Glucoselipid Biosurfactant Biosynthesis Operon of Rouxiella badensis DSM 100043T: Screening, Identification, and Heterologous Expression in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacterial Strains
2.2. Genomic Library Screening
2.2.1. Preparation of Genomic DNA
2.2.2. Creation of Genomic Library
2.2.3. High-Throughput Conjugation and Screening
2.2.4. Bioinformatic Analysis of the Pathway Gene Products
2.2.5. Cloning of ORFs into the pET21a(+) System
2.3. Gene Permutation and Expression Analysis
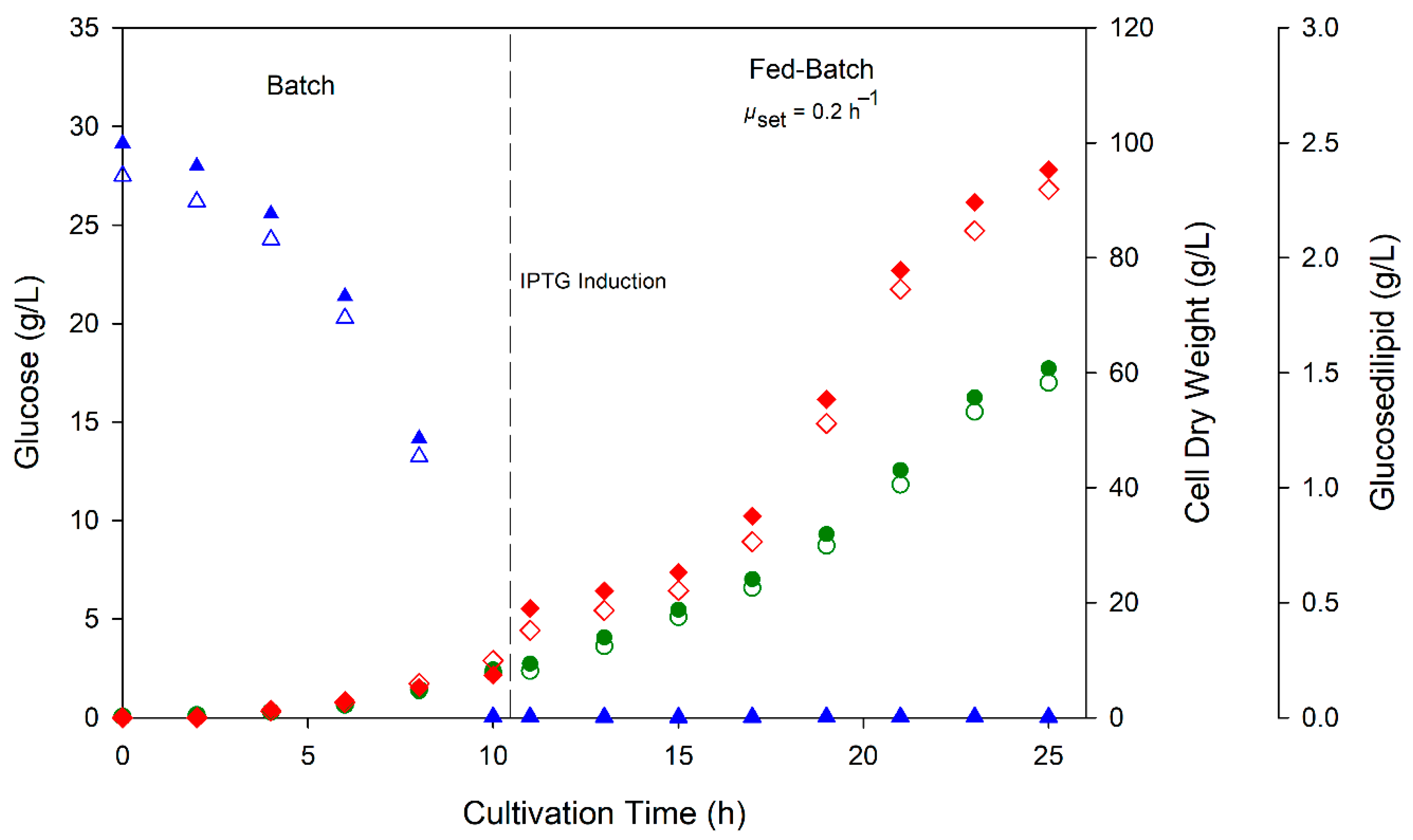
2.4. Fed-Batch Bioreactor Cultivation
2.5. Structure Elucidation of Produced Glucoselipids
2.5.1. Glucoselipids Extraction and Purification
2.5.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.5.3. Mass Spectrometry Characterization
3. Results
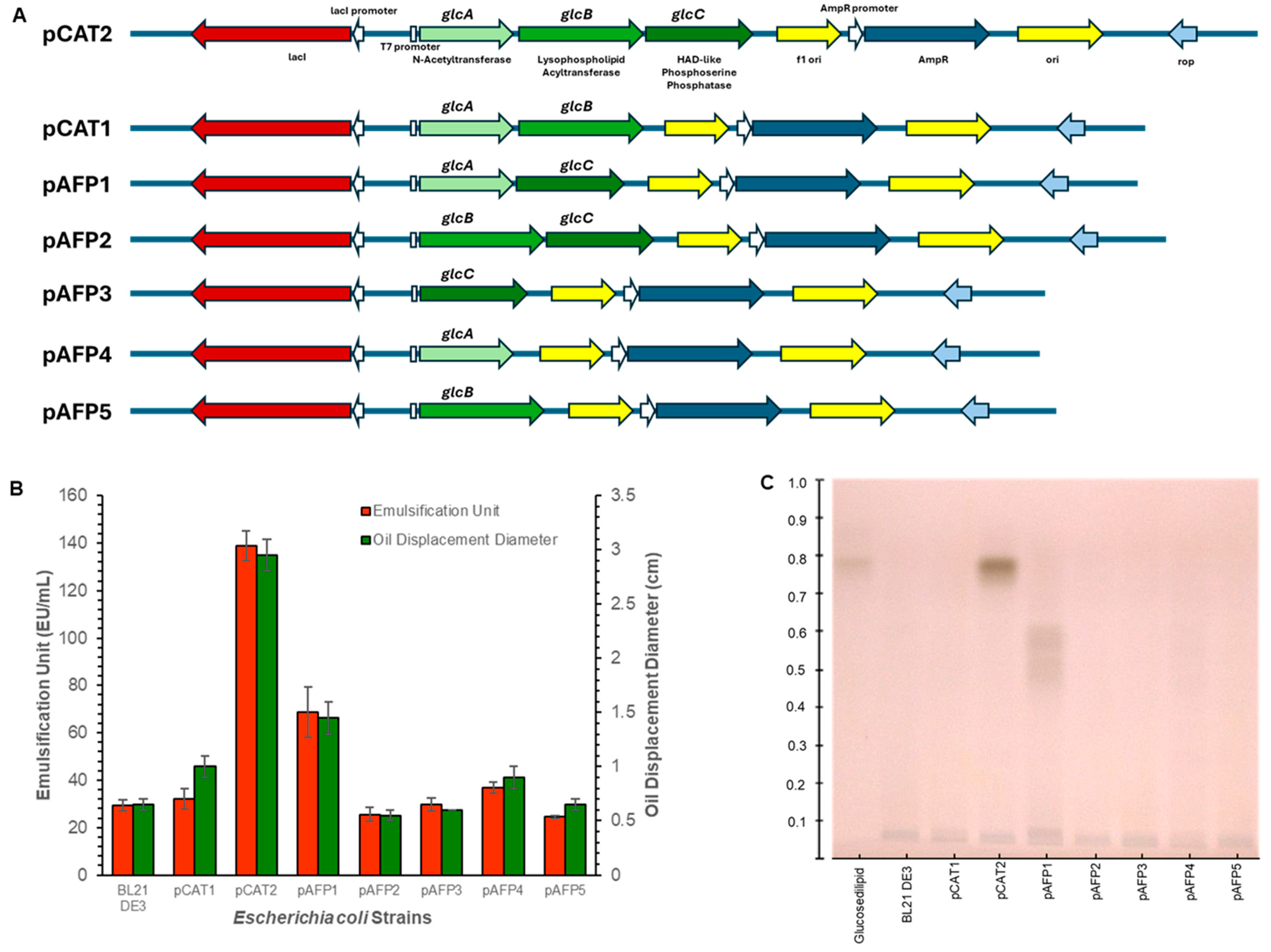
3.1. Identification of Genes Responsible for Glucoselipid Biosynthesis and Delineation of Their Functional Roles
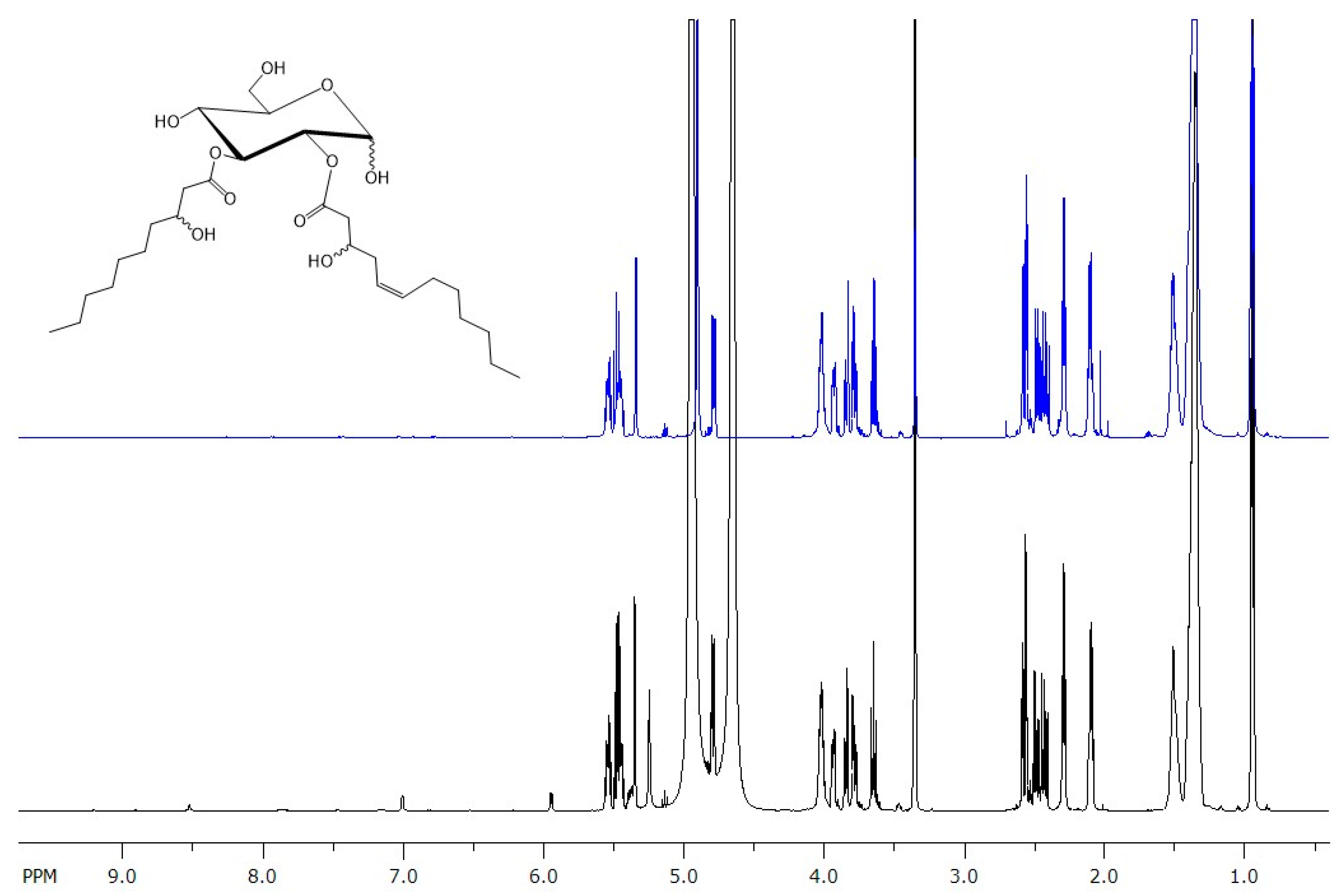
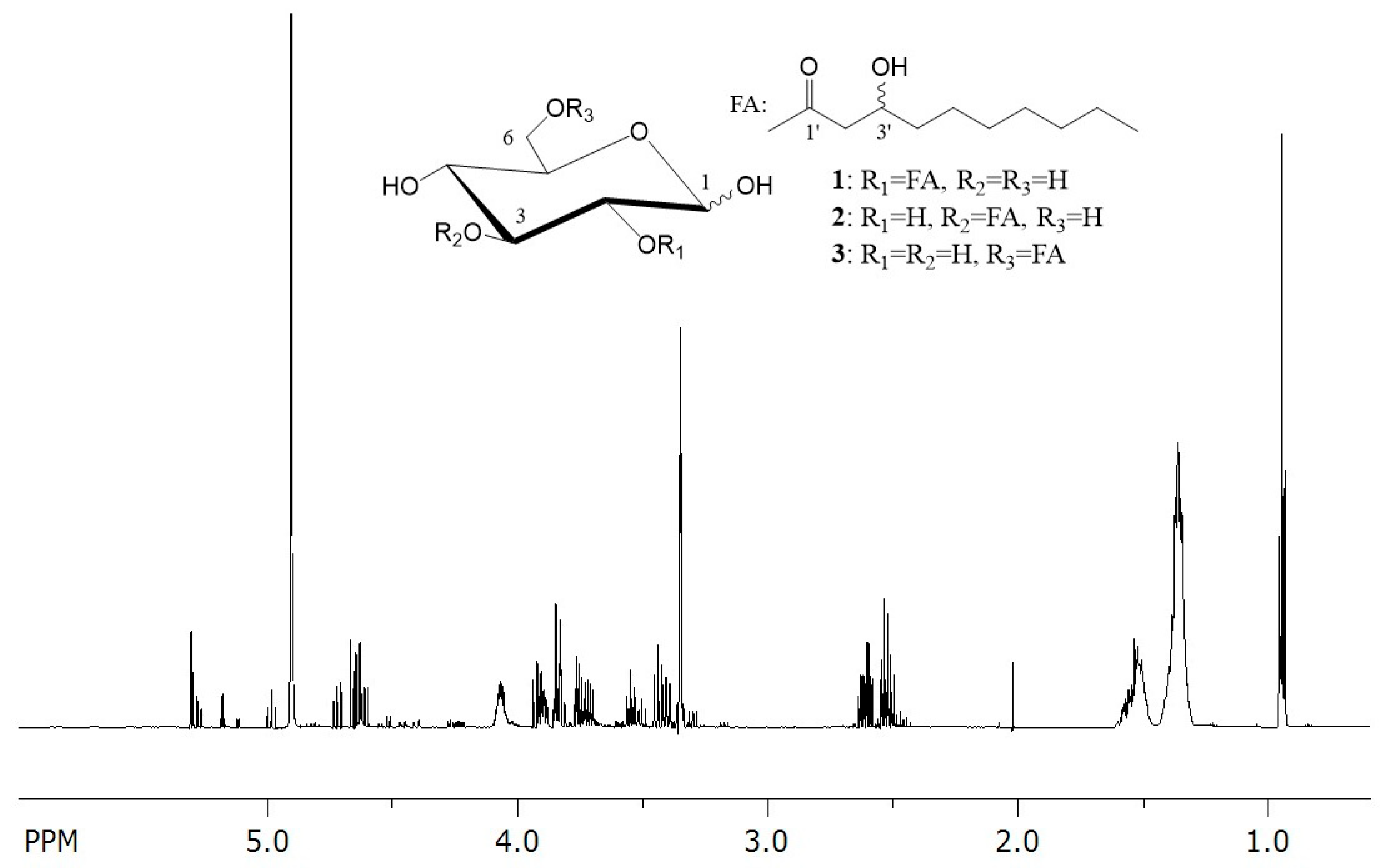
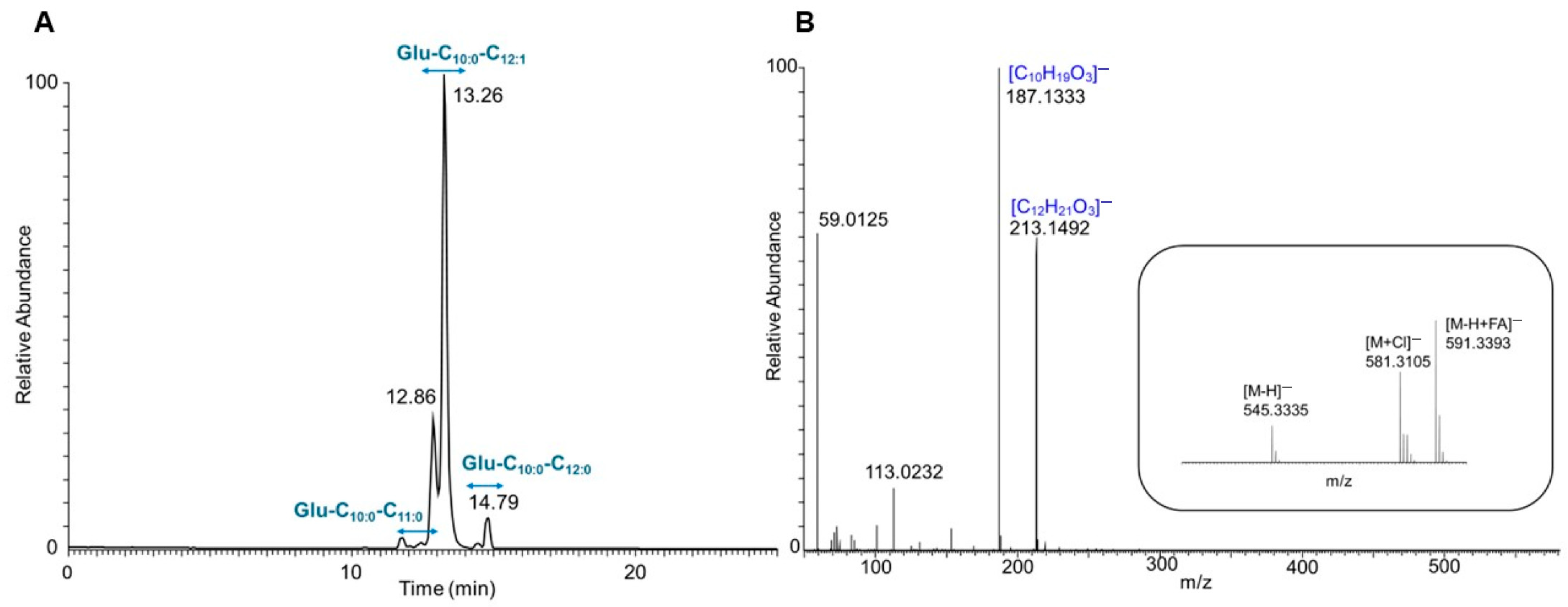
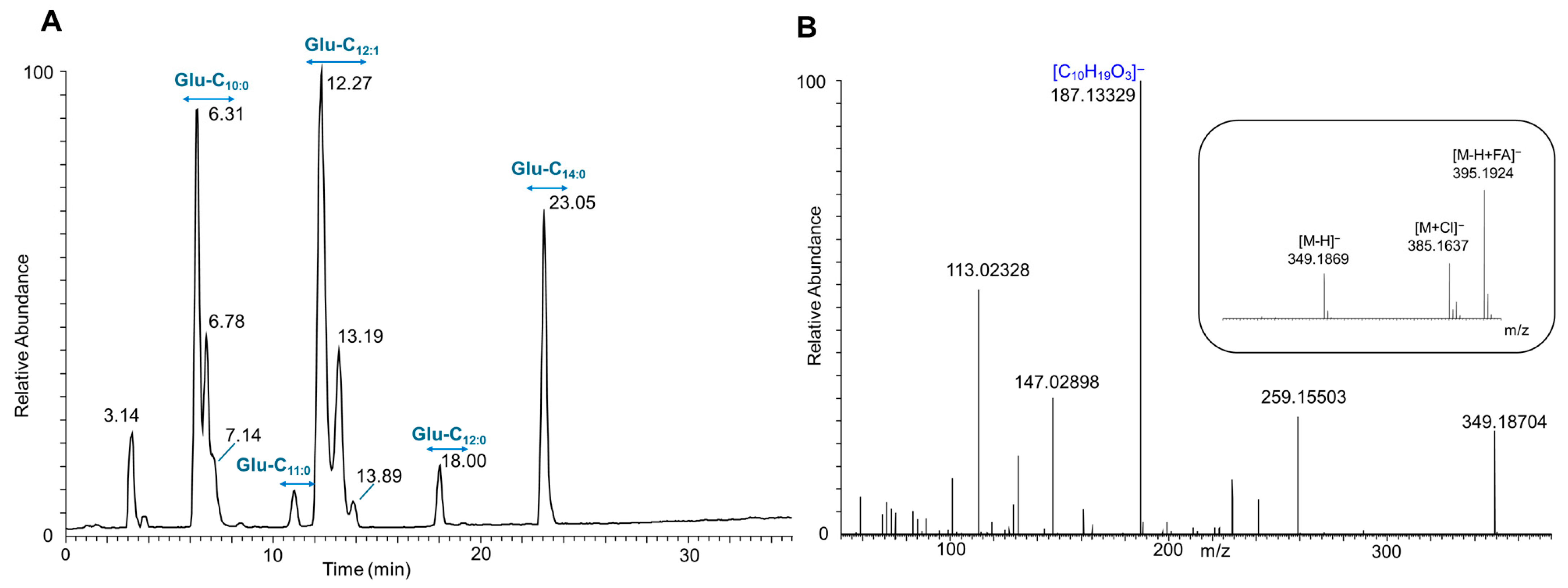
3.2. Structural Characterization of Produced Glucoselipids
3.3. Fed-Batch Bioreactor Cultivation of E. coli pCAT2 for Glucosedilipid Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| CDW | Cell dry weight |
| CMC | Critical micelle concentration |
| COSY | Correlation Spectroscopy |
| DNA | Deoxyribonucleic Acid |
| DPA | Diphenylamine-aniline-phosphoric acid |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HPTLC | High-performance thin-layer chromatography |
| HSQC | Heteronuclear Single-Quantum Coherence |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| LB | Luria–Bertani |
| LC-ESI/MS | Liquid chromatography/electrospray ionization mass spectrometry |
| MSM | Mineral salt medium |
| NMR | Nuclear magnetic resonance |
| OD600 | Optical density at 600 nm |
| ORF | Open reading frame |
| PCR | Polymerase chain reaction |
| Rf | Retardation factor |
| RT | Retention time |
| TOCSY | Total Correlation Spectroscopy |
| TIC | Total ion chromatogram |
| TLC | Thin-layer chromatography |
| XIC | Extracted ion chromatogram |
References
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Acta BBA—Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2018, 3, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskaya, E.; Kulakovskaya, T. Extracellular Glycolipids of Yeasts: Biodiversity, Biochemistry, and Prospects; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Zhang, J.; Soetaert, W. Microbial synthesis of sophorolipids. Process. Biochem. 2011, 46, 821–833. [Google Scholar] [CrossRef]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S.; et al. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Kügler, J.H.; Muhle-Goll, C.; Hansen, S.H.; Völp, A.R.; Kirschhöfer, F.; Kühl, B.; Brenner-Weiss, G.; Luy, B.; Syldatk, C.; Hausmann, R. Glycolipids produced by Rouxiella sp. DSM 100043 and isolation of the biosurfactants via foam-fractionation. AMB Express 2015, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Le Flèche-Matéos, A.; Kugler, J.H.; Hansen, S.H.; Syldatk, C.; Hausmann, R.; Lomprez, F.; Vandenbogaert, M.; Manuguerra, J.-C.; Grimont, P.A.D. Rouxiella badensis sp. nov. and Rouxiella silvae sp. nov. isolated from peat bog soil and emendation description of the genus Rouxiella. Int. J. Syst. Evol. Microbiol. 2017, 67, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Harahap, A.F.P.; Conrad, J.; Wolf, M.; Pfannstiel, J.; Klaiber, I.; Grether, J.; Hiller, E.; Vahidinasab, M.; Salminen, H.; Treinen, C.; et al. Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T. Molecules 2025, 30, 1798. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.R.; Meyer, H.; Yakimov, M. Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim. Biophys. Acta—Lipids Lipid Metab. 1998, 1393, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kaneda, K.; Ishizuka, I.; Toida, T.; Yano, I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J. Bacteriol. 1990, 172, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J.; Liles, M.; Mann, D.; Riesenfeld, C.; Goodman, R.M. Cloning the metagenome: Culture-independent access to thediversity and functions of the uncultivated microbial world. Methods Microbiol. 2002, 33, 241–255. [Google Scholar] [CrossRef]
- Martinez, A.; Kolvek, S.J.; Yip, C.L.T.; Hopke, J.; Brown, K.A.; MacNeil, I.A.; Osburne, M.S. Genetically Modified Bacterial Strains and Novel Bacterial Artificial Chromosome Shuttle Vectors for Constructing Environmental Libraries and Detecting Heterologous Natural Products in Multiple Expression Hosts. Appl. Environ. Microbiol. 2004, 70, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.; Kunorozva, L.; Klaiber, I.; Henkel, M.; Pfannstiel, J.; Van Zyl, L.J.; Hausmann, R.; Burger, A.; Trindade, M. Novel metagenome-derived ornithine lipids identified by functional screening for biosurfactants. Appl. Microbiol. Biotechnol. 2019, 103, 4429–4441. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, F. Novel or improved expression and screening systems for high—Throughput discovery of new bioactive compounds from marine metagenomic libraries. In Marine Microbial Biodiversity, Bioinformatics, and Biotechnology; European Union’s Seventh Framework Programme: Brussels, Belgium, 2014. Available online: https://api.semanticscholar.org/CorpusID:221376119 (accessed on 22 May 2025).
- Wang, Y.; Zhang, Z.; Ruan, J. A proposal to transfer Microbispora bispora (Lechevalier 1965) to a new genus, Thermobispora gen. nov., as Thermobispora bispora comb. nov. Int. J. Syst. Bacteriol. 1996, 46, 933–938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liles, M.R.; Williamson, L.L.; Rodbumrer, J.; Torsvik, V.; Goodman, R.M.; Handelsman, J. Recovery, Purification, and Cloning of High-Molecular-Weight DNA from Soil Microorganisms. Appl. Environ. Microbiol. 2008, 74, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pandya, C.; Liu, C.; Al-Obaidi, N.F.; Wang, M.; Zheng, L.; Keating, S.T.; Aono, M.; Love, J.D.; Evans, B.; et al. Panoramic view of a superfamily of phosphatases through substrate profiling. Proc. Natl. Acad. Sci. USA 2015, 112, E1974–E1983. [Google Scholar] [CrossRef] [PubMed]
- Moi, D.; Bernard, C.; Steinegger, M.; Nevers, Y.; Langleib, M.; Dessimoz, C. Structural phylogenetics unravels the evolutionary diversification of communication systems in gram-positive bacteria and their viruses. bioRxiv 2023. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2013, 51, W587–W592. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Booth, T.J.; Van Wersch, B.; Van Grieken, L.; Medema, M.H.; Chooi, Y.H. cblaster: A remote search tool for rapid identification and visualization of homologous gene clusters. Bioinform. Adv. 2021, 1, vbab016. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A ‘one for all, all for one’ bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011, 498, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Chopade, B.A. Studies on bioemulsifier production by Acinetobacter strains isolated from healthy human skin. J. Appl. Microbiol. 2001, 91, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1488, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, D.; Schulz, V.; Knorre, W.; Pohl, H.-D.; Korz, D.; Sanders, E.; Roß, A.; Deckwer, W.-D. High cell density cultivation of Escherichia coli at controlled specific growth rate. J. Biotechnol. 1991, 20, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Henkel, M.; Zwick, M.; Beuker, J.; Willenbacher, J.; Baumann, S.; Oswald, F.; Neumann, A.; Siemann-Herzberg, M.; Syldatk, C.; Hausmann, R. Teaching bioprocess engineering to undergraduates: Multidisciplinary hands-on training in a one-week practical course. Biochem. Mol. Biol. Educ. 2015, 43, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Hiller, E.; Off, M.; Hermann, A.; Vahidinasab, M.; Perino, E.H.B.; Lilge, L.; Hausmann, R. The influence of growth rate-controlling feeding strategy on the surfactin production in Bacillus subtilis bioreactor processes. Microb. Cell Fact. 2024, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential Agents for Controlling Cellular Communication, Motility, and Antagonism. Front. Mol. Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tyson, C.; Gitaitis, R.; Kvitko, B.; Dutta, B. Rouxiella badensis, a new bacterial pathogen of onion causing bulb rot. Front. Microbiol. 2022, 13, 1054813. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, S.; Guidi, C.; De Mey, M. ‘Metabolic Burden’ Explained: Stress Symptoms and Its Related Responses Induced by (Over)Expression of (Heterologous) Proteins in Escherichia coli; BioMed Central Ltd.: London, UK, 2024. [Google Scholar] [CrossRef]
- Lennen, R.M.; Kruziki, M.A.; Kumar, K.; Zinkel, R.A.; Burnum, K.E.; Lipton, M.S.; Hoover, S.W.; Ranatunga, D.R.; Wittkopp, T.M.; Marner, W.D.; et al. Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 8114–8128. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Matsuoka, H.; Hirooka, K. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 2007, 66, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.T.; Mohammad, T.S.H.; Czub, M.P.; Majorek, K.A.; Arolli, X.; Variot, C.; Anonick, M.; Minor, W.; Ballicora, M.A.; Becker, D.P.; et al. Gcn5-Related N-Acetyltransferases (GNATs) with a Catalytic Serine Residue Can Play Ping-Pong Too. Front. Mol. Biosci. 2021, 8, 646046. [Google Scholar] [CrossRef] [PubMed]
- Asensio, T.; Dian, C.; Boyer, J.B.; Rivière, F.; Meinnel, T.; Giglione, C. A Continuous Assay Set to Screen and Characterize Novel Protein N-Acetyltransferases Unveils Rice General Control Non-repressible 5-Related N-Acetyltransferase2 Activity. Front. Plant Sci. 2022, 13, 832144. [Google Scholar] [CrossRef] [PubMed]
- Daigle, D.M.; Hughes, D.W.; Wright, G.D. Prodigious substrate specificity of AAC(6′)-APH(2″), an aminoglycoside antibiotic resistance determinant in enterococci and staphylococci. Chem. Biol. 1999, 6, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Kuhn, M.L.; Chruszcz, M.; Anderson, W.F.; Minor, W. Structural, functional, and inhibition studies of a Gcn5-related N-acetyltransferase (GNAT) superfamily protein PA4794: A new C-terminal lysine protein acetyltransferase from Pseudomonas aeruginosa. J. Biol. Chem. 2013, 288, 30223–30235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huffman, J.; Li, S.; Shen, Y.; Du, L. Unusual acylation of chloramphenicol in Lysobacter enzymogenes, a biocontrol agent with intrinsic resistance to multiple antibiotics. BMC Biotechnol. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Hubrich, F.; Bösch, N.M.; Chepkirui, C.; Morinaka, B.I.; Rust, M.; Gugger, M.; Robinson, S.L.; Vagstad, A.L.; Piel, J. Ribosomally derived lipopeptides containing distinct fatty acyl moieties. Proc. Natl. Acad. Sci. USA 2022, 119, e2113120119. [Google Scholar] [CrossRef] [PubMed]
- Frankel, B.A.; Blanchard, J.S. Mechanistic analysis of Mycobacterium tuberculosis Rv1347c, a lysine Nε-acyltransferase involved in mycobactin biosynthesis. Arch. Biochem. Biophys. 2008, 477, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.M.; Yao, J.; Gajewski, S.; Kumar, G.; Martin, E.W.; O Rock, C.; White, S.W. A two-helix motif positions the lysophosphatidic acid acyltransferase active site for catalysis within the membrane bilayer. Nat. Struct. Mol. Biol. 2017, 24, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Silvano, E.; Päuker, O.; Guillonneau, R.; Quareshy, M.; Murphy, A.; A Mausz, M.; Stirrup, R.; Rihtman, B.; Aguilo-Ferretjans, M.; et al. A novel class of sulfur-containing aminolipids widespread in marine roseobacters. ISME J. 2021, 15, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, G.; Heflik, L.; Czolkoss, S.; Heinrichs, F.; Kleetz, J.; Yesilyurt, C.; Tischler, D.; Westhoff, P.; Exterkate, M.; Aktas, M.; et al. Characterization of multiple lysophosphatidic acid acyltransferases in the plant pathogen Xanthomonas campestris. FEBS J. 2024, 291, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Aygun-Sunar, S.; Bilaloglu, R.; Goldfine, H.; Daldal, F. Rhodobacter capsulatus OlsA is a bifunctional enyzme active in both ornithine lipid and phosphatidic acid biosynthesis. J. Bacteriol. 2007, 189, 8564–8574. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Hrudikova, R.; Zotchev, S.B.; Ertesvåg, H. Identification of a new phosphatase enzyme potentially involved in the sugar phosphate stress response in Pseudomonas fluorescens. Appl. Environ. Microbiol. 2017, 83, e02361-16. [Google Scholar] [CrossRef] [PubMed]
- Kankanamge, L.S.P.; Ruffner, L.A.; Touch, M.M.; Pina, M.; Beuning, P.J.; Ondrechen, M.J. Functional annotation of haloacid dehalogenase superfamily structural genomics proteins. Biochem. J. 2023, 480, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Nocek, B.; Brown, G.; Makarova, K.S.; Flick, R.; Wolf, Y.I.; Khusnutdinova, A.; Evdokimova, E.; Jin, K.; Tan, K.; et al. Functional diversity of haloacid dehalogenase superfamily phosphatases from Saccharomyces cerevisiae: Biochemical, structural, and evolutionary insights. J. Biol. Chem. 2015, 290, 18678–18698. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Proudfoot, M.; Gonzalez, C.F.; Brown, G.; Omelchenko, M.V.; Borozan, I.; Carmel, L.; Wolf, Y.I.; Mori, H.; Savchenko, A.V.; et al. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J. Biol. Chem. 2006, 281, 36149–36161. [Google Scholar] [CrossRef] [PubMed]
- Bazire, A.; Dufour, A. The Pseudomonas aeruginosa rhlG and rhlAB genes are inversely regulated and RhlG is not required for rhamnolipid synthesis. BMC Microbiol. 2014, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Saerens, K.M.J.; Van Bogaert, I.N.A.; Soetaert, W. Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola. FEMS Yeast Res. 2015, 15, fov075. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Koike, H.; Fujii, T.; Morita, T. Targeted transcriptomic study of the implication of central metabolic pathways in mannosylerythritol lipids biosynthesis in Pseudozyma antarctica T-34. PLoS ONE 2020, 15, e0227295. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.F.; Gaspar, E.M.S.M. Simultaneous chromatographic separation of enantiomers, anomers and structural isomers of some biologically relevant monosaccharides. J. Chromatogr. A 2008, 1188, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Royce, L.A.; Liu, P.; Stebbins, M.J.; Hanson, B.C.; Jarboe, L.R. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl. Microbiol. Biotechnol. 2013, 97, 8317–8327. [Google Scholar] [CrossRef] [PubMed]

| Name | Genotype | Reference |
|---|---|---|
| Strains | ||
| Rouxiella badensis DSM 100043T | Wild-type | [8] |
| Escherichia coli BL21(DE3) | E. coli BL21 F- ompT hsdS(rB – mB –) dcm + Tetr gal λ(DE3) endA Hte [argU proL Camr] [argU ileY leuW Strep/Specr] | Agilent Technologies (Santa Clara, CA, USA) |
| EPI300 | F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL nupG trfA tonA dhfr | Epicentre (Illumina, San Diego, CA, USA) |
| K12 JM109 | [15] | |
| Pseudomonas putida MBD1 | P. putida KT2440 derivative; Kanr; φC31 attB site+ | [14] |
| 54F3 | P. putida MBD1 containing 54F3 fosmid for expression of lyso-ornithine lipid biosurfactant | [16] |
| Plasmids | ||
| pCCERI | pCC1FOS derivative, Cmr, Apr, φC31 integrase, attP | [17] |
| pER1.3.50.2 | pRK2013 derivative with trfA gene deleted, Kanr | [17] |
| Fosmid 1.8.H6 | pCCFOS1 clone containing 2,399,010 bp to 2,429,311 bp of R. badensis (CP114060) | This study |
| pET21a(+) | Expression vector with a C-terminal His-tag, Ampr, ori, T7 promoter and terminator, MCS | Novagen (Merck KGaA, Darmstadt, Germany) |
| pCAT2 | pET21a(+) derivative; ORF 1; ORF2; ORF3 | This study |
| pCAT1 | pET21a(+) derivative; ORF 1; ORF2 | This study |
| pAFP1 | pET21a(+) derivative; ORF 1; ORF3 | This study |
| pAFP2 | pET21a(+) derivative; ORF 2; ORF3 | This study |
| pAFP3 | pET21a(+) derivative; ORF3 | This study |
| pAFP4 | pET21a(+) derivative; ORF1 | This study |
| pAFP5 | pET21a(+) derivative; ORF 2 | This study |
| Parameters | E. coli pCAT2 Glucoselipids | E. coli pAFP1 Glucoselipids |
|---|---|---|
| Column temperature | 40 °C | 50 °C |
| Injection volume | 0.37 µL | 3.0 µL |
| Mobile phase | A: 0.2% formic acid in water B: 0.2% formic acid in acetonitrile | A: 0.2% formic acid in water B: 0.2% formic acid in methanol |
| Gradient elution | 50–53% B from 0 to 15 min, 53–67% B from 15 to 17 min, 67–75% B from 17 to 25 min, 75–90% B from 25 to 30 min, 90–90% B (isocratic) from 30 to 35 min, 90–50% B from 35 to 36 min, 50–50% B from 36 to 37 min | 45–53% B from 0 to 5 min, 53–59% B from 5 to 10 min, 59–90% B from 10 to 20 min, 90–90% B (isocratic) from 20 to 25 min, 90–45% B from 25 to 26 min |
| Scan range | 200–1500 m/z | 100–1400 m/z |
| (N)CE * | 32 | 10 |
| Protein Description | Organism | Prob. | E Value | Seq. Ident % | Accession |
|---|---|---|---|---|---|
| GlcA | |||||
| N-acetyltransferase domain-containing protein | Pseudomonas fluorescens | 1.00 | 8.92 × 10−23 | 40.4 | AF-A0A5E6RK03-F1-model_v4 |
| Crystal structure of a GNAT superfamily PA3944 acetyltransferase in complex with CoA | Pseudomonas aeruginosa PAO1 | 1.00 | 6.33 × 10−12 | 22 | 6EDD |
| GlcB | |||||
| Uncharacterized protein | Rouxiella badensis | 1.00 | 1.90 × 10−53 | 100 | AF-A0A1X0WHA5-F1-model_v4 |
| Crystal structure of the 1-acyl-sn-glycerophosphate (LPA) acyltransferase, PlsC | Thermotoga maritima MSB8 | 1.00 | 9.26 × 10−6 | 14.7 | 5KYM |
| GlcC | |||||
| Uncharacterized protein | Serratia sp. M24T3 | 1.00 | 1.18 × 10−39 | 77.8 | AF-I0QXG7-F1-model_v4 |
| Crystal structure of a phosphoserine phosphohydrolase-like protein | Francisella tularensis SCHU S4 | 1.00 | 7.70 × 10−13 | 23.4 | 3KD3 |
| Strain | Glucoselipid | Molecular Formula | RT * [min] | m/z [M-H]− | Fatty Acid’s m/z [M-H]− | Relative Abundance (%) | |
|---|---|---|---|---|---|---|---|
| E. coli pCAT2 | Glu-C10:0-C11:0 | C27H50O10 | 12.3 | 533.333 | C11H21O3− (201) | C10H19O3− (187) | 4.54 |
| 12.8 | |||||||
| Glu-C10:0-C12:1 | C28H50O10 | 12.8 | 545.333 | C12H21O3− (213) | C10H19O3− (187) | 91.04 | |
| 13.2 | |||||||
| Glu-C10:0-C12:0 | C28H52O10 | 14.4 | 547.349 | C12H23O3− (215) | C10H19O3− (187) | 4.42 | |
| 14.7 | |||||||
| E. coli pAFP1 | Glu-C10:0 | C16H30O8 | 6.3 | 349.186 | C10H19O3− (187) | - | 33.95 |
| 6.7 | |||||||
| 7.1 | |||||||
| Glu-C11:0 | C17H32O8 | 11.0 | 363.202 | C11H21O3− (201) | - | 3.63 | |
| 11.8 | |||||||
| Glu-C12:0 | C18H34O8 | 18.0 | 377.218 | C12H23O3− (215) | - | 4.07 | |
| 18.4 | |||||||
| Glu-C12:1 | C18H32O8 | 12.3 | 375.202 | C12H21O3− (213) | - | 41.44 | |
| 13.1 | |||||||
| 13.8 | |||||||
| Glu-C14:0 | C20H38O8 | 23.0 | 405.249 | C14H27O3− (243) | - | 16.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap, A.F.P.; Treinen, C.; Zyl, L.J.V.; Williams, W.T.; Conrad, J.; Pfannstiel, J.; Klaiber, I.; Grether, J.; Hiller, E.; Vahidinasab, M.; et al. Glucoselipid Biosurfactant Biosynthesis Operon of Rouxiella badensis DSM 100043T: Screening, Identification, and Heterologous Expression in Escherichia coli. Microorganisms 2025, 13, 1664. https://doi.org/10.3390/microorganisms13071664
Harahap AFP, Treinen C, Zyl LJV, Williams WT, Conrad J, Pfannstiel J, Klaiber I, Grether J, Hiller E, Vahidinasab M, et al. Glucoselipid Biosurfactant Biosynthesis Operon of Rouxiella badensis DSM 100043T: Screening, Identification, and Heterologous Expression in Escherichia coli. Microorganisms. 2025; 13(7):1664. https://doi.org/10.3390/microorganisms13071664
Chicago/Turabian StyleHarahap, Andre Fahriz Perdana, Chantal Treinen, Leonardo Joaquim Van Zyl, Wesley Trevor Williams, Jürgen Conrad, Jens Pfannstiel, Iris Klaiber, Jakob Grether, Eric Hiller, Maliheh Vahidinasab, and et al. 2025. "Glucoselipid Biosurfactant Biosynthesis Operon of Rouxiella badensis DSM 100043T: Screening, Identification, and Heterologous Expression in Escherichia coli" Microorganisms 13, no. 7: 1664. https://doi.org/10.3390/microorganisms13071664
APA StyleHarahap, A. F. P., Treinen, C., Zyl, L. J. V., Williams, W. T., Conrad, J., Pfannstiel, J., Klaiber, I., Grether, J., Hiller, E., Vahidinasab, M., Perino, E. H. B., Lilge, L., Burger, A., Trindade, M., & Hausmann, R. (2025). Glucoselipid Biosurfactant Biosynthesis Operon of Rouxiella badensis DSM 100043T: Screening, Identification, and Heterologous Expression in Escherichia coli. Microorganisms, 13(7), 1664. https://doi.org/10.3390/microorganisms13071664







