Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives
Abstract
1. Introduction
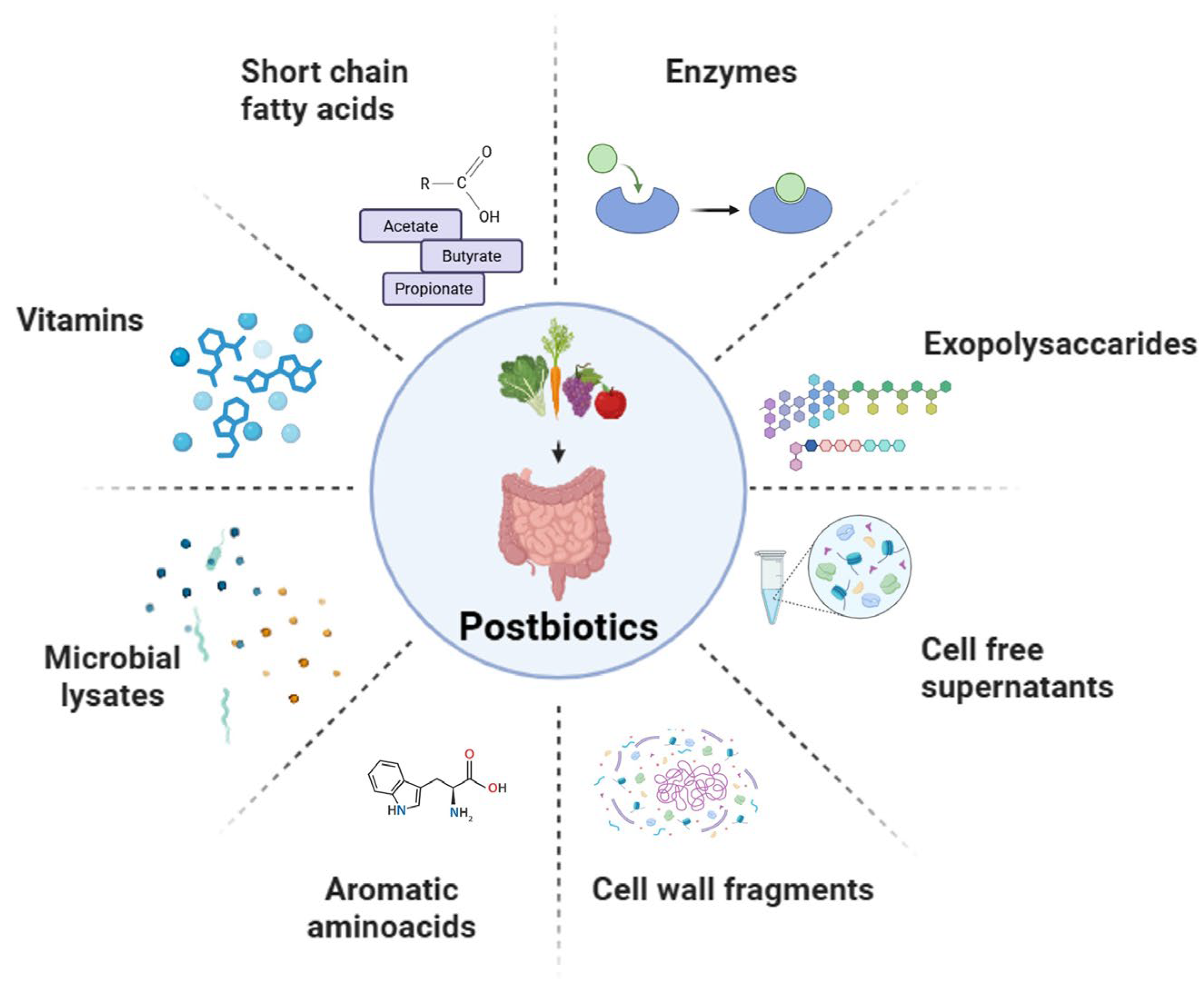
2. Sources and Classification of Postbiotics
2.1. Short-Chain Fatty Acids
2.2. Exopolysaccharides
2.3. Enzymes
2.4. Cell Wall Fragments
2.5. Cell-Free Supernatants
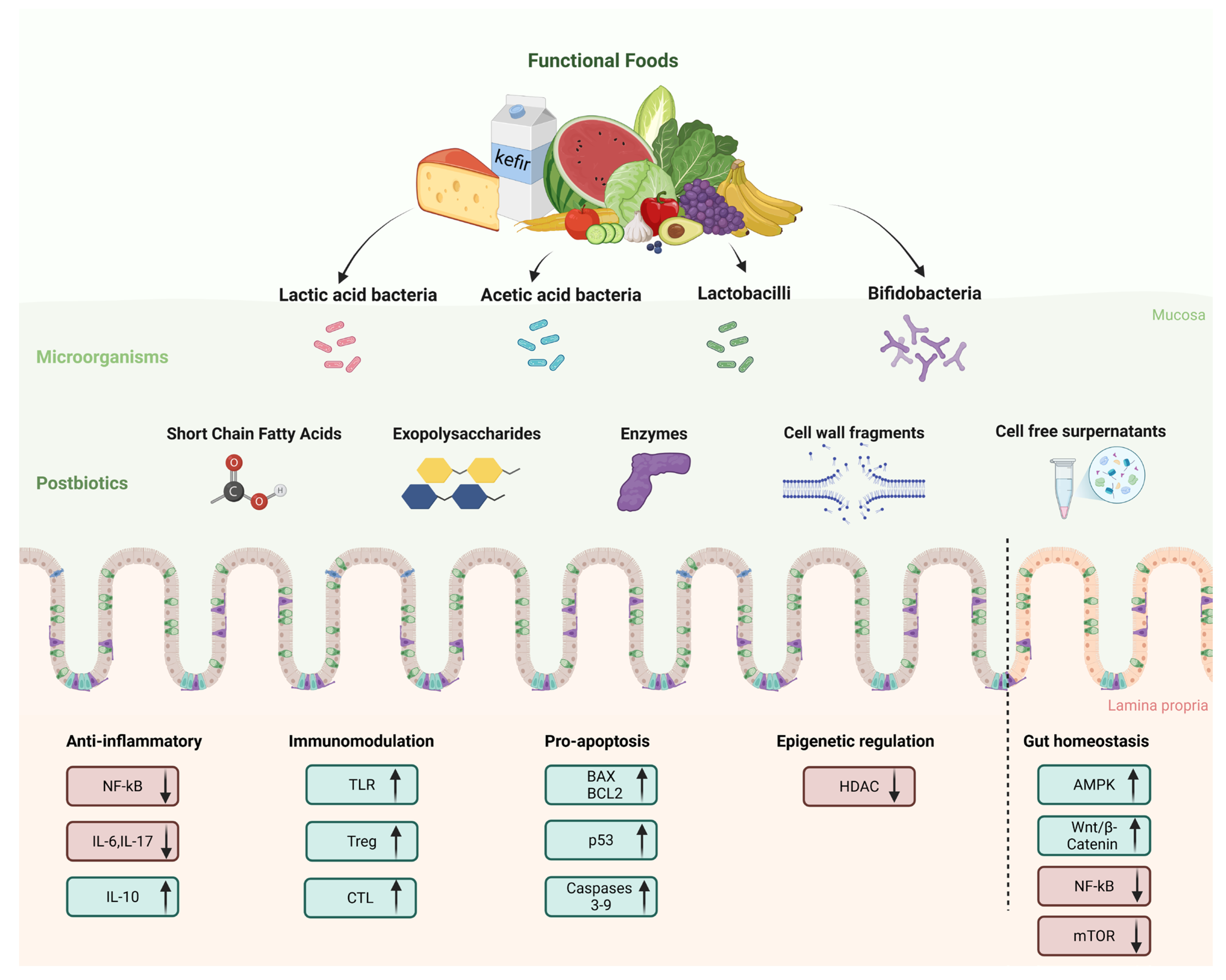
2.6. Functional Foods as Sources of Postbiotics
2.6.1. Sauerkraut (Fermented Cabbage)
2.6.2. Kefir
2.6.3. Kimchi
3. Mechanisms of Action of Postbiotics for Prevention and Management of Colorectal Cancer
3.1. Anti-Inflammatory and Immunomodulatory Effects
3.2. Apoptosis Induction and Tumor Suppression
3.3. Other Effects
4. Therapeutic Potential and Application in Biomedical Systems: Current Evidence from Preclinical Studies
4.1. Methodologies and Software
4.2. Studies on Cell Lines
4.3. In Vivo Studies
4.4. Investigating Postbiotic Safety and Effects Using Advanced Preclinical Models
5. Clinical Evidence, Formulation, and Delivery of Postbiotics
5.1. Clinical Evidence
5.2. Postbiotic Formulation and Delivery
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the Gut Microbiota in Anticancer Therapy: From Molecular Mechanisms to Clinical Applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Li, Q.; Yu, J. Gut Microbiota Modulation: A Novel Strategy for Prevention and Treatment of Colorectal Cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Murgiano, M.; Bartocci, B.; Puca, P.; di Vincenzo, F.; Del Gaudio, A.; Papa, A.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. Int. J. Mol. Sci. 2025, 26, 3059. [Google Scholar] [CrossRef]
- Senthilkumar, H.; Arumugam, M. Gut Microbiota: A Hidden Player in Polycystic Ovary Syndrome. J. Transl. Med. 2025, 23, 443. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, J.; Yu, P.; Qiu, T.; Jiang, S.; Yu, R. Unlocking the Power of Probiotics, Postbiotics: Targeting Apoptosis for the Treatment and Prevention of Digestive Diseases. Front. Nutr. 2025, 12, 1570268. [Google Scholar] [CrossRef]
- Mishra, S.; Jain, S.; Agadzi, B.; Yadav, H. A Cascade of Microbiota-Leaky Gut-Inflammation- Is It a Key Player in Metabolic Disorders? Curr. Obes. Rep. 2025, 14, 32. [Google Scholar] [CrossRef]
- Golden, A.; Williams, C.; Yadav, H.; Masternak, M.M.; Labyak, C.; Holland, P.J.; Arikawa, A.Y.; Jain, S. The Selection of Participants for Interventional Microbiota Trials Involving Cognitively Impaired Older Adults. Geroscience 2025, 1–10. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for Food: The Impact of Diet on Gut Microbiota and Human Health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Green, K.M.; Rawat, M. A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicro. Prot. 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Abbasi, A. Molecular Mechanisms of Postbiotics in Colorectal Cancer Prevention and Treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 1787–1803. [Google Scholar] [CrossRef]
- Feizi, H.; Plotnikov, A.; Rezaee, M.A.; Ganbarov, K.; Kamounah, F.S.; Nikitin, S.; Kadkhoda, H.; Gholizadeh, P.; Pagliano, P.; Kafil, H.S. Postbiotics versus Probiotics in Early-Onset Colorectal Cancer. Crit. Rev. Food Sci. Nutr. 2024, 64, 3573–3582. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Z.; Wang, Y.; Cai, S.; Qiao, Z.; Hu, X.; Wang, T.; Yi, J. Preventive Methods for Colorectal Cancer Through Dietary Interventions: A Focus on Gut Microbiota Modulation. Food Rev. Int. 2025, 41, 720–748. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Li, Y.; Liu, X.; Fang, L.; Jiang, Z. Probiotics and the Role of Dietary Substrates in Maintaining the Gut Health: Use of Live Microbes and Their Products for Anticancer Effects against Colorectal Cancer. J. Microbiol. Biotechnol. 2024, 34, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhou, R.W.; Harpaz, N.; Itzkowitz, S.H.; Parsons, R.E. Molecular Mechanisms in Colitis-Associated Colorectal Cancer. Oncogenesis 2023, 12, 48. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal Cancer Incidence, Mortality, and Stage Distribution in European Countries in the Colorectal Cancer Screening Era: An International Population-Based Study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Asefa, Z.; Belay, A.; Welelaw, E.; Haile, M. Postbiotics and Their Biotherapeutic Potential for Chronic Disease and Their Feature Perspective: A Review. Front. Microbiomes 2025, 4, 1489339. [Google Scholar] [CrossRef]
- Hijová, E. Postbiotics as Metabolites and Their Biotherapeutic Potential. Int. J. Mol. Sci. 2024, 25, 5441. [Google Scholar] [CrossRef]
- Suthar, P.; Kumar, S.; Kumar, V.; Sharma, V.; Dhiman, A. Postbiotics: An Exposition on next Generation Functional Food Compounds- Opportunities and Challenges. Crit. Rev. Food Sci. Nutr. 2025, 65, 1163–1182. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Saedi, S.; Derakhshan, S.; Hasani, A.; Khoshbaten, M.; Poortahmasebi, V.; Milani, P.G.; Sadeghi, J. Recent Advances in Gut Microbiome Modulation: Effect of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Inflammatory Bowel Disease Prevention and Treatment. Curr. Microbiol. 2024, 82, 12. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Martino, L.D.; Li, J. Natural Polysaccharides-Based Postbiotics and Their Potential Applications. Explor. Med. 2024, 5, 444–458. [Google Scholar] [CrossRef]
- Jin, X.; Nguyen, T.T.M.; Yi, E.-J.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Kim, M.-J.; Yi, T.-H. Emerging Trends in Skin Anti-Photoaging by Lactic Acid Bacteria: A Focus on Postbiotics. Chemistry 2024, 6, 1495–1508. [Google Scholar] [CrossRef]
- Harat, S.G.; Pourjafar, H. Health Benefits and Safety of Postbiotics Derived from Different Probiotic Species. Curr. Pharm. Des. 2025, 31, 116–127. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Rahman, U.-U.-; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S.; et al. Recent Advances in the Therapeutic Application of Short-Chain Fatty Acids (SCFAs): An Updated Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary Fiber-Derived Short-Chain Fatty Acids: A Potential Therapeutic Target to Alleviate Obesity-Related Nonalcoholic Fatty Liver Disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as Potential New Therapeutic Agents for Metabolic Disorders Management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Yin, M.O.L.; Heaney, L.M. The Athlete and Gut Microbiome: Short-Chain Fatty Acids as Potential Ergogenic Aids for Exercise and Training. Int. J. Sports Med. 2021, 42, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Reynés, B.; Palou, M.; Rodríguez, A.M.; Palou, A. Regulation of Adaptive Thermogenesis and Browning by Prebiotics and Postbiotics. Front. Physiol. 2018, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.a.O. Fermentation of Prebiotics by Human Colonic Microbiota in Vitro and Short-Chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Fang, H.; Rodrigues E-Lacerda, R.; Barra, N.G.; Kukje Zada, D.; Robin, N.; Mehra, A.; Schertzer, J.D. Postbiotic Impact on Host Metabolism and Immunity Provides Therapeutic Potential in Metabolic Disease. Endocr. Rev. 2025, 46, 60–79. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Zhang, Z.; Zhang, J.; Hu, H.; Lan, H.; Hong, W.; Yang, Z. Characterization of a Postbiotic Exopolysaccharide Produced by Lacticaseibacillus Paracasei ET-22 with Antioxidant and Anti-Inflammatory Efficacy. Int. J. Biol. Macromol. 2025, 306, 141608. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.H.; Law, J.W.F.; Ser, H.L.; Khaw, K.Y.; Letchumanan, V.; Lee, L.H.; Goh, B.H. Harnessing the Potentialities of Probiotics, Prebiotics, Synbiotics, Paraprobiotics, and Postbiotics for Shrimp Farming. Rev. Aquac. 2022, 14, 1478–1557. [Google Scholar] [CrossRef]
- Kango, N.; Nath, S. Prebiotics, Probiotics and Postbiotics: The Changing Paradigm of Functional Foods. J. Diet. Suppl. 2024, 21, 709–735. [Google Scholar] [CrossRef]
- Vijayaganapathi, A.; Anju, K.A.; Shree Kumari, G.R.; Subathra Devi, C.; Vaithilingam, M. Chapter 28—Antiatherosclerotic Effects of Postbiotics. In Postbiotics; Dharumadurai, D., Halami, P.M., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2025; pp. 513–528. ISBN 978-0-443-22188-0. [Google Scholar]
- Yang, Y.; Fan, G.; Lan, J.; Li, X.; Li, X.; Liu, R. Polysaccharide-Mediated Modulation of Gut Microbiota in the Treatment of Liver Diseases: Promising Approach with Significant Challenges. Int. J. Biol. Macromol. 2024, 280, 135566. [Google Scholar] [CrossRef]
- Gezginç, Y.; Karabekmez-erdem, T.; Tatar, H.D.; Ayman, S.; Ganiyusufoğlu, E.; Dayısoylu, K.S. Health Promoting Benefits of Postbiotics Produced by Lactic Acid Bacteria: Exopolysaccharide. Biotech Stud. 2022, 31, 61–70. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics Produced by Lactic Acid Bacteria: The next Frontier in Food Safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Chae, H.S.; Jeong, S.G.; Ham, J.S.; Im, S.K.; Ahn, C.N.; Lee, J.M. In Vitro Antioxidative Properties of Lactobacilli. Asian-Australas. J. Anim. Sci. 2005, 19, 262–265. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; LeBlanc, J.G.; Perdigón, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral Administration of a Catalase-Producing Lactococcus Lactis Can Prevent a Chemically Induced Colon Cancer in Mice. J. Med. Microbiol. 2008, 57, 100–105. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Cao, Q.; Zhang, C.; Dong, Z.; Feng, D.; Ye, H.; Zuo, J. Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens. Toxins 2022, 14, 830. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of Probiotics, Prebiotics, and Prebiotic-like Components in Common Functional Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Yang, W.; Jiang, S.; Li, Y. Supplementation with Exogenous Catalase from Penicillium Notatum in the Diet Ameliorates Lipopolysaccharide-Induced Intestinal Oxidative Damage through Affecting Intestinal Antioxidant Capacity and Microbiota in Weaned Pigs. Microbiol Spectr 2021, 9, e00654-21. [Google Scholar] [CrossRef]
- Wei, L.; Wang, B.; Bai, J.; Zhang, Y.; Liu, C.; Suo, H.; Wang, C. Postbiotics Are a Candidate for New Functional Foods. Food Chem. X 2024, 23, 101650. [Google Scholar] [CrossRef]
- Jung, B.-J.; Kim, H.; Chung, D.-K. Differential Immunostimulatory Effects of Lipoteichoic Acids Isolated from Four Strains of Lactiplantibacillus Plantarum. Appl. Sci. 2022, 12, 954. [Google Scholar] [CrossRef]
- Evangelista, A.G.; Corrêa, J.A.F.; Dos Santos, J.V.G.; Matté, E.H.C.; Milek, M.M.; Biauki, G.C.; Costa, L.B.; Luciano, F.B. Cell-Free Supernatants Produced by Lactic Acid Bacteria Reduce Salmonella Population in Vitro. Microbiol. 2021, 167, 001102. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-Free Culture Supernatant of Bifidobacterium Breve CNCM I-4035 Decreases Pro-Inflammatory Cytokines in Human Dendritic Cells Challenged with Salmonella Typhi through TLR Activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Marco, M.L. The Fermented Cabbage Metabolome and Its Protection against Cytokine-Induced Intestinal Barrier Disruption of Caco-2 Monolayers. Appl. Environ. Microbiol. 2025, 91, e02234-24. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breidt, F.; Lu, Z.; Fleming, H.P. DNA Fingerprinting of Lactic Acid Bacteria in Sauerkraut Fermentations. Appl. Env. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial Community Analysis of Sauerkraut Fermentation Reveals a Stable and Rapidly Established Community. Foods 2018, 7, 77. [Google Scholar] [CrossRef]
- Wilburn, J.R.; Ryan, E.P. Chapter 1—Fermented Foods in Health Promotion and Disease Prevention: An overview. In Fermented Foods in Health and Disease Prevention; Elsevier eBooks: Amsterdam, The Netherlands, 2017; pp. 3–19. [Google Scholar] [CrossRef]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards Understanding Molecular Modes of Probiotic Action. Curr. Opin. Biotechnol. 2006, 17, 204–210. [Google Scholar] [CrossRef]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and Short Chain Fatty Acids Produced by Microbial Fermentation Downregulate Proinflammatory Responses in Intestinal Epithelial Cells and Myeloid Cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, T.-Y.; Kim, Y.; Lee, S.-H.; Kim, S.; Kang, S.W.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Pedersen, M.G.B.; Søndergaard, E.; Nielsen, C.B.; Johannsen, M.; Gormsen, L.C.; Møller, N.; Jessen, N.; Rittig, N. Oral Lactate Slows Gastric Emptying and Suppresses Appetite in Young Males. Clin. Nutr. 2022, 41, 517–525. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, R.; Li, X.; Yao, Z.; Zhang, H.; Li, H.; Chen, W. Unexpected Immunoregulation Effects of D-Lactate, Different from L-Lactate. Food Agric. Immunol. 2022, 33, 286–301. [Google Scholar] [CrossRef]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of Dietary Acetic Acid Supplementation on Plasma Glucose, Lipid Profiles, and Body Mass Index in Human Adults: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2021, 121, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; de Moraes, W.M.A.M.; da Silva, G.A.R.; Prestes, J.; Schoenfeld, B.J. Vinegar (Acetic Acid) Intake on Glucose Metabolism: A Narrative Review. Clin. Nutr. ESPEN 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of Fermentation on Antioxidant Properties of Red Cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.-C.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoids, Polyphenols and Micronutrient Profiles of Brassica Oleraceae and Plum Varieties and Their Contribution to Measures of Total Antioxidant Capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Sidro, B.; Ullate, M.; Frias, J.; Vidal-Valverde, C. White Cabbage Fermentation Improves Ascorbigen Content, Antioxidant and Nitric Oxide Production Inhibitory Activity in LPS-Induced Macrophages. LWT—Food Sci. Technol. 2012, 46, 77–83. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Bartoszek, A.; Baer-Dubowska, W. Modulation of Rat Hepatic and Kidney Phase II Enzymes by Cabbage Juices: Comparison with the Effects of Indole-3-Carbinol and Phenethyl Isothiocyanate. Br. J. Nutr. 2011, 105, 816–826. [Google Scholar] [CrossRef]
- Siddeeg, A.; Afzaal, M.; Saeed, F.; Ali, R.; Shah, Y.A.; Shehzadi, U.; Ateeq, H.; Waris, N.; Hussain, M.; Raza, M.A.; et al. Recent Updates and Perspectives of Fermented Healthy Super Food Sauerkraut: A Review. Int. J. Food Prop. 2022, 25, 2320–2331. [Google Scholar] [CrossRef]
- Peters, A.; Krumbholz, P.; Jäger, E.; Heintz-Buschart, A.; Çakir, M.V.; Rothemund, S.; Gaudl, A.; Ceglarek, U.; Schöneberg, T.; Stäubert, C. Metabolites of Lactic Acid Bacteria Present in Fermented Foods Are Highly Potent Agonists of Human Hydroxycarboxylic Acid Receptor 3. PLOS Genet. 2019, 15, e1008145. [Google Scholar] [CrossRef]
- Kasperek, M.C.; Velasquez Galeas, A.; Caetano-Silva, M.E.; Xie, Z.; Ulanov, A.; La Frano, M.; Devkota, S.; Miller, M.J.; Allen, J.M. Microbial Aromatic Amino Acid Metabolism Is Modifiable in Fermented Food Matrices to Promote Bioactivity. Food Chem. 2024, 454, 139798. [Google Scholar] [CrossRef]
- Shelton, C.D.; Sing, E.; Mo, J.; Shealy, N.G.; Yoo, W.; Thomas, J.; Fitz, G.N.; Castro, P.R.; Hickman, T.T.; Torres, T.P.; et al. An Early-Life Microbiota Metabolite Protects against Obesity by Regulating Intestinal Lipid Metabolism. Cell Host Microbe 2023, 31, 1604–1619.e10. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-Mediated Immune System Imprinting Early in Life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite Profile Changes and Increased Antioxidative and Antiinflammatory Activities of Mixed Vegetables after Fermentation by Lactobacillus Plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of Kefir Drinks Produced by Backslopping Method Using Kefir Grains from Bosnia and Herzegovina: Microbial Dynamics and Volatilome Profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk Kefir: Composition, Microbial Cultures, Biological Activities, and Related Products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef]
- Barros, S.É.d.L.; Rocha, C.d.S.; Moura, M.S.B.d.; Barcelos, M.P.; Silva, C.H.T.d.P.d.; Hage-Melim, L.I.d.S. Potential Beneficial Effects of Kefir and Its Postbiotic, Kefiran, on Child Food Allergy. Food Funct. 2021, 12, 3770–3786. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and Healing Activity of Kefir and Kefiran Extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Seo, K.-H.; Gyu Lee, H.; Young Eor, J.; Jin Jeon, H.; Yokoyama, W.; Kim, H. Effects of Kefir Lactic Acid Bacteria-Derived Postbiotic Components on High Fat Diet-Induced Gut Microbiota and Obesity. Food Res. Int. 2022, 157, 111445. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.d.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R.; Sharma, A.; Lee, H.-J. Kefir: A Fermented Plethora of Symbiotic Microbiome and Health. J. Ethn. Foods 2024, 11, 35. [Google Scholar] [CrossRef]
- Nugroho, D.; Thinthasit, A.; Surya, E.; Hartati; Oh, J.-S.; Jang, J.-G.; Benchawattananon, R.; Surya, R. Immunoenhancing and Antioxidant Potentials of Kimchi, an Ethnic Food from Korea, as a Probiotic and Postbiotic Food. J. Ethn. Foods 2024, 11, 12. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J.D. Diet, Microbiota, and Microbial Metabolites in Colon Cancer Risk in Rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef]
- Chen, J.; Pitmon, E.; Wang, K. Microbiome, Inflammation and Colorectal Cancer. Semin. Immunol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary Fiber and SCFAs in the Regulation of Mucosal Immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-Protein-Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Lanis, J.M.; Alexeev, E.E.; Curtis, V.F.; Kitzenberg, D.A.; Kao, D.J.; Battista, K.D.; Gerich, M.E.; Glover, L.E.; Kominsky, D.J.; Colgan, S.P. Tryptophan Metabolite Activation of the Aryl Hydrocarbon Receptor Regulates IL-10 Receptor Expression on Intestinal Epithelia. Mucosal Immunol. 2017, 10, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, P.; Xie, Q.; Wang, N.; Li, H.; Smith, E.E.; Li, C.; Liu, F.; Huo, G.; Li, B. Protective Effects of Tryptophan-Catabolizing Lactobacillus Plantarum KLDS 1.0386 against Dextran Sodium Sulfate-Induced Colitis in Mice. Food Funct. 2020, 11, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus Reuteri Induces Gut Intraepithelial CD4+CD8αα+ T Cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Rabah, H.; Ménard, O.; Gaucher, F.; do Carmo, F.L.R.; Dupont, D.; Jan, G. Cheese Matrix Protects the Immunomodulatory Surface Protein SlpB of Propionibacterium Freudenreichii during in Vitro Digestion. Food Res. Int. 2018, 106, 712–721. [Google Scholar] [CrossRef]
- Taverniti, V.; Stuknyte, M.; Minuzzo, M.; Arioli, S.; De Noni, I.; Scabiosi, C.; Cordova, Z.M.; Junttila, I.; Hämäläinen, S.; Turpeinen, H.; et al. S-Layer Protein Mediates the Stimulatory Effect of Lactobacillus Helveticus MIMLh5 on Innate Immunity. Appl. Environ. Microbiol. 2013, 79, 1221–1231. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, T.; Zhang, P.; Ma, Y.; Qin, H. Lactobacillus Plantarum Surface Layer Adhesive Protein Protects Intestinal Epithelial Cells against Tight Junction Injury Induced by Enteropathogenic Escherichia Coli. Mol. Biol. Rep. 2011, 38, 3471–3480. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial Teichoic Acids Reverse Predominant IL-12 Production Induced by Certain Lactobacillus Strains into Predominant IL-10 Production via TLR2-Dependent ERK Activation in Macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef]
- Tomkovich, S.; Jobin, C. Microbiota and Host Immune Responses: A Love–Hate Relationship. Immunology 2016, 147, 1–10. [Google Scholar] [CrossRef]
- Zadeh, M.; Khan, M.W.; Goh, Y.J.; Selle, K.; Owen, J.L.; Klaenhammer, T.; Mohamadzadeh, M. Induction of Intestinal Pro-Inflammatory Immune Responses by Lipoteichoic Acid. J. Inflamm. 2012, 9, 7. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Colorectal Tumorigenesis via Epigenetic Regulation of CD8+ T Cell Immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Bäuerl, C.; Coll-Marqués, J.M.; Tarazona-González, C.; Pérez-Martínez, G. Lactobacillus Casei Extracellular Vesicles Stimulate EGFR Pathway Likely Due to the Presence of Proteins P40 and P75 Bound to Their Surface. Sci. Rep. 2020, 10, 19237. [Google Scholar] [CrossRef]
- Lu, R.; Shang, M.; Zhang, Y.-G.; Jiao, Y.; Xia, Y.; Garrett, S.; Bakke, D.; Bäuerl, C.; Martinez, G.P.; Kim, C.-H.; et al. Lactic Acid Bacteria Isolated from Korean Kimchi Activate the Vitamin D Receptor–Autophagy Signaling Pathways. Inflamm. Bowel Dis. 2020, 26, 1199–1211. [Google Scholar] [CrossRef]
- Abbasi, A.; Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Baghbanzadeh, A. Antigenotoxicity and Cytotoxic Potentials of Cell-Free Supernatants Derived from Saccharomyces Cerevisiae Var. Boulardii on HT-29 Human Colon Cancer Cell Lines. Probiotics Antimicro. Prot. 2023, 15, 1583–1595. [Google Scholar] [CrossRef]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-Killed Probiotic Bacteria Induce Apoptosis of HT-29 Human Colon Adenocarcinoma Cell Line via the Regulation of Bax/Bcl2 and Caspases Pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-Derived Ferrichrome Inhibits Colon Cancer Progression via JNK-Mediated Apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Holley, D.; Collins, L.B.; Montgomery, S.A.; Whitmore, A.C.; Hillhouse, A.; Curry, K.P.; Renner, S.W.; Greenwalt, A.; Ryan, E.P.; et al. A Gnotobiotic Mouse Model Demonstrates That Dietary Fiber Protects against Colorectal Tumorigenesis in a Microbiota- and Butyrate-Dependent Manner. Cancer Discov. 2014, 4, 1387–1397. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Z.; Han, S.; Lu, X. Butyrate Inhibits the Proliferation and Induces the Apoptosis of Colorectal Cancer HCT116 Cells via the Deactivation of MTOR/S6K1 Signaling Mediated Partly by SIRT1 Downregulation. Mol. Med. Rep. 2019, 19, 3941–3947. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Kim, K.; Son, M.-Y.; Min, J.-K.; Kim, J.; Han, T.-S.; Kim, D.-S.; Cho, H.-S. Downregulation of PRMT1, a Histone Arginine Methyltransferase, by Sodium Propionate Induces Cell Apoptosis in Colon Cancer. Oncol. Rep. 2019, 41, 1691–1699. [Google Scholar] [CrossRef]
- Tarasenko, N.; Nudelman, A.; Tarasenko, I.; Entin-Meer, M.; Hass-Kogan, D.; Inbal, A.; Rephaeli, A. Histone Deacetylase Inhibitors: The Anticancer, Antimetastatic and Antiangiogenic Activities of AN-7 Are Superior to Those of the Clinically Tested AN-9 (Pivanex). Clin. Exp. Metastasis 2008, 25, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Humphreys, K.J.; Simpson, K.J.; McKinnon, R.A.; Meech, R.; Michael, M.Z. Functional High-Throughput Screen Identifies MicroRNAs That Promote Butyrate-Induced Death in Colorectal Cancer Cells. Mol. Ther. Nucleic Acids 2022, 30, 30–47. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Fullaondo, A.; Odriozola, I.; Odriozola, A. Chapter Eight—Microbiota and Beneficial Metabolites in Colorectal Cancer. In Advances in Genetics; Martínez, A.O., Ed.; Advances in Host Genetics and Microbiome in Colorectal Cancer-Related Phenotypes; Academic Press: Cambridge, MA, USA, 2024; Volume 112, pp. 367–409. [Google Scholar]
- Da, M.; Sun, J.; Ma, C.; Li, D.; Dong, L.; Wang, L.-S.; Chen, F. Postbiotics: Enhancing Human Health with a Novel Concept. eFood 2024, 5, e180. [Google Scholar] [CrossRef]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic Postbiotic from Lactobacillus Paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Vedrine, M.L.; Hanlon, J.; Bevan, R.; Floyd, P.; Brown, T.; Matthies, F. Extensive Literature Search, Selection for Relevance and Data Extraction of Studies Related to the Toxicity of PCDD/Fs and DL-PCBs in Humans. EFSA Support. Publ. 2018, 15, 1136E. [Google Scholar] [CrossRef]
- D’Amore, T.; Chaari, M.; Falco, G.; De Gregorio, G.; Zaraî Jaouadi, N.; Ali, D.S.; Sarkar, T.; Smaoui, S. When Sustainability Meets Health and Innovation: The Case of Citrus by-Products for Cancer Chemoprevention and Applications in Functional Foods. Biocatal. Agric. Biotechnol. 2024, 58, 103163. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus Rhamnosus GG Decrease Colon Cancer Cell Invasion In Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Elham, N.; Naheed, M.; Elahe, M.; Hossein, M.M.; Majid, T. Selective Cytotoxic Effect of Probiotic, Paraprobiotic and Postbiotics of L. Casei Strains against Colorectal Cancer Cells: Invitro Studies. Braz. J. Pharm. Sci. 2022, 58, e19400. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Hsieh, Y.-M.; Huang, C.-C.; Tsai, C.-C. Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules 2017, 22, 107. [Google Scholar] [CrossRef]
- Jastrząb, R.; Tomecki, R.; Jurkiewicz, A.; Graczyk, D.; Szczepankowska, A.K.; Mytych, J.; Wolman, D.; Siedlecki, P. The Strain-Dependent Cytostatic Activity of Lactococcus Lactis on CRC Cell Lines Is Mediated through the Release of Arginine Deiminase. Microb. Cell Factories 2024, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, Z.; Mao, L.; Chen, S.; Sun, S. Sodium Butyrate Induces Autophagy in Colorectal Cancer Cells through LKB1/AMPK Signaling. J. Physiol. Biochem. 2019, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic Metabolites Produced by Lactobacillus Plantarum Strains Exert Selective Cytotoxicity Effects on Cancer Cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef]
- Macias-Diaz, A.; Lopez, J.J.; Bravo, M.; Jardín, I.; Garcia-Jimenez, W.L.; Blanco-Blanco, F.J.; Cerrato, R.; Rosado, J.A. Postbiotics of Lacticaseibacillus Paracasei CECT 9610 and Lactiplantibacillus Plantarum CECT 9608 Attenuates Store-Operated Calcium Entry and FAK Phosphorylation in Colorectal Cancer Cells. Mol. Oncol. 2024, 18, 1123–1142. [Google Scholar] [CrossRef]
- Deepak, V.; Pandian, S.R.K.; Sivasubramaniam, S.D.; Nellaiah, H.; Sundar, K. Optimization of Anticancer Exopolysaccharide Production from Probiotic Lactobacillus Acidophilus by Response Surface Methodology. Prep. Biochem. Biotechnol. 2016, 46, 288–297. [Google Scholar] [CrossRef]
- Cousin, F.J.; Jouan-Lanhouet, S.; Théret, N.; Brenner, C.; Jouan, E.; Moigne-Muller, G.L.; Dimanche-Boitrel, M.-T.; Jan, G. The Probiotic Propionibacterium Freudenreichii as a New Adjuvant for TRAIL-Based Therapy in Colorectal Cancer. Oncotarget 2016, 7, 7161–7178. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate Modulates Bacterial Adherence on LS174T Human Colorectal Cells by Stimulating Mucin Secretion and MAPK Signaling Pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Yang, L.; Xu, X. The Intervention of B. Longum Metabolites in Fnevs’ Carcinogenic Capacity: A Potential Double-Edged Sword. Exp. Cell Res. 2025, 445, 114407. [Google Scholar] [CrossRef]
- Erfanian, N.; Nasseri, S.; Miraki Feriz, A.; Safarpour, H.; Namaei, M.H. Characterization of Wnt Signaling Pathway under Treatment of Lactobacillus Acidophilus Postbiotic in Colorectal Cancer Using an Integrated in Silico and in Vitro Analysis. Sci. Rep. 2023, 13, 22988. [Google Scholar] [CrossRef]
- Erfanian, N.; Safarpour, H.; Tavakoli, T.; Mahdiabadi, M.A.; Nasseri, S.; Namaei, M.H. Investigating the Therapeutic Potential of Bifidobacterium Breve and Lactobacillus Rhamnosus Postbiotics through Apoptosis Induction in Colorectal HT-29 Cancer Cells. Iran. J. Microbiol. 2024, 16, 68–78. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Długosz, E.; Szulc-Dąbrowska, L.; Zielińska, D. Novel Gluconobacter Oxydans Strains Selected from Kombucha with Potential Postbiotic Activity. Appl. Microbiol. Biotechnol. 2024, 108, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, L.; Peek, R.M.; Acra, S.A.; Moore, D.J.; Wilson, K.T.; He, F.; Polk, D.B.; Yan, F. Supplementation of P40, a Lactobacillus Rhamnosus GG-Derived Protein, in Early Life Promotes Epidermal Growth Factor Receptor-Dependent Intestinal Development and Long-Term Health Outcomes. Mucosal Immunol. 2018, 11, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Administration of Metabiotics Extracted From Probiotic Lactobacillus Rhamnosus MD 14 Inhibit Experimental Colorectal Carcinogenesis by Targeting Wnt/β-Catenin Pathway. Front. Oncol. 2020, 10, 746. [Google Scholar] [CrossRef]
- Ma, F.; Song, Y.; Sun, M.; Wang, A.; Jiang, S.; Mu, G.; Tuo, Y. Exopolysaccharide Produced by Lactiplantibacillus Plantarum-12 Alleviates Intestinal Inflammation and Colon Cancer Symptoms by Modulating the Gut Microbiome and Metabolites of C57BL/6 Mice Treated by Azoxymethane/Dextran Sulfate Sodium Salt. Foods 2021, 10, 3060. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yu, B.; Zhou, J.; Zhang, J.; Zhang, R.; Xie, J.; Wang, Q.; Zhao, S. Lysates of Lactobacillus Acidophilus Combined with CTLA-4-Blocking Antibodies Enhance Antitumor Immunity in a Mouse Colon Cancer Model. Sci. Rep. 2019, 9, 20128. [Google Scholar] [CrossRef]
- Lee, P.-J.; Hung, C.-M.; Yang, A.-J.; Hou, C.-Y.; Chou, H.-W.; Chang, Y.-C.; Chu, W.-C.; Huang, W.-Y.; Kuo, W.-C.; Yang, C.-C.; et al. MS-20 Enhances the Gut Microbiota-Associated Antitumor Effects of Anti-PD1 Antibody. Gut Microbes 2024, 16, 2380061. [Google Scholar] [CrossRef]
- Oliero, M.; Cuisiniere, T.; Ajayi, A.S.; Gerkins, C.; Hajjar, R.; Fragoso, G.; Calvé, A.; Vennin Rendos, H.; Mathieu-Denoncourt, A.; Dagbert, F.; et al. Putrescine Supplementation Limits the Expansion of Pks+ Escherichia Coli and Tumor Development in the Colon. Cancer Res. Commun. 2024, 4, 1777–1792. [Google Scholar] [CrossRef]
- Zhong, B.; Zhao, Y.; Gao, L.; Yang, G.; Gao, Y.; Li, F.; Li, S. Anticancer Effects of Weizmannia Coagulans MZY531 Postbiotics in CT26 Colorectal Tumor-Bearing Mice by Regulating Apoptosis and Autophagy. Life 2024, 14, 1334. [Google Scholar] [CrossRef]
- Ma, C.; Duan, X.; Lei, X. 3D Cell Culture Model: From Ground Experiment to Microgravity Study. Front. Bioeng. Biotechnol. 2023, 11, 1136583. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; de Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Röder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D Model for CAR-mediated Cytotoxicity Using Patient-derived Colorectal Cancer Organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-Culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Kikuchi, I.S.; Cardoso Galante, R.S.; Dua, K.; Malipeddi, V.R.; Awasthi, R.; Ghisleni, D.D.M.; de Jesus Andreoli Pinto, T. Hydrogel Based Drug Delivery Systems: A Review with Special Emphasis on Challenges Associated with Decontamination of Hydrogels and Biomaterials. Curr. Drug Deliv. 2017, 14, 917–925. [Google Scholar] [CrossRef]
- Park, Y.; Huh, K.M.; Kang, S.-W. Applications of Biomaterials in 3D Cell Culture and Contributions of 3D Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Liu, M.; Prakash, S.; Han, J.; Ding, Z.; Zhao, Y.; Wang, Z. Dynamic Crosslinked and Injectable Biohydrogels as Extracellular Matrix Mimics for the Delivery of Antibiotics and 3D Cell Culture. RSC Adv. 2020, 10, 19587–19599. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and Organs-on-Chips: Insights into Human Gut-Microbe Interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Bartfeld, S. Modeling Infectious Diseases and Host-Microbe Interactions in Gastrointestinal Organoids. Dev. Biol. 2016, 420, 262–270. [Google Scholar] [CrossRef]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling Cryptosporidium Infection in Human Small Intestinal and Lung Organoids. Nat. Microbiol. 2018, 3, 814–823. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Zhang, L.; Liu, Z. Comparative Analysis of Organoid, Air-Liquid Interface, and Direct Infection Models for Studying Pathogen-Host Interactions in Endometrial Tissue. Sci. Rep. 2025, 15, 8531. [Google Scholar] [CrossRef]
- Workman, M.J.; Gleeson, J.P.; Troisi, E.J.; Estrada, H.Q.; Kerns, S.J.; Hinojosa, C.D.; Hamilton, G.A.; Targan, S.R.; Svendsen, C.N.; Barrett, R.J. Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 669–677.e2. [Google Scholar] [CrossRef]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus Gallinarum Modulates the Gut Microbiota and Produces Anti-Cancer Metabolites to Protect against Colorectal Tumourigenesis. Gut 2022, 71, 2011–2021. [Google Scholar] [CrossRef]
- Cho, Y.; Sung, M.-H.; Kang, H.-T.; Lee, J.H. Establishment of an Apical-Out Organoid Model for Directly Assessing the Function of Postbiotics. J. Microbiol. Biotechnol. 2024, 34, 2184–2191. [Google Scholar] [CrossRef]
- Lee, H.; Jung, K.B.; Kwon, O.; Son, Y.S.; Choi, E.; Yu, W.D.; Son, N.; Jeon, J.H.; Jo, H.; Yang, H.; et al. Limosilactobacillus Reuteri DS0384 Promotes Intestinal Epithelial Maturation via the Postbiotic Effect in Human Intestinal Organoids and Infant Mice. Gut Microbes 2022, 14, 2121580. [Google Scholar] [CrossRef]
- Furone, F.; Bellomo, C.; Carpinelli, M.; Nicoletti, M.; Hewa-Munasinghege, F.N.; Mordaa, M.; Mandile, R.; Barone, M.V.; Nanayakkara, M. The Protective Role of Lactobacillus Rhamnosus GG Postbiotic on the Alteration of Autophagy and Inflammation Pathways Induced by Gliadin in Intestinal Models. Front. Med. 2023, 10, 1085578. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; van der Lugt, B.; Duivenvoorde, L.P.M.; Vos, A.P.; Bastiaan-Net, S.; Tomassen, M.M.M.; Verbokkem, J.A.C.; Blok-Heimerikx, E.; Hooiveld, G.J.E.J.; van Baarlen, P.; et al. Comparison of IPSC-Derived Human Intestinal Epithelial Cells with Caco-2 Cells and Human in Vivo Data after Exposure to Lactiplantibacillus Plantarum WCFS1. Sci. Rep. 2024, 14, 26464. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Bajramagic, S.; Hodzic, E.; Mulabdic, A.; Holjan, S.; Smajlovic, S.V.; Rovcanin, A. Usage of Probiotics and Its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Med. Arch. 2019, 73, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, F.; Adewiah, S.; Fatchiyah, F. The Level Short Chain Fatty Acids and HSP 70 in Colorectal Cancer and Non-Colorectal Cancer. Acta Inf. Med. 2018, 26, 160–163. [Google Scholar] [CrossRef]
- Liu, L.; Sadaghian Sadabad, M.; Gabarrini, G.; Lisotto, P.; von Martels, J.Z.H.; Wardill, H.R.; Dijkstra, G.; Steinert, R.E.; Harmsen, H.J.M. Riboflavin Supplementation Promotes Butyrate Production in the Absence of Gross Compositional Changes in the Gut Microbiota. Antioxid. Redox Signal. 2023, 38, 282–297. [Google Scholar] [CrossRef]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef]
- D’Argenio, G.; Mazzacca, G. Short-Chain Fatty Acid in the Human Colon. In Advances in Nutrition and Cancer 2; Zappia, V., Della Ragione, F., Barbarisi, A., Russo, G.L., Iacovo, R.D., Eds.; Springer: Boston, MA, USA, 1999; pp. 149–158. ISBN 978-1-4757-3230-6. [Google Scholar]
- Compare, D.; Nardone, G. Contribution of Gut Microbiota to Colonic and Extracolonic Cancer Development. Dig. Dis. 2011, 29, 554–561. [Google Scholar] [CrossRef]
- Cheng, P.; Zeng, W.; Li, L.; Huo, D.; Zeng, L.; Tan, J.; Zhou, J.; Sun, J.; Liu, G.; Li, Y.; et al. PLGA-PNIPAM Microspheres Loaded with the Gastrointestinal Nutrient NaB Ameliorate Cardiac Dysfunction by Activating Sirt3 in Acute Myocardial Infarction. Adv. Sci. 2016, 3, 1600254. [Google Scholar] [CrossRef]
- Sabatino, A.D.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral Butyrate for Mildly to Moderately Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef]
- Chien, S.-T.; Suydam, I.T.; Woodrow, K.A. Prodrug Approaches for the Development of a Long-Acting Drug Delivery Systems. Adv. Drug Deliv. Rev. 2023, 198, 114860. [Google Scholar] [CrossRef]
- Egorin, M.J.; Yuan, Z.-M.; Sentz, D.L.; Plaisance, K.; Eiseman, J.L. Plasma Pharmacokinetics of Butyrate after Intravenous Administration of Sodium Butyrate or Oral Administration of Tributyrin or Sodium Butyrate to Mice and Rats. Cancer Chemother. Pharmacol. 1999, 43, 445–453. [Google Scholar] [CrossRef]
- Patnaik, A.; Rowinsky, E.K.; Villalona, M.A.; Hammond, L.A.; Britten, C.D.; Siu, L.L.; Goetz, A.; Felton, S.A.; Burton, S.; Valone, F.H.; et al. A Phase I Study of Pivaloyloxymethyl Butyrate, a Prodrug of the Differentiating Agent Butyric Acid, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2002, 8, 2142–2148. [Google Scholar] [PubMed]
- Russo, R.; Santarcangelo, C.; Badolati, N.; Sommella, E.; De Filippis, A.; Dacrema, M.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. In Vivo Bioavailability and in Vitro Toxicological Evaluation of the New Butyric Acid Releaser N-(1-Carbamoyl-2-Phenyl-Ethyl) Butyramide. Biomed. Pharmacother. 2021, 137, 111385. [Google Scholar] [CrossRef] [PubMed]
- Annison, G.; Illman, R.J.; Topping, D.L. Acetylated, Propionylated or Butyrylated Starches Raise Large Bowel Short-Chain Fatty Acids Preferentially When Fed to Rats. J. Nutr. 2003, 133, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Saad, S.; Tillett, B.J.; McGuire, H.M.; Bordbar, S.; Yap, Y.A.; Nguyen, L.T.; Wilkins, M.R.; Corley, S.; Brodie, S.; et al. Metabolite-Based Dietary Supplementation in Human Type 1 Diabetes Is Associated with Microbiota and Immune Modulation. Microbiome 2022, 10, 9. [Google Scholar] [CrossRef]
- Toden, S.; Lockett, T.J.; Topping, D.L.; Scherer, B.L.; Watson, E.-J.L.; Southwood, J.G.; Clarke, J.M. Butyrylated Starch Affects Colorectal Cancer Markers Beneficially and Dose-Dependently in Genotoxin-Treated Rats. Cancer Biol. Ther. 2014, 15, 1515–1523. [Google Scholar] [CrossRef]
- Clarke, J.M.; Young, G.P.; Topping, D.L.; Bird, A.R.; Cobiac, L.; Scherer, B.L.; Winkler, J.G.; Lockett, T.J. Butyrate Delivered by Butyrylated Starch Increases Distal Colonic Epithelial Apoptosis in Carcinogen-Treated Rats. Carcinogenesis 2012, 33, 197–202. [Google Scholar] [CrossRef]
- Dianzani, C.; Foglietta, F.; Ferrara, B.; Rosa, A.C.; Muntoni, E.; Gasco, P.; Pepa, C.D.; Canaparo, R.; Serpe, L. Solid Lipid Nanoparticles Delivering Anti-Inflammatory Drugs to Treat Inflammatory Bowel Disease: Effects in an in Vivo Model. World J. Gastroenterol. 2017, 23, 4200–4210. [Google Scholar] [CrossRef]
- Mu, Y.; Kinashi, Y.; Li, J.; Yoshikawa, T.; Kishimura, A.; Tanaka, M.; Matsui, T.; Mori, T.; Hase, K.; Katayama, Y. Polyvinyl Butyrate Nanoparticles as Butyrate Donors for Colitis Treatment. ACS Appl. Bio Mater. 2021, 4, 2335–2341. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, R.; Kang, Z.; Cao, Z.; Liu, N.; Shen, J.; Wang, C.; Pan, F.; Zhou, X.; Liu, Z.; et al. Delivery of Short Chain Fatty Acid Butyrate to Overcome Fusobacterium Nucleatum-Induced Chemoresistance. J. Control. Release 2023, 363, 43–56. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Liu, L.; Wang, B.; Walker, W.A.; Acra, S.A.; Yan, F. Activation of Epidermal Growth Factor Receptor Mediates Mucin Production Stimulated by P40, a Lactobacillus Rhamnosus GG-Derived Protein *. J. Biol. Chem. 2014, 289, 20234–20244. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Characterization of a Probiotic-Derived Soluble Protein Which Reveals a Mechanism of Preventive and Treatment Effects of Probiotics on Intestinal Inflammatory Diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus Rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yang, X.; Shang, L. Microfluidic-derived montmorillonite composite microparticles for oral codelivery of probiotic biofilm and postbiotics. Sci. Adv. 2025, 11, eadt2131. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zeng, H.; Xu, D.; Li, W.; Wang, Y. Advances in prebiotic carbohydrate–based targeted delivery: Overcoming gastrointestinal challenges for bioactive ingredients. Food Chem. 2025, 466, 142210. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, C.; Du, T.; Zhuo, J.; Wang, C.; Li, B.; Xu, J.; Fan, M.; Wang, J.; Zhang, W. Cytoprotection of probiotics by nanoencapsulation for advanced functions. Trends Food Sci. Technol. 2023, 142, 104227. [Google Scholar] [CrossRef]
- Cuevas-González, P.; Liceaga, A.; Aguilar-Toalá, J. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Schnorr, C.E.; Pasquali, M.A.d.B. Paraprobiotics and postbiotics—Current state of scientific research and future trends toward the development of functional foods. Foods 2023, 12, 2394. [Google Scholar] [CrossRef]
- Taşkoparan, Ş.; Altınay, C.; Özer, H.B. Recent updates of probiotic dairy-based beverages. Food Funct. 2025, 16, 1656–1669. [Google Scholar] [CrossRef]

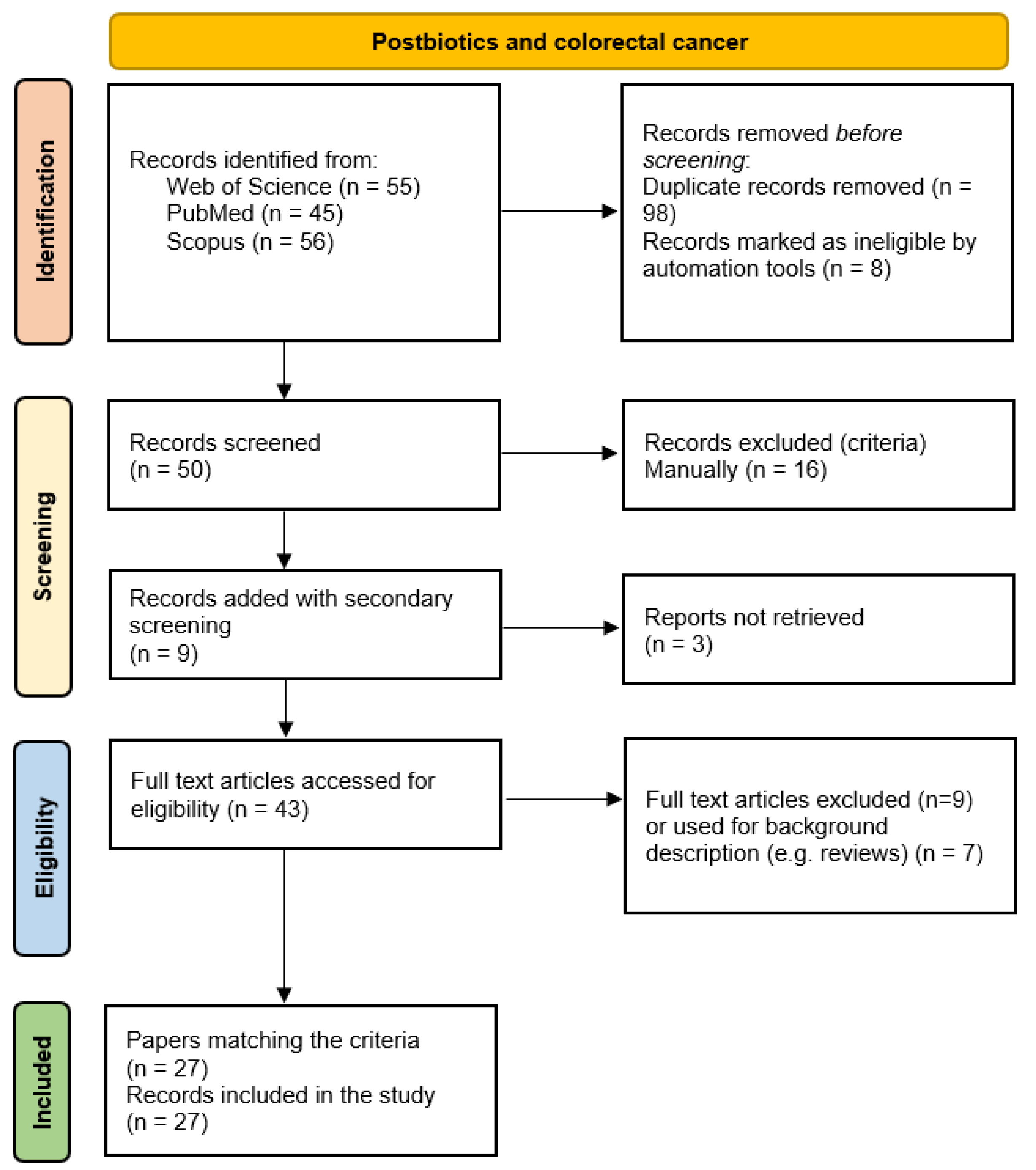
| Criterion | Decision | |
|---|---|---|
| Inclusion | Exclusion | |
| Default keywords and search terms exist as a whole or at least in the title, keywords, or abstract of the article | × | |
| The article is published in a peer-reviewed scientific journal | × | |
| The article is written in English | × | |
| Studies where terms such as prebiotics were referred to; however, supernatant/heat-killed cultures were used for testing/assessing | × | |
| Studies on diseases considered high risk factors for colorectal cancer, and a relevant study model was developed/used | × | |
| Duplicate records | × | |
| The full text is not available | × | |
| Articles published before 2010 | × | |
| Only testing live microorganisms | × | |
| Studies on gut microbiota transplantation | × | |
| ID | Model | Study Type | Microorganism Strain/Species | Molecules of Interest | Events | Pathway/Gene Involved | Notes | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | HCT-116 | in vitro | Lactobacillus casei and Lactobacillus rhamnosus GG | Cell-free supernatant | Decreasing matrix metalloproteinase-9 (MMP-9) and increasing the tight junction protein zona occludens-1 (ZO-1) levels | cell invasion | [131] | |
| 2 | HT-29 | in vitro | 7 strains of Lactobacillus | Cell-free supernatant | Lactate dehydrogenase regulation | apoptosis | [133] | |
| 3 | HCT-116 HT-29 | in vitro | synthetic | sodium butyrate | Autophagy | LKB1–AMPK pathway | [135] | |
| 4 | HT-29 | in vitro | Lactobacillus plantarum | bacteriocins | Antiproliferative effect | apoptosis | study using several cancer cell lines | [136] |
| 5 | Caco-2 | in vitro | Lactobacillus acidophilus | exopolysaccharide | Upregulation of the expression of PPAR-γ | [138] | ||
| 6 | HT-29 HCT-116 | in vitro | Propionibacterium freudenreichii | culture supernatant, metabolites (propionate/acetate) | Increased pro-apoptotic gene expression (TRAIL-R2/DR5) and decreased anti-apoptotic gene expression (FLIP, XIAP); death receptors (TRAIL-R1/DR4, TRAIL-R2/DR5) and caspases (caspase-8, -9, and -3) activation; Bcl-2 expression inhibition | extrinsic apoptotic pathway | in combination with TNF-Related Apoptosis-Inducing Ligand (TRAIL) | [139] |
| 7 | LS174T | in vitro | Lactobacillus acidophilus and Bifidobacterium longum | butyrate | dose-dependent increase in mucin protein contents; increased transcriptional levels of MUC3, MUC4, and MUC12 | MAPK signaling pathway | doses: 6 or 9 mM | [140] |
| 8 | scRNA-seq analysis and DEGs analysis HT-29 human dermal fibroblasts | in silico in vitro | Lactobacillus acidophilus ATCC4356 | cell-free supernatant | Cell cycle arrest at G1 phase, anti-proliferative and anti-migration effects, and anti-proliferative activity on control fibroblasts. | Wnt signaling (SFRP1, SFRP2, SFRP4, MMP7) | [142] | |
| 9 | HT-29 human dermal fibroblast | in vitro | Bifidobacterium breve Lactobacillus rhamnosus | cell-free supernatant | Anti-proliferation, anti-migration, and apoptosis-related effects | apoptosis: Bax/Bcl2/caspase-3; Wnt signaling: RSPO2, NGF, MMP7 | [143] | |
| 10 | Caco-2 | in vitro | Lactobacillus casei | cell-free supernatant | Tumor cell cytotoxic effect | apoptosis | comparison of probiotic (live), paraprobiotic (heat-killed), and postbiotics (CFS) | [132] |
| 11 | HT-29 | in vitro | Gluconobacter oxydans strains isolated from Kombucha (KNS30, KNS31, KNS32, K1, and K2) | gluconic acid, glucuronic acid, acetic acid, pyruvic acid, fumaric acid, and lactic acid | Tumor cell cytotoxic effect | apoptotic/necrotic: annexin V and PI positive | study using gastric cell line: AGS; HUVEC cell lines used as control | [144] |
| 12 | HT-29 HCT-116 | in vitro | Lactobacillus lactis | cell-free supernatant | Depletion of arginine, decreased levels of c-Myc, and reduced phosphorylation of p70-S6 kinase | cell cycle arrest | [134] | |
| 13 | NCM460 Caco-2 HT-29 | in vitro | Lacticaseibacillus paracasei and Lactiplantibacillus plantarum | heat-inactivated cultures | Downregulation of Orai1 and STIM1 | FAK pathway (Store-operated calcium entry) | [137] | |
| 14 | HT-29 | in vitro | Saccharomyces boulardii | cell-free supernatant | Increased expression of Caspase 3 and PTEN genes; decreased expression of RelA and Bcl-XL genes | apoptosis | [117] | |
| 15 | HT-29 Fnevs infection model | in vitro | Bifidobacterium longum | cell-free supernatant | Inhibition of proliferation, migration, and invasion | inhibitory effects on the expression of specific oncogenes (e.g., Myc, IL16, KCNN2, ACSBG1, Pum1, MET, NR5A2) | controversial results | [141] |
| 16 | mouse colon carcinoma CT26.WT tumor cells were injected subcutaneously into BALB/c mice | in vivo | Weizmannia coagulans MZY531 | powder of W. coagulans MZY531; oligosaccharide suspension | Inhibition of tumor growth by modulating apoptosis and autophagy in tumor cells | apoptosis: Bax/Bcl2/caspase-3 and JAK2/STAT3 autophagy: PI3K/AKT/mTOR and TGF-β/SMAD4 | [151] | |
| 17 | Sprague–Dawley rats | in vivo | Lactobacillus rhamnosus MD 14 | metabiotic extract (acetate, butyrate, propionate, acetamide, thiocyanic acid, and oxalic acid) | Downregulation of oncogenes (K-ras, β-catenin, Cox-2, NF-κB) and upregulation of the TP53 gene leading to almost normal colon histology | Wnt/β-Catenin Pathway | active components in the metabiotic extract were characterized by LC-MS | [146] |
| 18 | xenograft mouse model CT-26 cells subcutaneously injected into BALB/c mice | in vivo ex vivo | multiple strains of probiotics and yeast | MS-20 “Symbiota®” in combination with anti-programmed cell death 1 (PD1) antibody | Inhibited colon and lung cancer growth | CD8+ T cells and PD1 expression | fecal samples from six patients were used for ex vivo evaluation | [149] |
| 19 | C57BL/6 mouse model where cancer was induced via AOM/DSS administration | in vivo | Escherichia coli Nissle 1917 | putrescine | Inhibition of the growth of the pathogenic strain pks+ E. coli NC101; reduced the number and size of colonic tumors, regulation of inflammatory cytokines; shift in the composition of gut microbiota | cell proliferation; fecal Lcn-2 marker of inflammation in inflammatory bowel diseases, TNFα, IL6, and IL10; 16S rRNA amplicon sequencing | [150] | |
| 20 | xenograft models obtained by injecting SW620 cells into male BALB/c nude mice Caco-2/bbe SKCO-1 SW620 | in vivo in vitro | Lactobacillus casei ATCC334 | ferrichrome | Activation of the JNK-DDIT3-mediated apoptotic pathway | JNK-DDIT3-mediated apoptotic pathway | effect of ferrichrome was compared with 5-FU and cisplatin | [119] |
| 21 | C57BL/6 mouse model where cancer was induced via AOM/DSS administration | in vivo | Lactiplantibacillus plantarum-12 | exopolysaccharide | Activation of caspase cascade and NF-κB signaling (IκB-α, p65, p-p65, p38, and p-p38) | inflammatory signaling and apoptosis | additional untargeted fecal metabolomic analysis | [147] |
| 22 | BALB/c mice CRC models induced via AOM/DSS administration | in vivo | Lactobacillus acidophilus | lysates | Increased CD8 + T cells and effector memory T cells, decreased Treg and M2 macrophages | TLR signaling pathway | combination with CTLA-4-blocking antibodies | [148] |
| 23 | C57B/6 mouse model CRC cell lines Organoids from CRC patients | in vitro in vivo organoids | Lactobacillus gallinarum | cell-free supernatant (indole-3-lactic acid, most enriched metabolite) | Antitumorigenic role: proliferation, apoptosis, cell cycle distribution, gut microbiota modulation | cell proliferation apoptosis | [169] | |
| 24 | Organoids derived from C57BL/6 male mice small intestines and colon | in vitro in vivo organoids | Lactiplantibacillus plantarum KM2 and Bacillus velezensis KMU01 | cell-free supernatant | Inflammatory response; LPS-induced and mitochondrial homeostasis through mitophagy and mitochondrial biogenesis | COX-2 decreased; expression of tight-junction markers ZO-1, claudin, and occludin increased, and expression of mitochondrial homeostasis factors PINK1, parkin, and PGC1a also increased. | [170] | |
| 25 | hPSC-derived intestinal organoids C57BL Mice Caco-2 | in vitro in vivo organoids | Limosilactobacillus reuteri DS0384 | N-carbamyl glutamic acid (NCG) | Intestinal epithelial maturation, inflammatory response, and intestinal epithelial barrier integrity | mature specific marker: (CDX2), (OLFM4), (DEFA5 and LYZ), (KRT20, CREB3L3, DPP4, LCT, SLC5A1, and MUC13); Inflammatory pathway: (IFNγ)/TNFα, IL-1β, IL-6, IL-8, and TNFα; localization of zonula occludens-1 | [171] | |
| 26 | Caco-2 Organoids derived from biopsies of a celiac disease patient | in vitro organoids | Lactobacillus rhamnosus GG | cell-free supernatant | Alteration in autophagy and inflammation pathways induced by gliadin in celiac disease | mTOR pathway: phosphorylation of p70S6K, p4EBP-1; inflammatory marker: NF- kb; autophagy: LC3II and p62 protein, SQSTM1 autophagosome membrane marker | [172] | |
| 27 | Caco-2 hiPSC-derived IEC monolayers | in vitro advanced patient-derived in vitro | Lactiplantibacillus plantarum | heat-killed | Inflammatory response | IL-8, REG3α, and HBD2 | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, T.; Zolfanelli, C.; Lauciello, V.; Di Ciancia, A.; Vagliasindi, A.; Smaoui, S.; Varzakas, T. Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms 2025, 13, 1335. https://doi.org/10.3390/microorganisms13061335
D’Amore T, Zolfanelli C, Lauciello V, Di Ciancia A, Vagliasindi A, Smaoui S, Varzakas T. Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms. 2025; 13(6):1335. https://doi.org/10.3390/microorganisms13061335
Chicago/Turabian StyleD’Amore, Teresa, Cinzia Zolfanelli, Vincenzo Lauciello, Alessio Di Ciancia, Alessio Vagliasindi, Slim Smaoui, and Theodoros Varzakas. 2025. "Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives" Microorganisms 13, no. 6: 1335. https://doi.org/10.3390/microorganisms13061335
APA StyleD’Amore, T., Zolfanelli, C., Lauciello, V., Di Ciancia, A., Vagliasindi, A., Smaoui, S., & Varzakas, T. (2025). Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms, 13(6), 1335. https://doi.org/10.3390/microorganisms13061335









