L-Arabinose Alters the E. coli Transcriptome to Favor Biofilm Growth and Enhances Survival During Fluoroquinolone Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Selection and Cultivation
2.2. Growth Curves
2.3. Crystal Violet Assay
2.4. Biofilm Growth on Agar and EPS Harvesting
2.5. Confocal Laser Scanning Microscopy (CLSM)
2.6. RNA Harvesting
2.7. RNA-Seq Data Collection and Analysis
2.8. RT-PCR/qPCR
3. Results
3.1. Arabinose and Levofloxacin Influence E. coli Planktonic Cell and Biofilm Growth
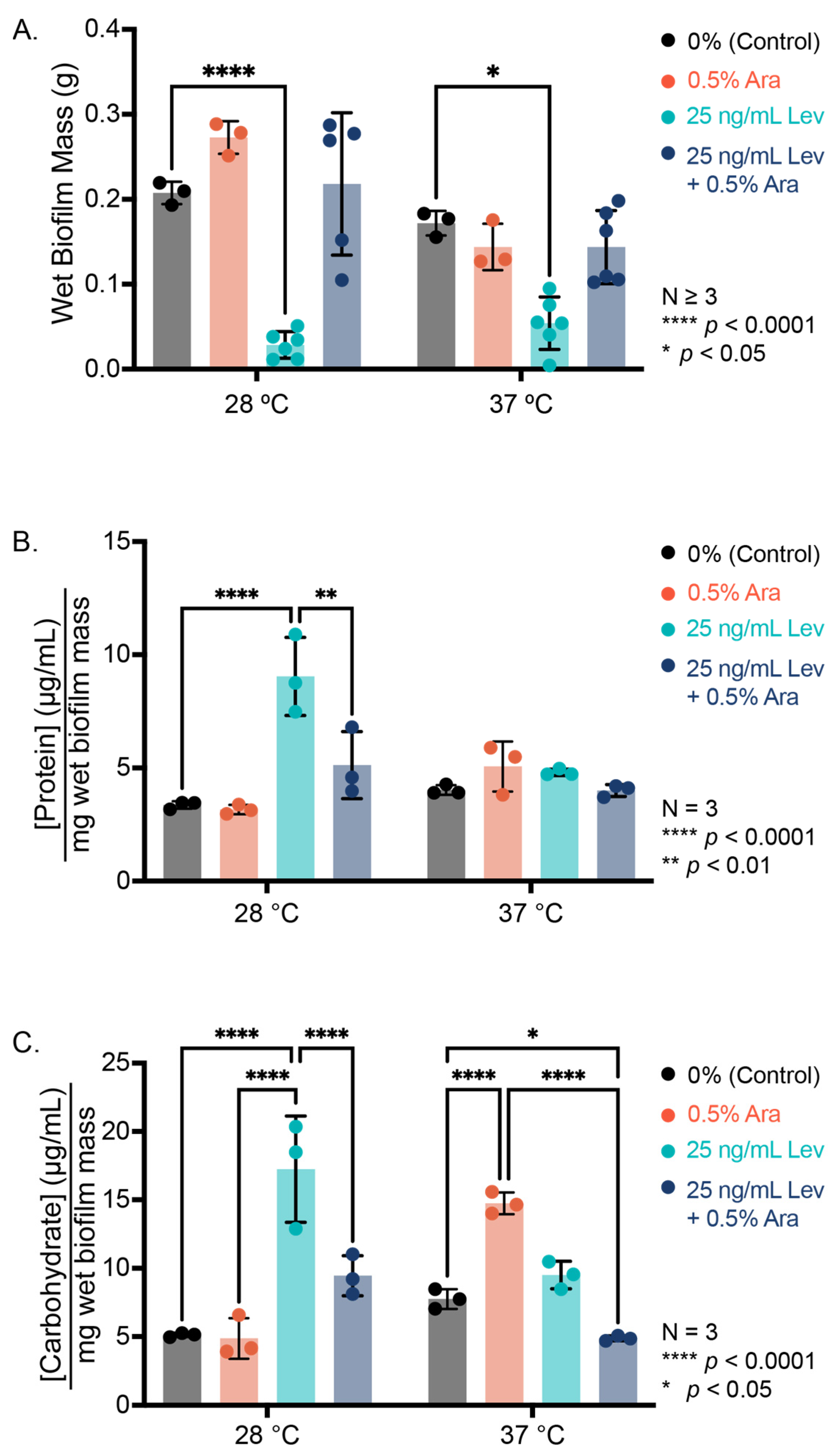
3.2. Arabinose Addition Changes Biofilm EPS Composition
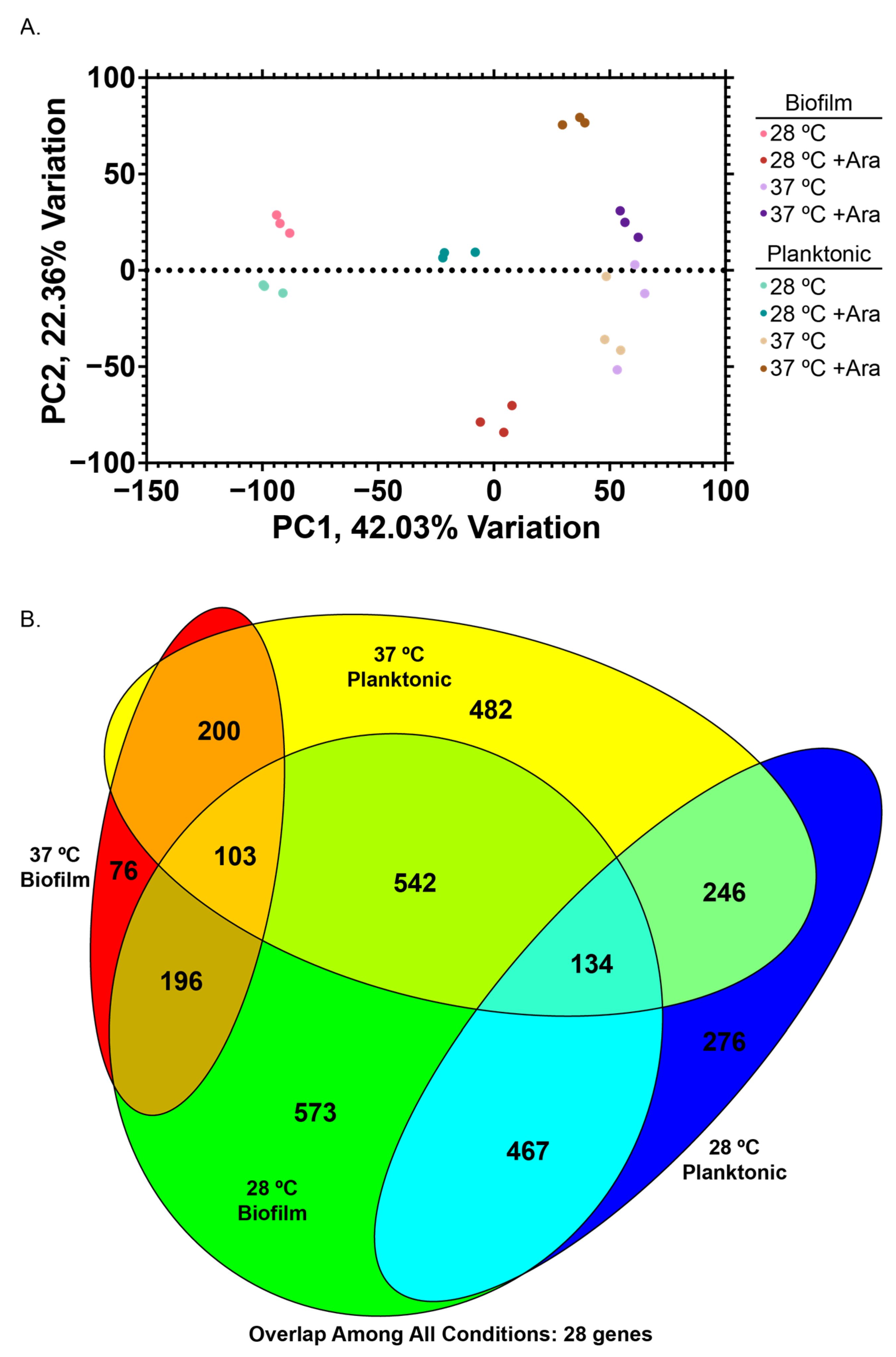
3.3. Examining Changes to the E. coli Transcriptome with Arabinose Addition
3.4. Influence of Arabinose on Gene Expression at 37 °C
3.5. Influence of Arabinose on Gene Expression at 28 °C
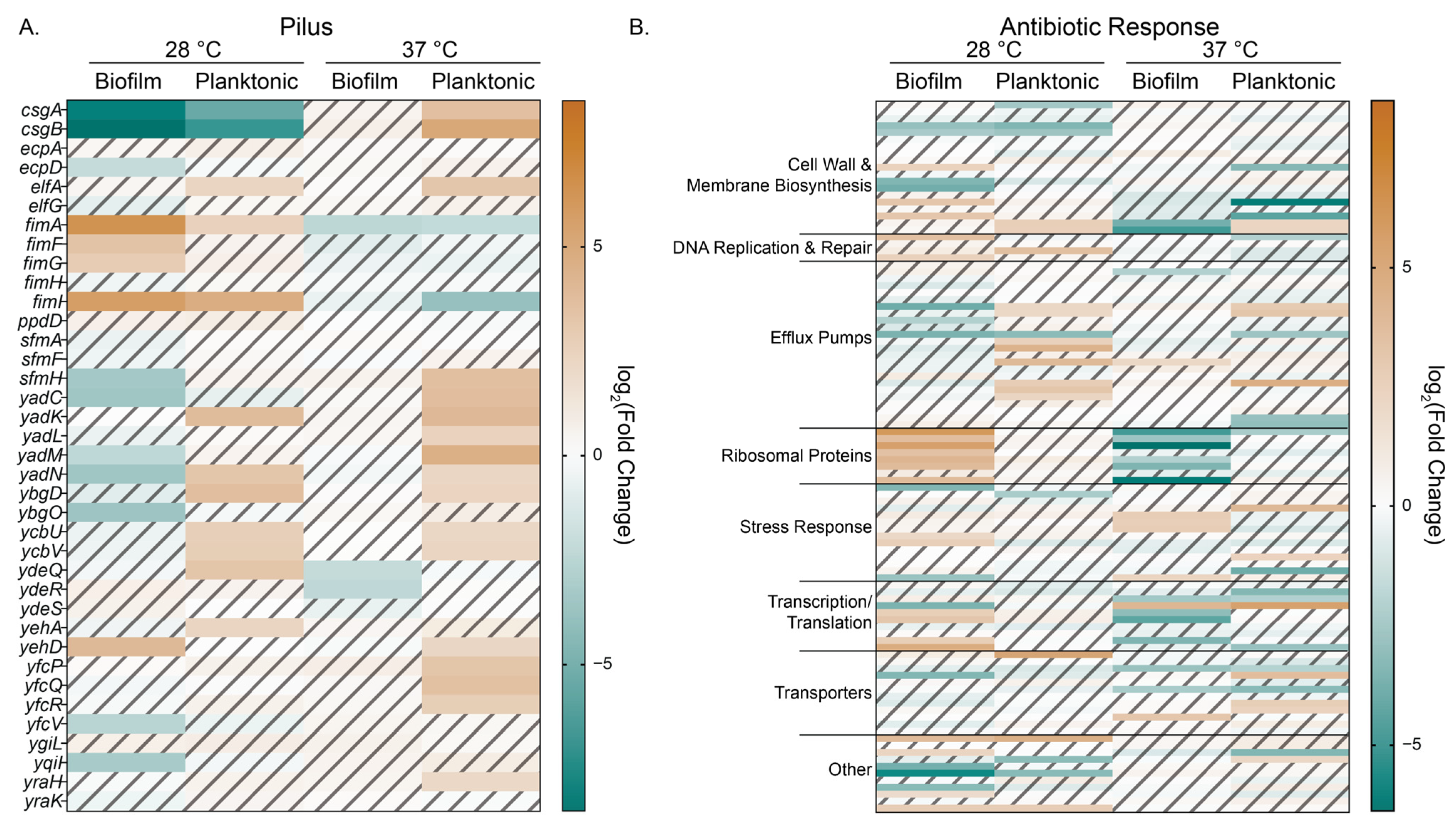
3.6. Arabinose Changes Expression of Genes RELATED to Extracellular Structures, Efflux, Transporters, and Antibiotic Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Extracellular polymeric substance |
| DEG | Differentially expressed gene |
| Ara | L-arabinose |
| Lev | Levofloxacin |
References
- Ganipisetti, V.M.; Dudiki, N.; Athavale, A. A Diagnostic Quandary of Escherichia coli Pneumonia: A Case Report and Literature Review. Cureus 2023, 15, e39668. [Google Scholar] [CrossRef] [PubMed]
- Houdt, R.V.; Michiels, C.W. Role of Bacterial Cell Surface Structures in Escherichia coli Biofilm Formation. Res. Microbiol. 2005, 156, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. PH Regulates Genes for Flagellar Motility, Catabolism, and Oxidative Stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L.; Marshall, J.; Holmes, A.; Wright, K.M.; Rossez, Y.; Merget, B.; Humphris, S.; Toth, I.; Jackson, R.W.; Holden, N.J. The Role of L-Arabinose Metabolism for Escherichia coli O157:H7 in Edible Plants. Microbiology 2021, 167, 001070. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Wang, X.; Rao, C.V. Reciprocal Regulation of L-Arabinose and d-Xylose Metabolism in Escherichia coli. J. Bacteriol. 2016, 198, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.A.; Rao, C.V. Regulation of Arabinose and Xylose Metabolism in Escherichia coli. Appl. Environ. Microb. 2010, 76, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shen, P.; He, Y.; Zhang, Y.; Liu, J. Spatial Transcriptome Uncovers Rich Coordination of Metabolism in E. coli K12 Biofilm. Nat. Chem. Biol. 2023, 19, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Bufe, T.; Hennig, A.; Klumpp, J.; Weiss, A.; Nieselt, K.; Schmidt, H. Differential Transcriptome Analysis of Enterohemorrhagic Escherichia coli Strains Reveals Differences in Response to Plant-Derived Compounds. BMC Microbiol. 2019, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Pascual, F.; Lempp, M.; Nosho, K.; Jeckel, H.; Jo, J.K.; Neuhaus, K.; Hartmann, R.; Jelli, E.; Hansen, M.F.; Price-Whelan, A.; et al. Spatial Alanine Metabolism Determines Local Growth Dynamics of Escherichia coli Colonies. eLife 2021, 10, e70794. [Google Scholar] [CrossRef] [PubMed]
- Hantus, C.E.; Moppel, I.J.; Frizzell, J.K.; Francis, A.E.; Nagashima, K.; Ryno, L.M. L-Rhamnose Globally Changes the Transcriptome of Planktonic and Biofilm Escherichia coli Cells and Modulates Biofilm Growth. Microorganisms 2024, 12, 1911. [Google Scholar] [CrossRef] [PubMed]
- Fehér, C.; Gál, B.; Fehér, A.; Barta, Z.; Réczey, K. Investigation of Commercial Enzyme Preparations for Selective Release of Arabinose from Corn Fibre. J. Chem. Technol. Biotechnol. 2015, 90, 1329–1337. [Google Scholar] [CrossRef]
- Schleif, R. AraC Protein, Regulation of the l-Arabinose Operon in Escherichia coli, and the Light Switch Mechanism of AraC Action. FEMS Microbiol. Rev. 2010, 34, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Schleif, R. Regulation of the L-Arabinose Operon of Escherichia coli. Trends Genet. 2000, 16, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, J.K.; Taylor, R.L.; Ryno, L.M. Constitutive Activation of RpoH and the Addition of L-Arabinose Influence Antibiotic Sensitivity of PHL628 E. Coli. Antibiotics 2024, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Longin, R.; Prigent-Combaret, C.; Dorel, C.; Hooreman, M.; Lejeune, P. Isolation of an Escherichia coli K-12 Mutant Strain Able to Form Biofilms on Inert Surfaces: Involvement of a New OmpR Allele That Increases Curli Expression. J. Bacteriol. 1998, 180, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Benamara, H.; Rihouey, C.; Jouenne, T.; Alexandre, S. Impact of the Biofilm Mode of Growth on the Inner Membrane Phospholipid Composition and Lipid Domains in Pseudomonas Aeruginosa. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 98–105. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. Jove 2011, 47, 2437. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A. Colorimetric Method for Determination of Sugars and Related Substances. Analytical 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Marchand, S.J.L.; Wang, Y.; Duncan, M.K. A Simple Method for Quantitating Confocal Fluorescent Images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.A.; Fass, J.N. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ files (Version 1.33) [Software]. 2011. Available online: https://github.com/najoshi/sickle (accessed on 1 June 2025).
- Buffalo, V.; Fass, J. Scythe—A Bayesian Adapter Trimmer (Version 0.994 BETA). [Software] 2014. Available online: https://github.com/vsbuffalo/scythe (accessed on 1 June 2025).
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating High-Throughput Genomic Analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences. F1000Research 2016, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome. Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J. Eulerr: Area-Proportional Euler and Venn Diagrams with Ellipses, R. package version 7.0.2; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.; Mi, H. PANTHER: Making Genome-scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.D.; Paley, S.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Moore, L.R.; Subhraveti, P.; Gama-Castro, S.; Tierrafria, V.H.; et al. The EcoCyc Database (2023). EcoSal. Plus 2023, 11, eesp00022023. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.D.; Paladino, M.M.; Nagashima, K.; Brezel, E.R.; Holtzman, J.S.; Urso, S.J.; Ryno, L.M. Temperature-Dependent Influence of FliA Overexpression on PHL628 E. Coli Biofilm Growth and Composition. Front. Cell. Infect. Microbiol. 2021, 11, 775270. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.M.; Peters, J.E.; Hay, A.G. Global Analysis of Candidate Genes Important for Fitness in a Competitive Biofilm Using DNA-Array-Based Transposon Mapping. Microbiology 2006, 152, 2233–2245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boulanger, E.F.; Sabag-Daigle, A.; Thirugnanasambantham, P.; Gopalan, V.; Ahmer, B.M.M. Sugar-Phosphate Toxicities. Microbiol. Mol. Biol. Rev. 2021, 85, e00123-21. [Google Scholar] [CrossRef] [PubMed]
- Aidelberg, G.; Towbin, B.D.; Rothschild, D.; Dekel, E.; Bren, A.; Alon, U. Hierarchy of Non-Glucose Sugars in Escherichia coli. BMC Syst. Biol. 2014, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Song, S.; Park, C. Priority of Pentose Utilization at the Level of Transcription: Arabinose, Xylose, and Ribose Operons. Mol. Cells 1998, 8, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Muroi, H.; Sunada, C.; Taniguchi, M.; Oi, S. Determination of Effector Molecules in L-Arabinose-Induced Bulge Formation and Lysis of Escherichia coli IFO 3545. Microbiology 1991, 137, 1163–1169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Englesberg, E.; Anderson, R.L.; Weinberg, R.; Lee, N.; Hoffee, P.; Huttenhauer, G.; Boyer, H. L-Arabinose-Sensitive, l-Ribulose 5-Phosphate 4-Epimerase-Deficient Mutants of Escherichia coli. J. Bacteriol. 1962, 84, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Curtis, J.C.; Plotkin, B.J. Insulin Regulation of Escherichia coli Abiotic Biofilm Formation: Effect of Nutrients and Growth Conditions. Antibiotics 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Saint-Ruf, C.; Garfa-Traoré, M.; Collin, V.; Cordier, C.; Franceschi, C.; Matic, I. Massive Diversification in Aging Colonies of Escherichia coli. J. Bacteriol. 2014, 196, 3059–3073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, Y.; Zhang, B.; Zhang, Y.-H.P.J. Spatially Structured Exchange of Metabolites Enhances Bacterial Survival and Resilience in Biofilms. Nat. Commun. 2024, 15, 7575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, Y.; Jin, X.; Wu, Q.; Bai, F.; Liu, J. Persistent Glucose Consumption under Antibiotic Treatment Protects Bacterial Community. Nat. Chem. Biol. 2025, 21, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, B.A.; Gil-Gil, T.; Manuel, J.A.; Smith, A.P.; Margollis, E.; Baquero, F.; Levin, B.R. What’s the Matter with MICs: Bacterial Nutrition, Limiting Resources, and Antibiotic Pharmacodynamics. Microbiol. Spectr. 2023, 11, e04091-22. [Google Scholar] [CrossRef] [PubMed]
- Malouin, F.; Chamberland, S.; Brochu, N.; Parr, T.R. Influence of Growth Media on Escherichia coli Cell Composition and Ceftazidime Susceptibility. Antimicrob. Agents Chemother. 1991, 35, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.; Hankemeier, T.; Hasselt, J.G.C. van Nutrient Conditions Affect Antimicrobial Pharmacodynamics in Pseudomonas Aeruginosa. Microbiol. Spectr. 2024, 13, e01409-24. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.R.; Gomes, L.C.; Araújo, J.D.P.; Miranda, J.M.; Simões, M.; Melo, L.F.; Mergulhão, F.J. The Effect of Glucose Concentration and Shaking Conditions on Escherichia coli Biofilm Formation in Microtiter Plates. Chem. Eng. Sci. 2013, 94, 192–199. [Google Scholar] [CrossRef]
- Ahmad, M.; Aduru, S.V.; Smith, R.P.; Zhao, Z.; Lopatkin, A.J. The Role of Bacterial Metabolism in Antimicrobial Resistance. Nat. Rev. Microbiol. 2025, 23, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.M.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic Efficacy Is Linked to Bacterial Cellular Respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Bacterial Metabolism-Inspired Molecules to Modulate Antibiotic Efficacy. J. Antimicrob. Chemother. 2019, 74, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Singh, S.; Pethe, K. Inhibiting Respiration as a Novel Antibiotic Strategy. Curr. Opin. Microbiol. 2023, 74, 102327. [Google Scholar] [CrossRef] [PubMed]
- Adamus-Białek, W.; Wawszczak, M.; Arabski, M.; Majchrzak, M.; Gulba, M.; Jarych, D.; Parniewski, P.; Głuszek, S. Ciprofloxacin, Amoxicillin, and Aminoglycosides Stimulate Genetic and Phenotypic Changes in Uropathogenic Escherichia coli Strains. Virulence 2019, 10, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J. In Vitro Effects of Sub-inhibitory Concentrations of Amoxicillin on Physiological Responses and Virulence Determinants in a Commensal Strain of Escherichia coli. J. Appl. Microbiol. 2021, 131, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Raeispour, M.; Ranjbar, R. Antibiotic Resistance, Virulence Factors and Genotyping of Uropathogenic Escherichia coli Strains. Antimicrob. Resist. Infect. Control 2018, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Korea, C.; Badouraly, R.; Prevost, M.; Ghigo, J.; Beloin, C. Escherichia coli K-12 Possesses Multiple Cryptic but Functional Chaperone–Usher Fimbriae with Distinct Surface Specificities. Environ. Microbiol. 2010, 12, 1957–1977. [Google Scholar] [CrossRef] [PubMed]
- Koita, K.; Rao, C.V. Identification and Analysis of the Putative Pentose Sugar Efflux Transporters in Escherichia coli. PLoS ONE 2012, 7, e43700. [Google Scholar] [CrossRef] [PubMed]
- Yogiara; Kim, D.; Hwang, J.-K.; Pan, J.-G. Escherichia coli ASKA Clone Library Harboring TRNA-Specific Adenosine Deaminase (TadA) Reveals Resistance towards Xanthorrhizol. Molecules 2015, 20, 16290–16305. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Enokida, H.; Hayami, H.; Kawahara, M.; Nakagawa, M. Genome-wide Transcriptome Analysis of Fluoroquinolone Resistance in Clinical Isolates of Escherichia coli. Int. J. Urol. 2012, 19, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, H.; Lv, Z.; Hu, Y.; Wang, H.; Shu, F.; Zhu, C.; Xue, T. The Two-Component System CpxRA Affects Antibiotic Susceptibility and Biofilm Formation in Avian Pathogenic Escherichia coli. Anim. Open Access J. 2023, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Li, Q.; Chen, X.; Ni, J.; Shang, F.; Xue, T. Role of LsrR in the Regulation of Antibiotic Sensitivity in Avian Pathogenic Escherichia coli. Poult. Sci. 2020, 99, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.C.; Black, E.P.; Hou, Z.; Sugarwara, M.; Sadowski, M.J.; Diez-Gonzalez, F. Transcriptional Responses of Escherichia coli K-12 and O157:H7 Associated with Lettuce Leaves. Appl. Environ. Microb. 2012, 78, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Qi, K.; Wang, S.; Chen, X.; Ni, J.; Deng, R.; Shang, F.; Xue, T. McbR is involved in biofilm formation and H2 O2 stress response in avian pathogenic Escherichia coli X40. Poult. Sci. 2019, 98, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
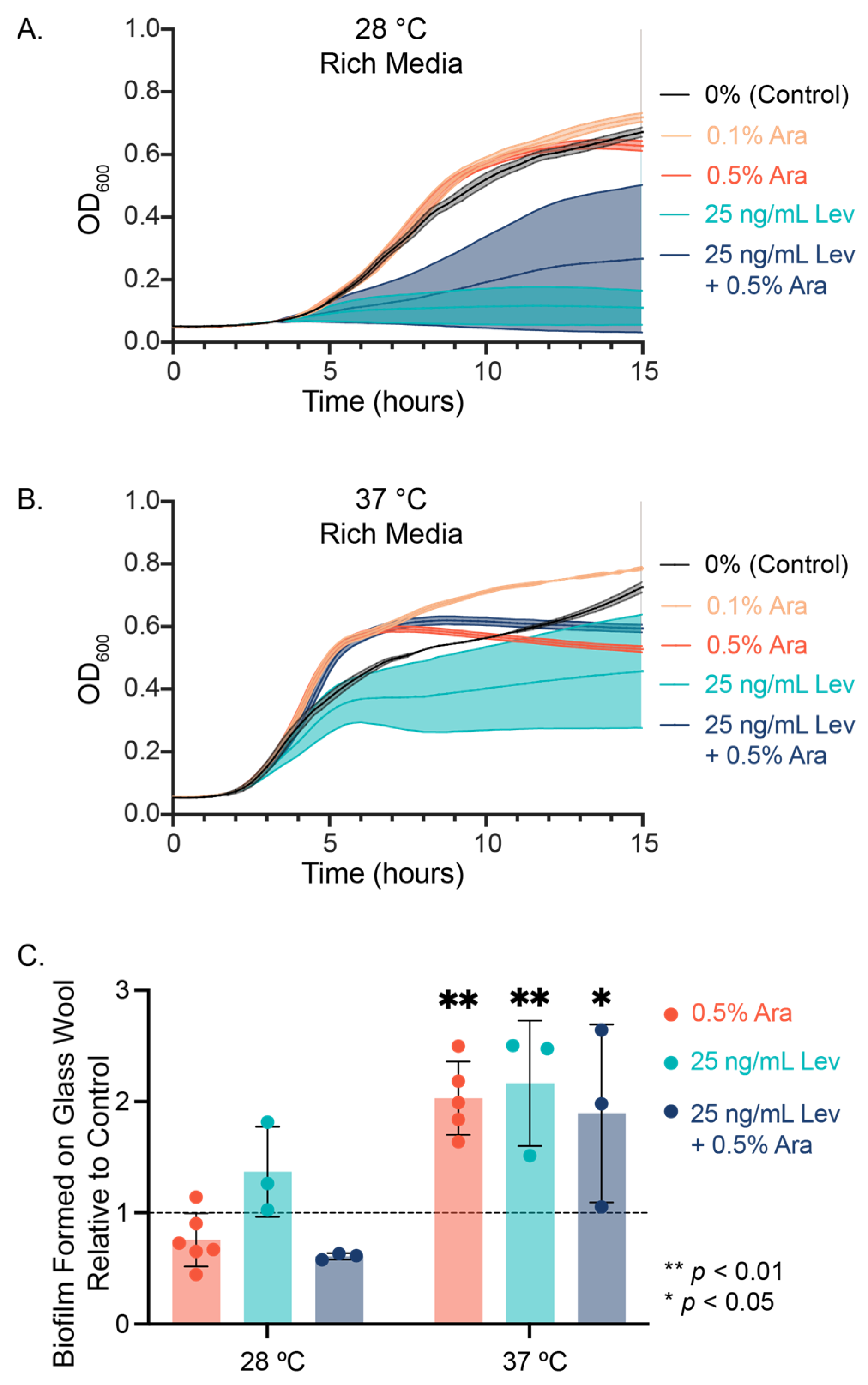


| Rate Constant (h−1) | Maximum Growth (Abs at 600 nm) | |||
|---|---|---|---|---|
| 28 °C | 37 °C | 28 °C | 37 °C | |
| 0% | 0.500 ± 0.007 | 0.54 ± 0.02 | 0.672 ± 0.004 | 0.639 ± 0.004 |
| 0.1% (w/w) Ara | 0.552 ± 0.009 | 0.674 ± 0.02 | 0.715 ± 0.004 | 0.740 ± 0.003 * |
| 0.5% (w/w) Ara | 0.614 ± 0.010 § | 1.34 ± 0.04 *,§ | 0.649 ± 0.003 | 0.567 ± 0.002 *,§ |
| 25 ng/mL Lev | 0.386 ± 0.09 | 0.74 ± 0.09 | 0.118 ± 0.005 * | 0.423 ± 0.01 * |
| 25 ng/mL Lev + 0.5% (w/w) Ara | 0.24 ± 0.07 *,‡ | 1.12 ± 0.02 *,†,‡ | 0.34 ± 0.09 *,†,‡ | 0.613 ± 0.002 †,‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin, K.M.; Frizzell, J.K.; Neighmond, A.A.; Moppel, I.J.; Ryno, L.M. L-Arabinose Alters the E. coli Transcriptome to Favor Biofilm Growth and Enhances Survival During Fluoroquinolone Stress. Microorganisms 2025, 13, 1665. https://doi.org/10.3390/microorganisms13071665
Austin KM, Frizzell JK, Neighmond AA, Moppel IJ, Ryno LM. L-Arabinose Alters the E. coli Transcriptome to Favor Biofilm Growth and Enhances Survival During Fluoroquinolone Stress. Microorganisms. 2025; 13(7):1665. https://doi.org/10.3390/microorganisms13071665
Chicago/Turabian StyleAustin, Katherine M., Jenna K. Frizzell, Audrey A. Neighmond, Isabella J. Moppel, and Lisa M. Ryno. 2025. "L-Arabinose Alters the E. coli Transcriptome to Favor Biofilm Growth and Enhances Survival During Fluoroquinolone Stress" Microorganisms 13, no. 7: 1665. https://doi.org/10.3390/microorganisms13071665
APA StyleAustin, K. M., Frizzell, J. K., Neighmond, A. A., Moppel, I. J., & Ryno, L. M. (2025). L-Arabinose Alters the E. coli Transcriptome to Favor Biofilm Growth and Enhances Survival During Fluoroquinolone Stress. Microorganisms, 13(7), 1665. https://doi.org/10.3390/microorganisms13071665





