Phosphoproteome Reveals the Role of Baicalin in Alleviating rPVL-Induced Cell Cycle Arrest in BMECs
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and rPVL Production and Purification
2.2. Cell Culture and Sample Treatment
2.3. SDT Digestion of Whole-Cell Lysates and LC-MS/MS Analysis in 4D Data-Independent Acquisition (DIA) Mode
2.4. Cell Cycle Analysis
2.5. Western Blot Analysis
2.6. Quantitative Immunofluorescence (IF) Analysis
2.7. Mouse Experimental Groups and Drug Treatments
2.8. Hematoxylin–Eosin (H&E) Staining
2.9. Quantitative Immunohistochemical Analysis
2.10. Statistical Analysis
3. Results
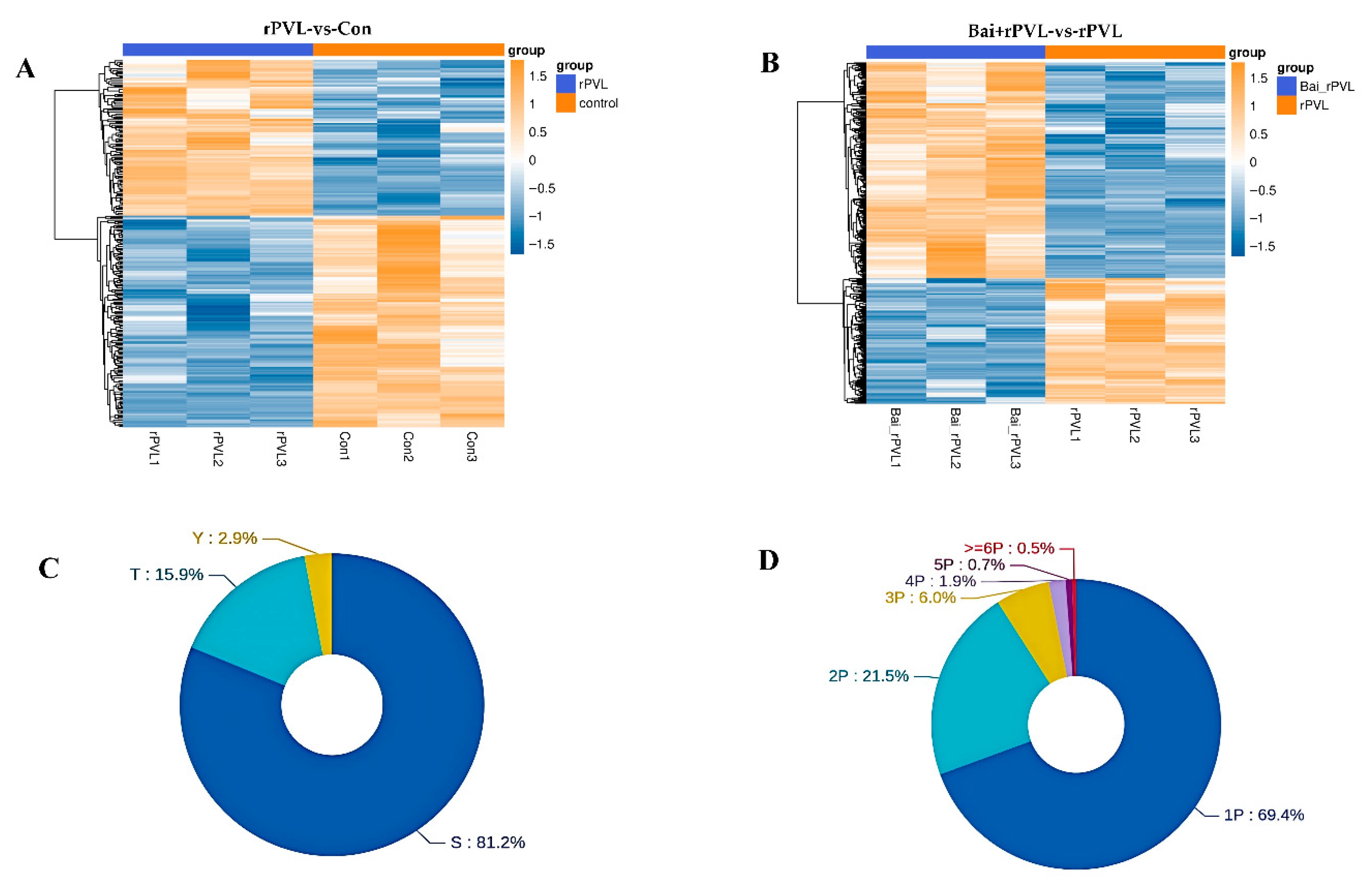
3.1. Differentially Expressed Phosphorylated Proteins (DEPPs)
3.2. KEGG Pathway and Gene Ontology (GO) Analysis of DEPPs
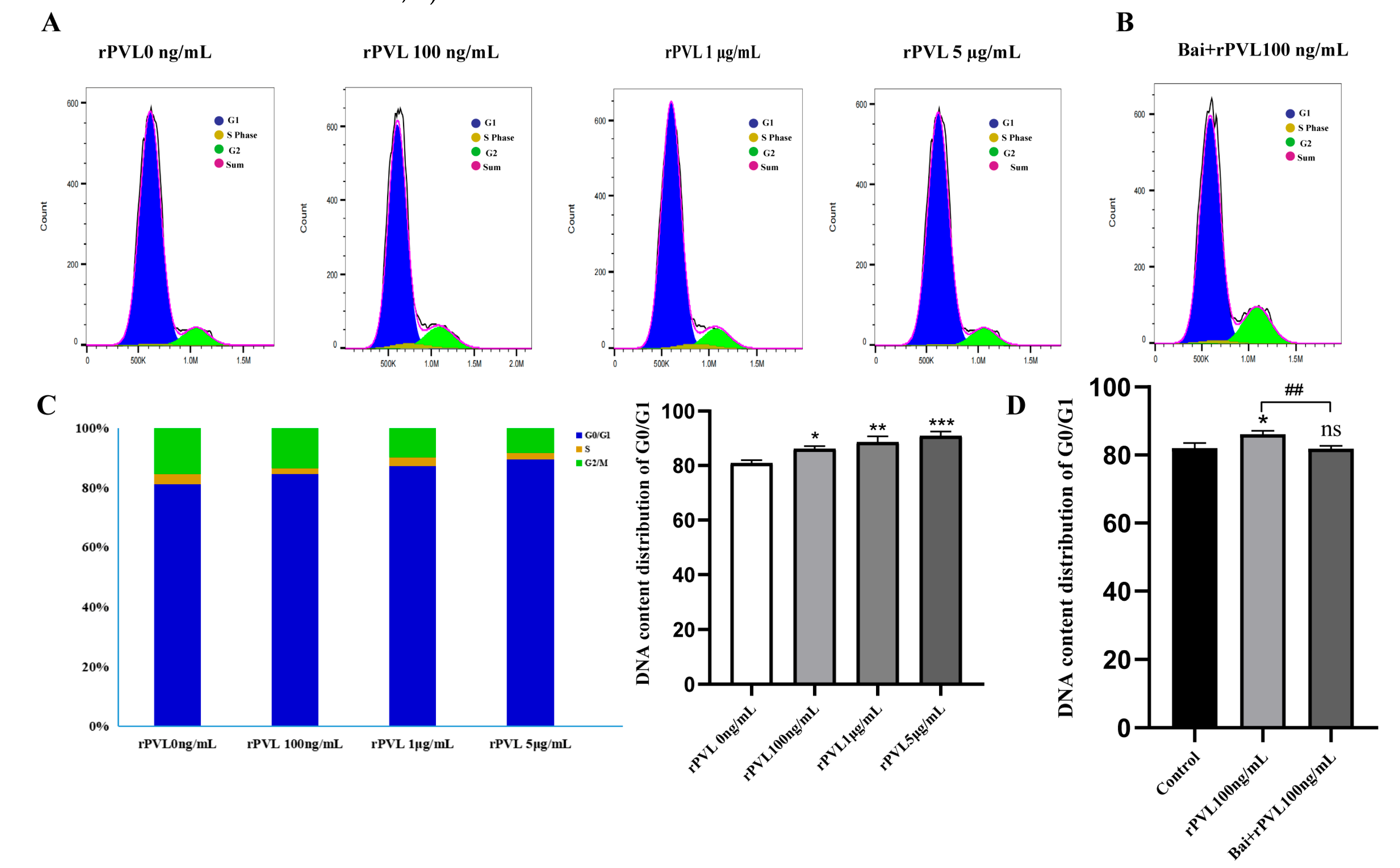
3.3. Baicalin Alleviated G0/G1 Cycle Arrest in the BMECs Induced by rPVL
3.4. Changes in the Expression of Cell-Cycle-Related Proteins In Vitro
3.5. Histopathological Changes in Mammary Tissue and Baicalin-Mediated Regulation of the Dephosphorylation of Cell-Cycle-Regulating Proteins Induced by rPVL In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Pu, W.; Su, Y.; Li, J.; Li, C.; Yang, Z.; Deng, H.; Ni, C. High Incidence of Oxacillin-Susceptible mecA-Positive Staphylococcus aureus (OS-MRSA) Associated with Bovine Mastitis in China. PLoS ONE 2014, 9, e88134. [Google Scholar]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Tucker, I.G.; Jain, R.; Alawi, F.; Nanjan, K.; Bork, O. Translational studies on a ready-to-use intramuscular injection of penethamate for bovine mastitis. Drug Deliv. Transl. Res. 2017, 8, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Robles, T.; Torres, V.J. Staphylococcus aureus Pore-Forming Toxins. In Staphylococcus aureus; Springer: Cham, Switzerland, 2016; pp. 121–144. [Google Scholar]
- Banerji, R.; Karkee, A.; Kanojiya, P.; Saroj, S.D. Pore-forming toxins of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2265–2285. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2015, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ponmalar, I.I.; Cheerla, R.; Ayappa, K.G.; Basu, J.K. Correlated protein conformational states and membrane dynamics during attack by pore-forming toxins. Proc. Natl. Acad. Sci. USA 2019, 116, 12839–12844. [Google Scholar] [CrossRef] [PubMed]
- Escajadillo, T.; Nizet, V. Pharmacological Targeting of Pore-Forming Toxins as Adjunctive Therapy for Invasive Bacterial Infection. Toxins 2018, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Bischofberger, M.; Pernot, L.; van der Goot, F.G.; Frêche, B. Bacterial pore-forming toxins: The (w)hole story? Cell. Mol. Life Sci. 2007, 65, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Woodin, A.M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem. J. 1959, 73, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Nemali, S.; Siemsen, D.W.; Nelson, L.K.; Bunger, P.L.; Faulkner, C.L.; Rainard, P.; Gauss, K.A.; Jutila, M.A.; Quinn, M.T. Molecular analysis of the bovine anaphylatoxin C5a receptor. J. Leukoc. Biol. 2008, 84, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y. Infectious status of mastitis in dairy of mastitis in dairy cattle induced by Staphylococcus aureus and its advances on epidemiological patterns and antimicrobial resistance in northern china. Acta Vet. Zootech. Sin. 2015, 46, 1477–1488. [Google Scholar]
- Ma, W.W.; Zhou, X.Z. Expression of panton-valenine leukocidin from staphylococcus aureus and Its Effects on Bovine Mammary Epithelial Cells. Acta Vet. Zootech. Sin. 2019, 50, 2105–2112. [Google Scholar]
- Sadat, A.; Shata, R.R.; Farag, A.M.M.; Ramadan, H.; Alkhedaide, A.; Soliman, M.M.; Elbadawy, M.; Abugomaa, A.; Awad, A. Prevalence and Characterization of PVL-Positive Staphylococcus aureus Isolated from Raw Cow’s Milk. Toxins 2022, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Seker, E.; Ozenc, E.; Turedi, O.K.; Yilmaz, M. Prevalence of mecA and pvl genes in coagulase negative staphylococci isolated from bovine mastitis in smallholder dairy farms in Turkey. Anim. Biotechnol. 2022, 34, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Shi, L.; Zhang, S.S.; Ding, P.S.; Ma, F.; Song, K.D.; Qiang, P.; Chang, W.J.; Dai, Y.Y.; Mei, Y.D.; et al. LukS-PV Induces Apoptosis via the SET8-H4K20me1-PIK3CB Axis in Human Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 11, 718–791. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Ma, F.; Wang, Z.; Nie, Z.; Xu, L.; Ding, P.; Ma, X. LukS-PV induces cell cycle arrest and apoptosis through p38/ERK MAPK signaling pathway in NSCLC cells. Biochem. Biophys. Res. Commun. 2020, 521, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-X.; Zhang, S.-S.; Dai, C.-Y.; Peng, J.; Pan, Q.; Xu, L.-F.; Ma, X.-L. LukS-PV-Regulated MicroRNA-125a-3p Promotes THP-1 Macrophages Differentiation and Apoptosis by Down-Regulating NF1 and Bcl-2. Cell. Physiol. Biochem. 2017, 44, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Xie, Q.; Chang, W.; Huo, X.; Chen, F.; Ma, X. LukS-PV induces mitochondrial-mediated apoptosis and G0/G1 cell cycle arrest in human acute myeloid leukemia THP-1 cells. Int. J. Biochem. Cell Biol. 2013, 45, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, B.; Luo, Z.-Q.; Qiu, J.; Zhou, X.; Li, G.; Zhang, B.; Deng, X.; Yang, Z.; Wang, J. The combination of osthole with baicalin protects mice from Staphylococcus aureus pneumonia. World J. Microbiol. Biotechnol. 2016, 33, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hai, Z.; Hou, L.; Liu, Y.; Zhang, D.; Zhou, X. Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 14520. [Google Scholar] [CrossRef] [PubMed]
- Heininen, J.; Erbacher, C.; Kotiaho, T.; Kostiainen, R.; Teppo, J. Enzymatic Phosphorylation of Oxidized Tyrosine Residues. J. Proteome Res. 2023, 22, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, P.; Wan, Q.; Ruan, X.; Wu, P.; Hao, Y.; Zhang, Z.; Sun, J.; Nie, W.; Chen, S. High-Coverage Four-Dimensional Data-Independent Acquisition Proteomics and Phosphoproteomics Enabled by Deep Learning-Driven Multidimensional Predictions. Anal. Chem. 2023, 95, 7495–7502. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R. Overview of Bacterial Protein Toxins from Pathogenic Bacteria: Mode of Action and Insights into Evolution. Toxins 2024, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and Characterization of Staphylococcus aureus Cultured From Raw Milk Taken From Dairy Cows with Mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Fritzwanker, S.; Schulz, S.; Kliewer, A. SR-17018 Stimulates Atypical µ-Opioid Receptor Phosphorylation and Dephosphorylation. Molecules 2021, 26, 4509. [Google Scholar] [CrossRef] [PubMed]
- Medema, R.H.; Lindqvist, A. Boosting and suppressing mitotic phosphorylation. Trends Biochem. Sci. 2011, 36, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Cell Cycle Progression Synchronization: An Overview. In Cell-Cycle Synchronization: Methods Protocols; Wang, Z., Ed.; Springer: New York, NY, USA, 2022; pp. 3–23. [Google Scholar]
- Roskoski, R. Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol. Res. 2016, 107, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Düster, R.; Anand, K.; Binder, S.C.; Schmitz, M.; Gatterdam, K.; Fisher, R.P.; Geyer, M. Structural basis of Cdk7 activation by dual T-loop phosphorylation. Nat. Commun. 2024, 15, 6597. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Semenza, G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Han, Y.; Pan, W.; Du, J.; Zuo, D.; Ba, Y.; Zhang, H. Tyrosine phosphatase SHP2 aggravates tumor progression and glycolysis by dephosphorylating PKM2 in gastric cancer. MedComm 2024, 5, e527. [Google Scholar] [CrossRef] [PubMed]
- Mandati, V.; Del Maestro, L.; Dingli, F.; Lombard, B.; Loew, D.; Molinie, N.; Romero, S.; Bouvard, D.; Louvard, D.; Gautreau, A.M.; et al. Phosphorylation of Merlin by Aurora A kinase appears necessary for mitotic progression. J. Biol. Chem. 2019, 294, 12992–13005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xue, T.; Shao, A.; Lang, Y.; Qin, C.; Zhao, M.; Kuang, Y.; Yu, Z.; Geng, Y.; Zhao, C.; et al. Bclaf1 regulates c-FLIP expression and protects cells from TNF-induced apoptosis and tissue injury. EMBO Rep. 2021, 23, e52702. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Sun, Z.; Duan, Y.; Wang, F.; Wei, G.; Yang, J.-H. Bcl-2-associated transcription factor 1 Ser290 phosphorylation mediates DNA damage response and regulates radiosensitivity in gastric cancer. J. Transl. Med. 2021, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, B.; Wu, D.; Zhang, F. BCLAF1 induces cisplatin resistance in lung cancer cells. Oncol. Lett. 2020, 20, 227. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Li, J.; Wang, J.; You, Q.; Zhang, D.; Zhou, X. Phosphoproteome Reveals the Role of Baicalin in Alleviating rPVL-Induced Cell Cycle Arrest in BMECs. Microorganisms 2025, 13, 1673. https://doi.org/10.3390/microorganisms13071673
Hou L, Li J, Wang J, You Q, Zhang D, Zhou X. Phosphoproteome Reveals the Role of Baicalin in Alleviating rPVL-Induced Cell Cycle Arrest in BMECs. Microorganisms. 2025; 13(7):1673. https://doi.org/10.3390/microorganisms13071673
Chicago/Turabian StyleHou, Ling, Jun Li, Juqing Wang, Qin You, Dongtao Zhang, and Xuezhang Zhou. 2025. "Phosphoproteome Reveals the Role of Baicalin in Alleviating rPVL-Induced Cell Cycle Arrest in BMECs" Microorganisms 13, no. 7: 1673. https://doi.org/10.3390/microorganisms13071673
APA StyleHou, L., Li, J., Wang, J., You, Q., Zhang, D., & Zhou, X. (2025). Phosphoproteome Reveals the Role of Baicalin in Alleviating rPVL-Induced Cell Cycle Arrest in BMECs. Microorganisms, 13(7), 1673. https://doi.org/10.3390/microorganisms13071673





