Encapsulation of Fresh Spirulina Biomass in Alginate Spheres for Yogurt Fortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Arthrospira platensis and Growth Conditions
2.2. Spirulina Spheres Production
2.3. Yogurt Preparation
2.4. Fortification of Yogurt with Spirulina Spheres (Main Trials)
2.5. pH and LABs Enumeration
2.6. Spirulina Viability Check
2.7. Statistical Analysis
3. Results
3.1. Preliminary Trial
3.2. Main Trials—pH and LABs
3.3. Main Trials—Viability of Spirulina Embedded in Alginate Spheres
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marjanovíc, B.; Benkovíc, M.; Jurina, T.; Cvetníc, T.S.; Valinger, D.; Jasenka Gajdoš Kljusuríc, J.G.; Tušek, A.J. Bioactive compounds from Spirulina spp.—Nutritional value, extraction, and application in food industry. Separations 2024, 11, 257. [Google Scholar] [CrossRef]
- Gogna, S.; Kaur, J.; Sharma, K.; Prasad, R.; Singh, J.; Bhadariya, V.; Kumar, P.; Jarial, S. Spirulina—An edible Cyanobacterium with potential therapeutic health benefits and toxicological consequences. J. Am. Nutr. Ass. 2023, 42, 559–572. [Google Scholar] [CrossRef]
- Sidari, R.; Tofalo, R. A comprehensive overview on microalgal-fortified/based food and beverages. Food Rev. Int. 2019, 35, 778–805. [Google Scholar] [CrossRef]
- Fan, Z.; Shahid, A.; Su, K.; Zhao, A.; Zhang, B.; Xu, J. Comprehensive analysis of the effects of fresh Spirulina microcapsules on protein cross-linking and structural changes in wheat noodles. Food Chem. 2025, 482, 144034. [Google Scholar] [CrossRef]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of microalgal biomass for innovative food production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Func. Foods 2019, 60, 103427. [Google Scholar] [CrossRef]
- Özyurt, G.; Uslu, L.; Durmuş, M.; Sakarya, Y.; Uzlaşir, T.; Küley, E. Chemical and physical characterization of microencapsulated Spirulina fermented with Lactobacillus plantarum. Algal Res. 2023, 73, 103149. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of different storage methods on bioactive compounds in Arthrospira platensis biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Luo, G.; Liu, H.; Yang, S.; Sun, Z.; Sun, L.; Wang, L. Manufacturing processes, additional nutritional value and versatile food applications of fresh microalgae Spirulina. Front. Nutr. 2024, 11, 1455553. [Google Scholar] [CrossRef]
- Ma, Z.; Ahmed, F.; Yuan, B.; Zhang, W. Fresh living Arthrospira as dietary supplements: Current status and challenges. Trends Food Sci. Technol. 2019, 88, 439–444. [Google Scholar] [CrossRef]
- Bchir, B.; Felfoul, I.; Bouaziz, M.A.; Gharred, T.; Yaich, H.; Noumi, E.; Snoussi, M.; Bejaoui, H.; Kenzali, Y.; Blecker, C.; et al. Investigation of physicochemical, nutritional, textural, and sensory properties of yoghurt fortified with fresh and dried Spirulina (Arthrospira platensis). Int. Food Res. J. 2019, 26, 1565–1576. [Google Scholar]
- Patel, P.; Jethani, H.; Radha, C.; Vijayendra, S.V.N.; Mudliar, S.N.; Sarada, R.; Chauhan, V.S. Development of a carotenoid enriched probiotic yogurt from fresh biomass of Spirulina and its characterization. J. Food Sci. Technol. 2019, 56, 3721–3731. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as natural fermentation booster for milk and soy fermented beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Cui, H.; Qin, S.; Ren, J.; Zhang, Z.; An, Q.; Zhang, N.; Yang, J.; Yang, Y.; Fan, G.; et al. Characterizing and decoding the key odor compounds of Spirulina platensis at different processing stages by sensomics. Food Chem. 2024, 461, 140944. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Machado, A.R.; Assis, L.M.; Costa, J.A.V.; Badiale-Furlong, E.; Motta, A.S.; Micheletto, Y.M.S.; Souza-Soares, L.A. Application of sonication and mixing for nanoencapsulation of the cyanobacterium Spirulina platensis in liposomes. Int. Food Res. J. 2015, 22, 96–101. [Google Scholar]
- Rajmohan, D.; Bellmer, D. Characterization of Spirulina-alginate beads formed using ionic gelation. Int. J. Food Sci. 2019, 2019, 7101279. [Google Scholar] [CrossRef]
- Zen, C.K.; Vicenzi Tiepo, C.B.; Vieira da Silva, R.; Oliveira Reinehr, C.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef]
- Nourmohammadi, N.; Soleimanian-Zada, S.; Shekarchizadeha, H. Effect of Spirulina (Arthrospira platensis) microencapsulated in alginate and whey protein concentrate addition on physicochemical and organoleptic properties of functional stirred yogurt. J. Sci. Food Agric. 2020, 100, 5260–5268. [Google Scholar] [CrossRef]
- Madkour, F.F.; Kamil, A.E.-W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef]
- Garofalo, G.; Ponte, M.; Busetta, G.; Tolone, M.; Bonanno, A.; Portolano, B.; Gaglio, R.; Erten, H.; Sardina, M.T.; Settanni, L. A thorough investigation of the microbiological, physicochemical, and sensory properties of ewe’s yoghurt fermented by a selected multi-strain starter culture. Foods 2023, 12, 3454. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Fadaei, V.; Mohamadi-Alasti, F.; Khosravi-Darani, K. Influence of Spirulina platensis powder on the starter culture viability in probiotic yoghurt containing spinach during cold storage. Eur. J. Exp. Biol. 2013, 3, 389–393. [Google Scholar]
- Agustini, T.W.; Soetrisnanto, D.; Ma’ruf, W.F. Study on chemical, physical, microbiological and sensory of yoghurt enriched by Spirulina platensis. Int. Food Res. J. 2017, 24, 367–371. [Google Scholar]
- Mocanu, G.; Botez, E.; Nistor, O.V.; Andronoiu, D.G.; Vlăsceanu, G. Influence of Spirulina platensis biomass over some starter culture of lactic bacteria. J. Agroaliment. Process. Technol. 2013, 19, 474–479. [Google Scholar]
- Alizadeh Khaledabad, M.; Ghasempour, Z.; Moghaddas Kia, E.; Rezazad Bari, M.; Zarrin, R. Probiotic yoghurt functionalised with microalgae and Zedo gum: Chemical, microbiological, rheological and sensory characteristics. Int. J. Dairy Technol. 2020, 73, 67–75. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Influence of incorporated Spirulina platensis on the growth of microflora and physicochemical properties of ayran as a functional food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Boosting effects of Spirulina platensis, whey protein, and probiotics on the growth of microflora and the nutritional value of ayran. Eng. Rep. 2020, 2, e12235. [Google Scholar] [CrossRef]
- de Caire, G.Z.; Parada, J.L. Effect of Spirulina platensis biomass on the growth of lactic acid bacteria in milk. World J. Microbiol. Biotechnol. 2000, 16, 563–565. [Google Scholar] [CrossRef]
- Guldas, M.; Irkin, R. Influence of Spirulina platensis powder on the microflora of yoghurt and acidophilus milk. Mljekarstvo časopis Unaprjeđenje Proizv. Prerade Mlijek 2010, 60, 237–243. [Google Scholar]
- Gyenis, B.; Szigeti, J.; Molnár, N.; Varga, L. Use of dried microalgal biomasses to stimulate acid production and growth of Lactobacillus plantarum and Enterococcus faecium in milk. Acta Ag. Kaposváriensis 2005, 9, 53–59. [Google Scholar]
- Parada, J. Lactic acid bacteria growth promoters from Spirulina platensis. Int. J. Food Microbiol. 1998, 45, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Pascal, G.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leuk. Biol. 2011, 89, 685–695. [Google Scholar]
- Ganchev, I. Impact of Spirulina platensis biomass on the viability of Lactobacillus delbrueckii subsp. bulgaricus strain during the freeze-drying process. BioTechnologia 2024, 105, 109–119. [Google Scholar] [CrossRef]
- Kahraman Ilıkkan, Ö.; Bağdat, E.Ş.; Yalçın, D. Evaluation of prebiotic, probiotic, and synbiotic potentials of microalgae. Food Health 2022, 8, 161–171. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Luwidharto, J.C.N.; Rahayu, E.S.; Suroto, D.A.; Wikandari, R.; Ulfah, A.; Utami, T. Effects of Spirulina platensis addition on growth of Lactobacillus plantarum Dad 13 and Streptococcus thermophilus Dad 11 in fermented milk and physicochemical characteristics of the product. Appl. Food Biotechnol. 2022, 9, 205–216. [Google Scholar]
- Dinçoğlu, A.H.; Akça, S.S.; Çalişkan, Z. Effect of Spirulina platensis on probiotic, nutritional, and quality properties of yogurt. Int. Food Res. J. 2024, 31, 157–168. [Google Scholar] [CrossRef]
- Niccolai, A.; Bažec, K.; Rodolfi, L.; Biondi, N.; Zlatić, E.; Jamnik, P.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (Spirulina) in a vegetal soybean drink for developing new functional lactose-free beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Yağmur, N.; Şahin, S.; Ersan, L.I. Bio-yoghurt enriched with Spirulina-encapsulated pomegranate peel: Impact on some metabolomics, antioxidative, textural and colour properties. Int. J. Dairy Sci. 2024, 77, 724–734. [Google Scholar]
- Yağmur, N.; Şahin, S.; Akyildiz, G. The effect of pomegranate peel extracts coated with Spirulina microalgae supplementation on physicochemical, microbiological and sensorial properties of yoghurts during storage. Int. J. Food Sci. Technol. 2023, 58, 3180–3188. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansa, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Badwan, A.A.; Abumalooh, A.; Sallam, E.; Abukalaf, A.; Jawan, O. A sustained release drug delivery system using calcium alginate beads. Drug Dev. Ind. Pharm. 1985, 11, 239–256. [Google Scholar] [CrossRef]
- Castillo-Barzola, A.; Paisig, H.L.; Faieta, M.; Jordán-Suárez, O.; Porras-Sosa, E.; Tuesta, T. Effect of microencapsulation by spray drying on the protein content of Spirulina (Arthrospira platensis). Ital. J. Food Sci. 2025, 37, 423–439. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Malletzidou, L.; Kyratzopoulou, E.; Kyzaki, N.; Nerantzis, E.; Kazakis, N.A. Near-Infrared Spectroscopy for growth estimation of Spirulina platensis cultures. Methods Protoc. 2024, 7, 91. [Google Scholar] [CrossRef]
- Venkatasamy, J.K.; Duraisamy, R.; Chockalingam, V. The growth performance of Spirulina platensis on media supplemented with digested poultry droppings slurry of biogas plant. Asian J. Biol. Life Sci. 2024, 13, 287–296. [Google Scholar] [CrossRef]
- Heidebach, T.; Forst, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Tovar López, S.; Rodríguez Andrade, V.A.; Reyes Salazar, C.O.; García Manríques, L.F.; Medina García, M.F.; Hernández de la Peña, F.J. Spheres of Spirulina and/or Nutraceuticals Impervious to Aqueous Media for Incorporation into Food Matrices and Methods and Processes for Producing Said Spheres. WO2017176103A1, 12 October 2017. [Google Scholar]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Fernando, P.U.A.I.; Kennedy, A.J.; Pokrzywinski, K.; Jernberg, J.; Thornell, T.; George, G.; Kosgei, G.K.; Wang, Y.; Coyne, K.J. Development of alginate beads for precise environmental release applications: A design of experiment based approach and analysis. J. Environ. Manag. 2024, 351, 119872. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.F.; Moreira, A.F.; Miguel, S.P.; Coutinho, P. Recent advances in microalgae encapsulation techniques for biomedical applications. Adv. Colloid Interface Sci. 2024, 333, 103297. [Google Scholar] [CrossRef] [PubMed]
- Mihafu, F.D.; Issa, J.Y.; Kamiyango, M.W. Implication of sensory evaluation and quality assessment in food product development: A review. Curr. Res. Nutr. Food Sci. 2020, 08, 690–702. [Google Scholar] [CrossRef]
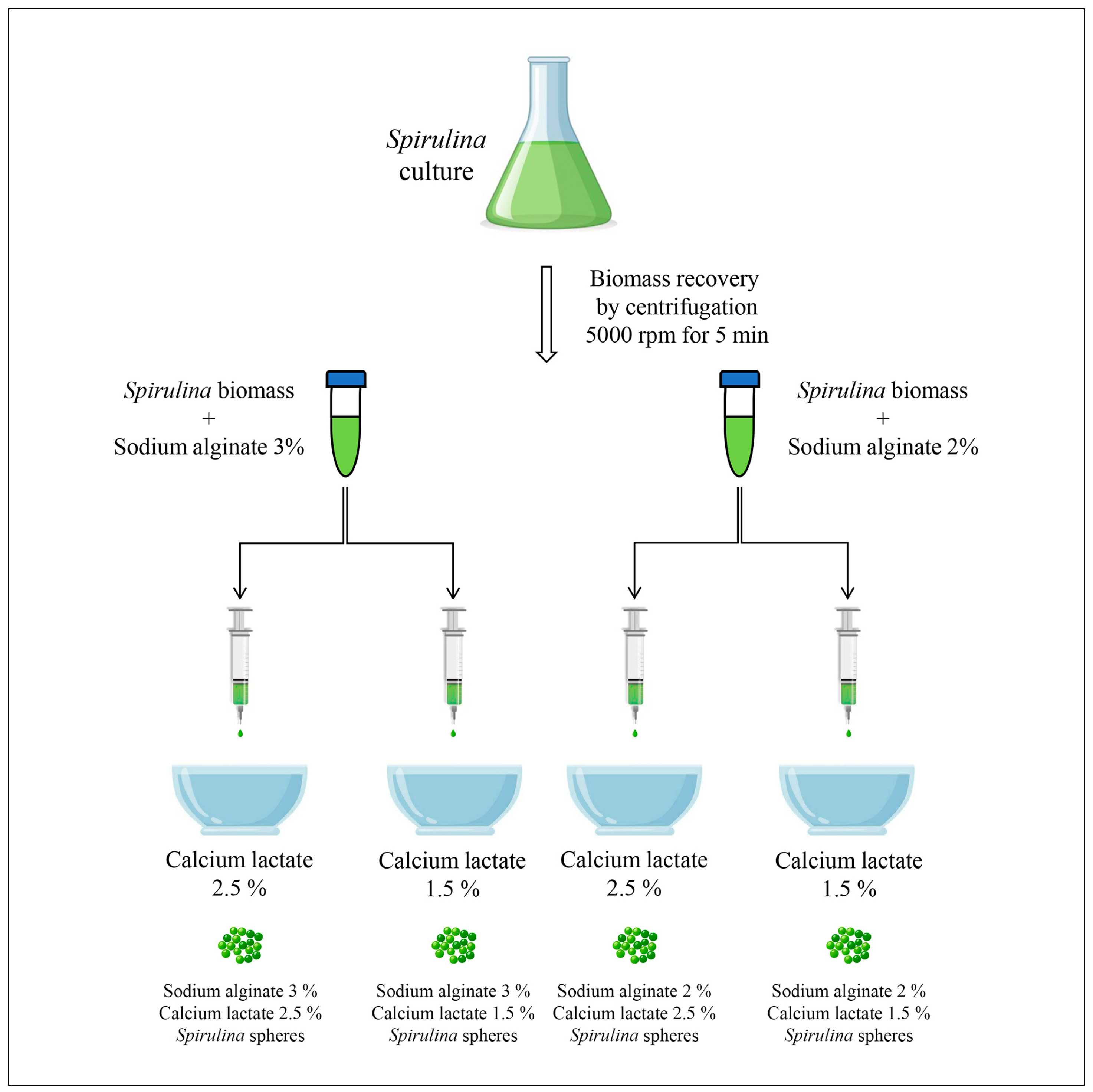

 ) compared to the control yogurt samples (
) compared to the control yogurt samples ( ) across 15 days. Values are mean ± standard deviation. Means with different superscript letters are significantly different (p < 0.05).
) across 15 days. Values are mean ± standard deviation. Means with different superscript letters are significantly different (p < 0.05).
 ) compared to the control yogurt samples (
) compared to the control yogurt samples ( ) across 15 days. Values are mean ± standard deviation. Means with different superscript letters are significantly different (p < 0.05).
) across 15 days. Values are mean ± standard deviation. Means with different superscript letters are significantly different (p < 0.05).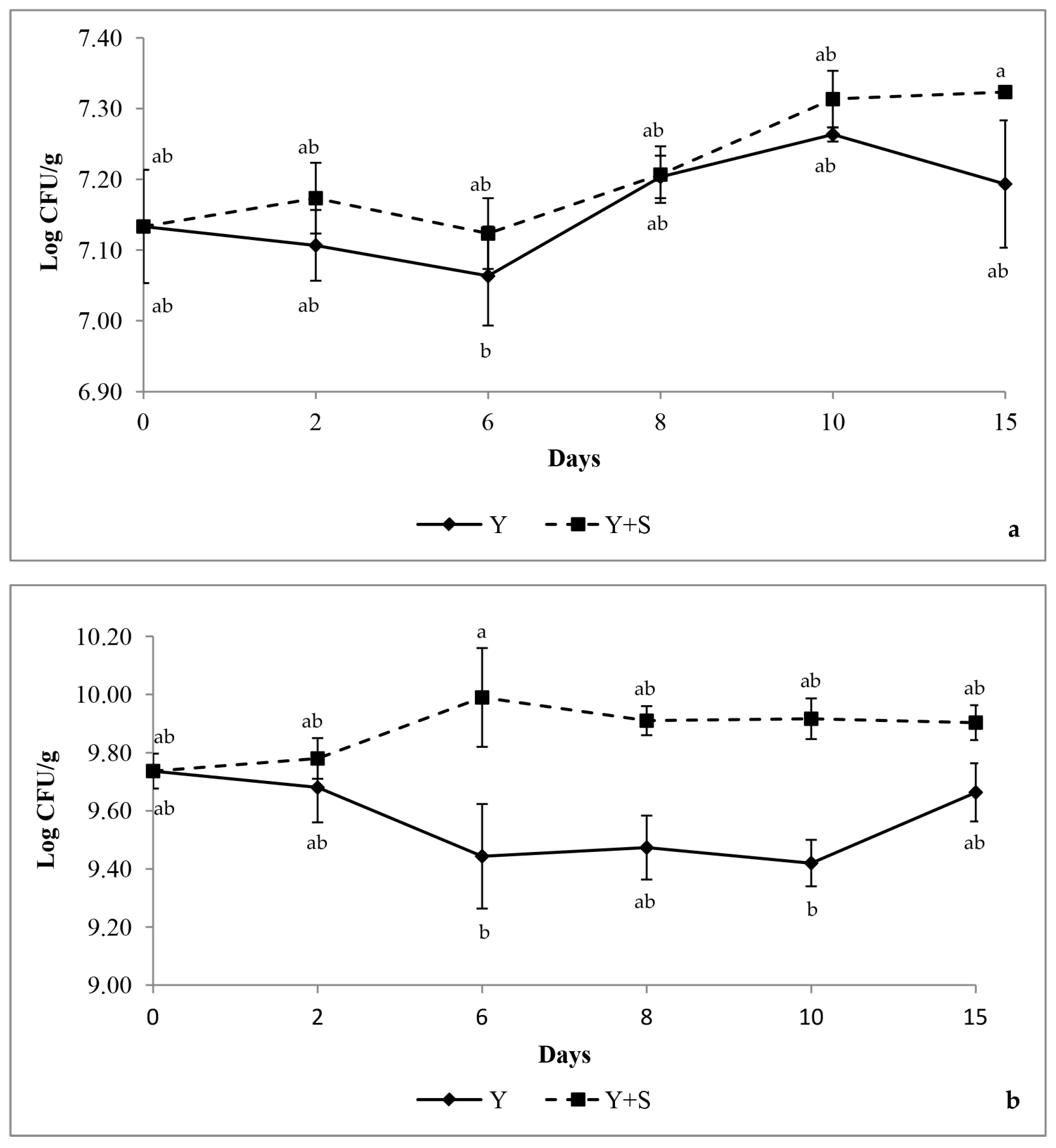

| Samples | Acronyms |
|---|---|
| Unenriched Spirulina yogurt | Y |
| Spirulina spheres | S |
| Yogurt enriched with Spirulina spheres | Y+S |
| Spheres Types | Diameters (mm) | Qualitative Characteristics |
|---|---|---|
| SA 3%—CL 2.5% | 8.8 ± 0.02 a | completely solidified and hard |
| SA 3%—CL 1.5% | 5.0 ± 0.01 b | thin external film, fairly resistant |
| SA 2%—CL 2.5% | 4.6 ± 0.02 c | thin external film, fairly resistant |
| SA 2%—CL 1.5% | 5.1 ± 0.02 b | easily breakable |
| Samples | Days | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 8 | 10 | 15 | |
| Y | 4.26 ± 0.00 a | 4.12 ± 0.01 bc | 4.10 ± 0.01 cd | 4.11 ± 0.00 cd | 4.11 ± 0.01 cd | 4.04 ± 0.00 e |
| Y+S | 4.26 ± 0.01 a | 4.14 ± 0.01 b | 4.11 ± 0.01 cd | 4.14 ± 0.00 b | 4.14 ± 0.01 b | 4.08 ± 0.01 d |
| Samples | Days | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 10 | 12 | 17 | |
| S | 0.254 ± 0.016 a | 0.256 ± 0.021 a | 0.250 ± 0.007 a | 0.252 ± 0.011 a | 0.246 ± 0.012 a | 0.255 ± 0.009 a | 0.2515 ± 0.005 a |
| Y+S | 0.251 ± 0.003 a | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b |
| Zarrouk medium | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.000 ± 0.000 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siclari, D.; Panuccio, M.R.; Sidari, R. Encapsulation of Fresh Spirulina Biomass in Alginate Spheres for Yogurt Fortification. Microorganisms 2025, 13, 1641. https://doi.org/10.3390/microorganisms13071641
Siclari D, Panuccio MR, Sidari R. Encapsulation of Fresh Spirulina Biomass in Alginate Spheres for Yogurt Fortification. Microorganisms. 2025; 13(7):1641. https://doi.org/10.3390/microorganisms13071641
Chicago/Turabian StyleSiclari, Domenico, Maria Rosaria Panuccio, and Rossana Sidari. 2025. "Encapsulation of Fresh Spirulina Biomass in Alginate Spheres for Yogurt Fortification" Microorganisms 13, no. 7: 1641. https://doi.org/10.3390/microorganisms13071641
APA StyleSiclari, D., Panuccio, M. R., & Sidari, R. (2025). Encapsulation of Fresh Spirulina Biomass in Alginate Spheres for Yogurt Fortification. Microorganisms, 13(7), 1641. https://doi.org/10.3390/microorganisms13071641






