leli, C.; Bottino, P.; Ferrara, L.; Matteo, L.D.; Gotta, F.; Vay, D.; Cornaglia, E.; Zenato, M.; Di Bella, C.; Scomparin, E.; et al. Application of Real-Time PCR Syndromic Panel on Lower Respiratory Tract Samples: Potential Use for Antimicrobial De-Escalation. Microorganisms 2025, 13, 1678. https://doi.org/10.3390/microorganisms13071678
Leli C, Bottino P, Ferrara L, Matteo LD, Gotta F, Vay D, Cornaglia E, Zenato M, Di Bella C, Scomparin E, et al. Application of Real-Time PCR Syndromic Panel on Lower Respiratory Tract Samples: Potential Use for Antimicrobial De-Escalation. Microorganisms. 2025; 13(7):1678. https://doi.org/10.3390/microorganisms13071678
Chicago/Turabian StyleLeli, Christian, Paolo Bottino, Lidia Ferrara, Luigi Di Matteo, Franca Gotta, Daria Vay, Elisa Cornaglia, Mattia Zenato, Chiara Di Bella, Elisabetta Scomparin, and et al. 2025. "Application of Real-Time PCR Syndromic Panel on Lower Respiratory Tract Samples: Potential Use for Antimicrobial De-Escalation" Microorganisms 13, no. 7: 1678. https://doi.org/10.3390/microorganisms13071678
APA StyleLeli, C., Bottino, P., Ferrara, L., Matteo, L. D., Gotta, F., Vay, D., Cornaglia, E., Zenato, M., Di Bella, C., Scomparin, E., Bolla, C., Bonato, V., Savi, L., Roveta, A., Maconi, A., & Rocchetti, A. (2025). Application of Real-Time PCR Syndromic Panel on Lower Respiratory Tract Samples: Potential Use for Antimicrobial De-Escalation. Microorganisms, 13(7), 1678. https://doi.org/10.3390/microorganisms13071678