Evaluation of the Application Effects of Siniperca chuatsi in Biofloc Systems: A Comparative Study on the Use of Bamboo Flour and Rice Straw as Carbon Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Start-Up of BFT Aquaculture Systems
2.2. Fish Stocking and Management
2.3. Sample Collection
2.4. Determination of TAN and NO2−-N
2.5. Histopathological Procedure
2.6. Bioinformatics Analysis
2.7. qPCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
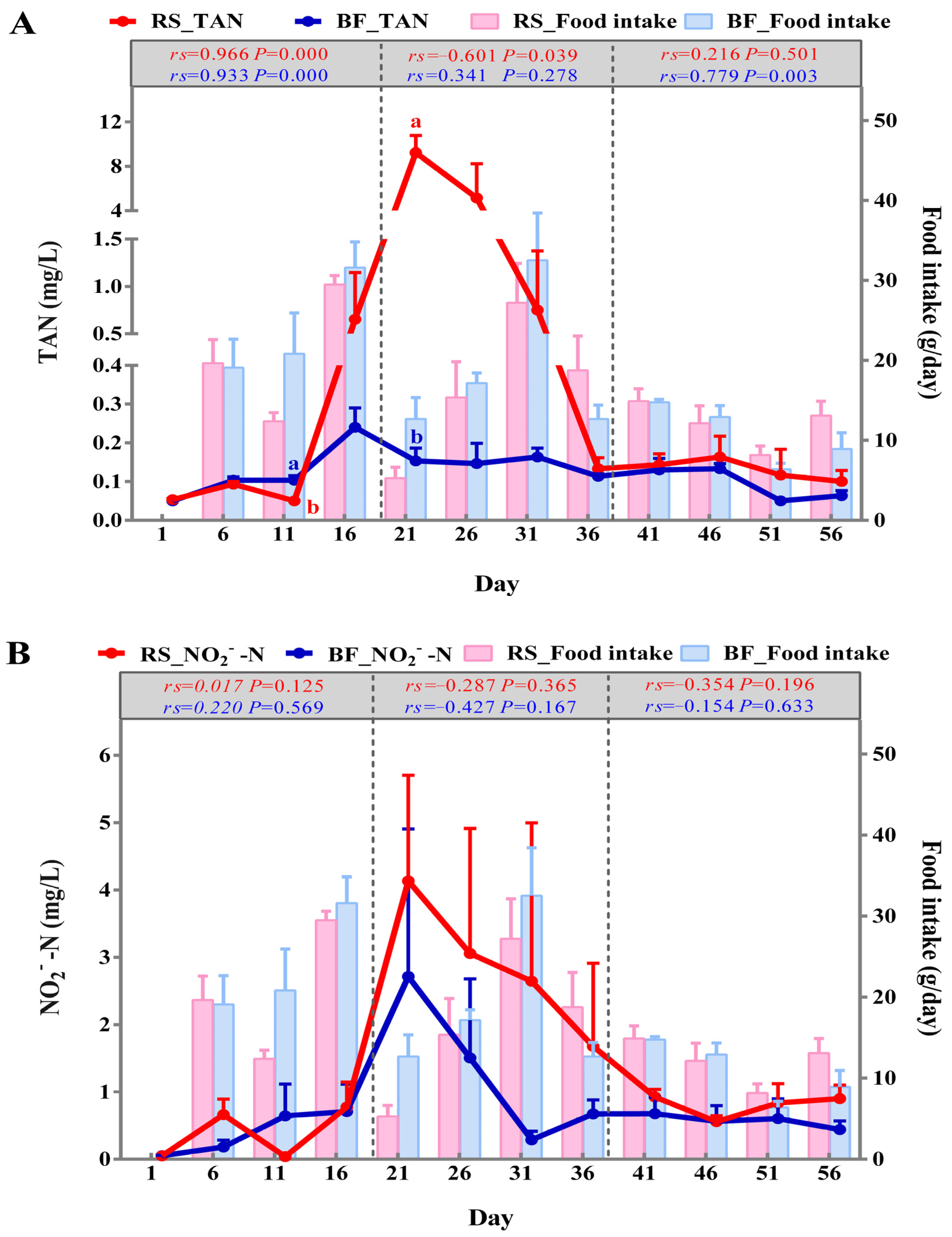
3.2. Changes of TAN and NO2−-N in the Water
3.3. Gill Histology
3.4. Microbiota Diversity Analysis
3.5. Bacterial Community Composition
3.6. Relative Expression Levels of Genes
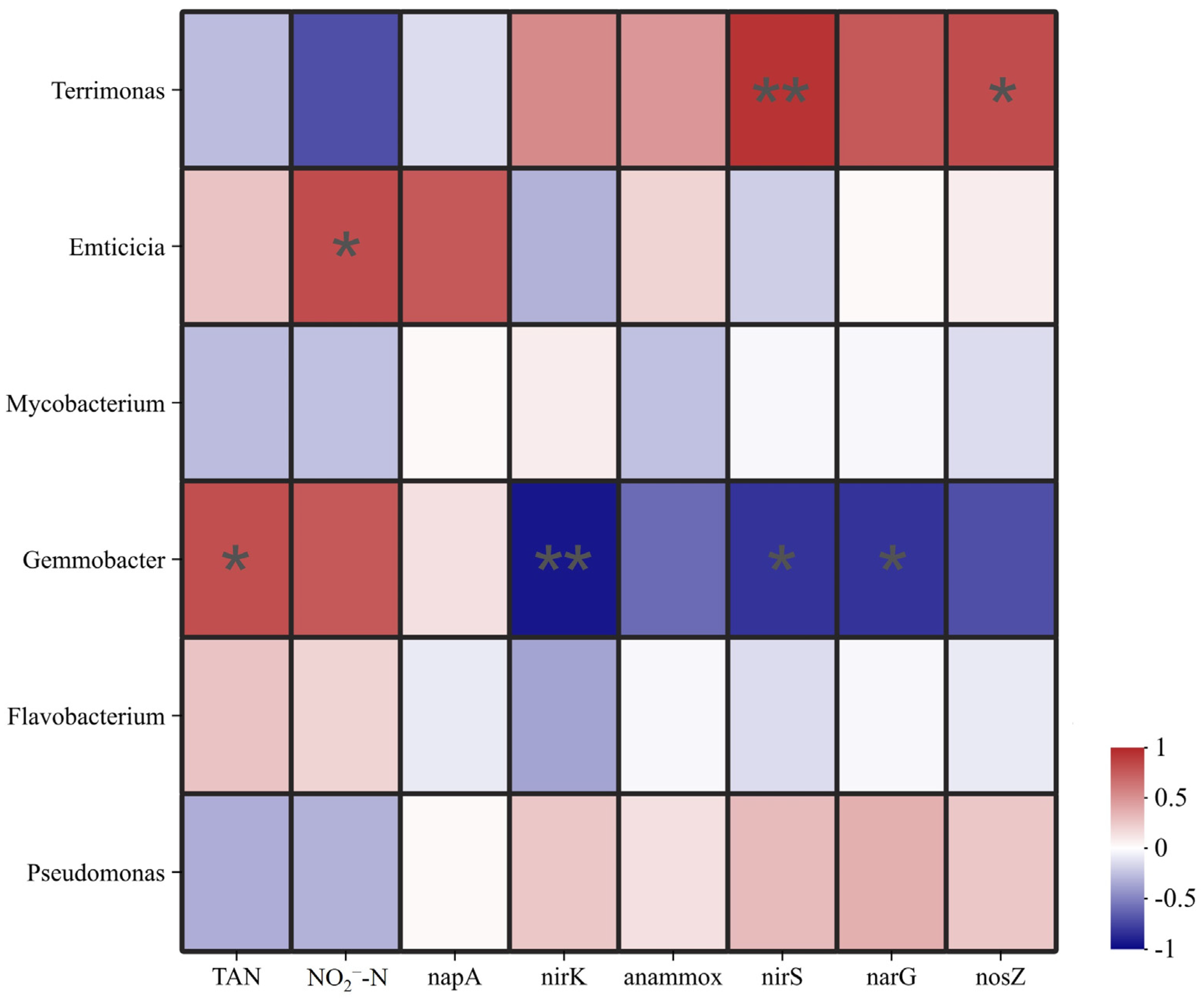
3.7. Correlation Analysis for Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Gao, H.Q. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2024; p. 25. [Google Scholar]
- He, S.; Li, L.; Lv, L.Y.; Cai, W.J.; Dou, Y.Q.; Li, J.; Tang, S.L.; Chen, X.; Zhang, Z.; Xu, J.; et al. Mandarin fish (Sinipercidae) genomes provide insights into innate predatory feeding. Commun. Biol. 2020, 3, 361. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Zeng, K.; Weng, S.P.; Chan, S.-M. Experimental transmission, pathogenicity and physical–chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.F.; He, S.; Li, J.; Huang, K.; Zhang, Y.P.; Huang, D. Lipid deposition pattern and adaptive strategy in response to dietary fat in Chinese perch (Siniperca chuatsi). Nutr. Metab. 2018, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Liang, X.F.; Liu, L.; He, S.; Kuang, Y.; Hoseinifar, S.H.; Dawar, F.U. Growth and Metabolic Response of Chinese Perch to Different Dietary Protein-to-Energy Ratios in Artificial Diets. Int. J. Mol. Sci. 2019, 20, 5983. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Lu, J.; Li, Z.; Shi, S.; Liu, Z. Effects of live and artificial feeds on the growth, digestion, immunity and intestinal microflora of mandarin fish hybrid (Siniperca chuatsi♀ × Siniperca scherzeri♂). Aquac. Res. 2017, 48, 4479–4485. [Google Scholar] [CrossRef]
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Bueno, G.W.; Bernal, F.E.M.; Roubach, R.; Skipper Skipper-Horton, J.O.; Sampaio, F.G.; Fialho, N.S.; Bureau, D.P. Modeling of waste outputs in the aquatic environment from a commercial cage farm under neotropical climate conditions. Aquac. Environ. Interact. 2023, 15, 133–144. [Google Scholar] [CrossRef]
- Ramli, N.M.; Verreth, J.A.J.; Yusoff, F.M.; Nurulhuda, K.; Nagao, N.; Verdegem, M.C.J. Integration of Algae to Improve Nitrogenous Waste Management in Recirculating Aquaculture Systems: A Review. Front. Bioeng. Biotechnol. 2020, 8, 1004. [Google Scholar] [CrossRef]
- Zhou, J.; Mogollon, J.M.; van Bodegom, P.M.; Barbarossa, V.; Beusen, A.H.W.; Scherer, L. Effects of Nitrogen Emissions on Fish Species Richness across the World’s Freshwater Ecoregions. Environ. Sci. Technol. 2023, 57, 8347–8354. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2019, 12, 878–908. [Google Scholar] [CrossRef]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems–A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2023, 907, 167911. [Google Scholar] [CrossRef] [PubMed]
- Kocour Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Máchová, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2016, 10, 525–542. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquac. 2020, 13, 1193–1222. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2020, 13, 676–705. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Robles-Porchas, G.R.; Gollas-Galván, T.; Martínez-Porchas, M.; Martínez-Cordova, L.R.; Miranda-Baeza, A.; Vargas-Albores, F. The nitrification process for nitrogen removal in biofloc system aquaculture. Rev. Aquac. 2020, 12, 2228–2249. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Deng, M.; Chen, J.; Gou, J.; Hou, J.; Li, D.; He, X. The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture 2018, 482, 103–110. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Wang, A.-L.; Liao, S.-A. Effect of different carbon sources on microbial community structure and composition of ex-situ biofloc formation. Aquaculture 2020, 515, 734492. [Google Scholar] [CrossRef]
- Xu, W.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Dauda, A.B.; Romano, N.; Ebrahimi, M.; Teh, J.C.; Ajadi, A.; Chong, C.M.; Karim, M.; Natrah, I.; Kamarudin, M.S. Influence of carbon/nitrogen ratios on biofloc production and biochemical composition and subsequent effects on the growth, physiological status and disease resistance of African catfish (Clarias gariepinus) cultured in glycerol-based biofloc systems. Aquaculture 2018, 483, 120–130. [Google Scholar] [CrossRef]
- Silva, U.L.; Vieira, L.C.; Mello, M.V.L.; Franca, E.J.; Falcon, D.R.; Correia, E.S. Response of phytoplankton to different carbon sources and C:N ratios in tilapia fingerling culture with bioflocs. Boletim Instituto Pesca 2018, 44, 155–160. [Google Scholar] [CrossRef]
- Day, S.B.; Salie, K.; Stander, H.B. A growth comparison among three commercial tilapia species in a biofloc system. Aquac. Int. 2016, 24, 1309–1322. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sajjadi, M.M.; Alizadeh, M.; Sourinejad, I. Nursery performance of Pacific white shrimp (Litopenaeus vannamei Boone, 1931) cultivated in a biofloc system: The effect of adding different carbon sources. Aquac. Res. 2017, 48, 1491–1501. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Q.; Luo, L.; Wang Ca Li, J.; Wang, L. Effect of feed C/N ratio promoted bioflocs on water quality and production performance of bottom and filter feeder carp in minimum-water exchanged pond polyculture system. Aquaculture 2014, 434, 442–448. [Google Scholar] [CrossRef]
- Fugimura, M.M.S.; dos Reis Flor, H.; de Melo, E.P.; da Costa, T.V.; Wasielesky, W.; Oshiro, L.M.Y. Brewery residues as a source of organic carbon in Litopenaeus schmitti white shrimp farms with BFT systems. Aquac. Int. 2014, 23, 509–522. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.C.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Li, C.; Deng, Y.; Tadda, M.A.; Lan, L.; Zhu, S.; Liu, D. Effects of different solid carbon sources on water quality, biofloc quality and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2018, 495, 919–931. [Google Scholar] [CrossRef]
- Serfling, S.A.; Microbial, f.l.o.c.s. Natural treatment method supports freshwater, marine species in recirculating systems. Glob. Aquac. Advocate 2006, 9, 34–36. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Yan, J.; Shi, K.; Pang, C.; Lester, E.; Wu, T. Influence of minerals on the thermal processing of bamboo with a suite of carbonaceous materials. Fuel 2016, 180, 256–262. [Google Scholar] [CrossRef]
- Zhou, B.; Duan, J.; Xue, L.; Zhang, J.; Yang, L. Effect of plant-based carbon source supplements on denitrification of synthetic wastewater: Focus on the microbiology. Environ. Sci. Pollut. Res. Int. 2019, 26, 24683–24694. [Google Scholar] [CrossRef] [PubMed]
- Addo, F.G.; Zhang, S.; Manirakiza, B.; Ma, Y.; Yuan, S.; Alklaf, S.A.; Guo, S.; Abakari, G. Brown sugar addition enhanced nutrient removal rates, growth performance, and bacterial community in a rice straw-based biofloc shrimp culture system. Aquaculture 2023, 567, 739274. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; No. 4503; SRAC Publication: Stoneville, MS, USA, 2013. [Google Scholar]
- Chen, S.; Zhang, Y.; Wei, Y.; Guo, Q.; Gan, L. Acetylcholinesterase activity, histopathological changes, lipid peroxidation and stress-related genes expression in Nile tilapia (Oreochromis niloticus) exposed to waterborne methidathion. Aquac. Rep. 2023, 33, 101757. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Q.; Deng, Z.; Cao, Z.; Jiang, J.; Chen, S.; Gan, L. Lactiplantibacillus plantarum fermented broth improved survival of marble goby (Oxyeleotris marmoratus) after skin abrasion by regulating skin mucus microbiota. Aquaculture 2023, 573, 739575. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci. Total Environ. 2018, 622–623, 121–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.F.; He, S.; Wang, J.; Li, L.; Zhang, Z.; Li, J.; Chen, X.; Li, L.; Alam, M.S. Metabolic responses of Chinese perch (Siniperca chuatsi) to different levels of dietary carbohydrate. Fish. Physiol. Biochem. 2021, 47, 1449–1465. [Google Scholar] [CrossRef]
- Li, L.; Fang, J.; Liang, X.F.; Alam, M.S.; Liu, L.; Yuan, X. Effect of feeding stimulants on growth performance, feed intake and appetite regulation of mandarin fish, Siniperca chuatsi. Aquac. Res. 2019, 50, 3684–3691. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Alizadeh, M.; Mohammadi, M.; Aliabad, H.S. Biofloc system applied to Nile tilapia (Oreochromis niloticus) farming using different carbon sources: Growth performance, carcass analysis, digestive and hepatic enzyme activity. Iran. J. Fish. Sci. 2021, 542, 490–513. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356–357, 147–152. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Akrami, R.; Najdegerami, E.H.; Ghiasvand, Z.; Koohsari, H. Effects of different protein levels and carbon sources on water quality, antioxidant status and performance of common carp (Cyprinus carpio) juveniles raised in biofloc based system. Aquaculture 2020, 516, 734639. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Biofloc Systems for Sustainable Production of Economically Important Aquatic Species: A Review. Sustainability 2021, 13, 7255. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, K.M.; Kim, J.H. Biofloc Technology in Fish Aquaculture: A Review. Antioxidants 2023, 12, 398. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tukmechi, A.; Farah, K.R. Use of different carbon sources for the biofloc system during the grow-out culture of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2018, 484, 259–267. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Sewid, A.H.; Nada, H.S.; Kamel, M.A.; El-Mandrawy, S.A.M.; Abdelhakim, T.M.N.; El-Murr, A.E.I.; Nahhas, N.E.; Hozzein, W.N.; Ibrahim, D. Mannanoligosaccharides as a Carbon Source in Biofloc Boost Dietary Plant Protein and Water Quality, Growth, Immunity and Aeromonas hydrophila Resistance in Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 1724. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Wei, H.; Zhu, X.; Han, D.; Jin, J.; Yang, Y.; Xie, S. Biofloc formation improves water quality and fish yield in a freshwater pond aquaculture system. Aquaculture 2019, 506, 256–269. [Google Scholar] [CrossRef]
- Tinh, T.H.; Koppenol, T.; Hai, T.N.; Verreth, J.A.J.; Verdegem, M.C.J. Effects of carbohydrate sources on a biofloc nursery system for whiteleg shrimp (Litopenaeus vannamei). Aquaculture 2021, 531, 735795. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Alizadeh, M.; Sharifinia, M. Effects of different carbon sources on water quality, biofloc quality, and growth performance of Nile tilapia (Oreochromis niloticus) fingerlings in a heterotrophic culture system. Aquac. Int. 2021, 29, 307–321. [Google Scholar] [CrossRef]
- Addo, F.G.; Zhang, S.; Manirakiza, B.; Ohore, O.E.; Shudong, Y. The impacts of straw substrate on biofloc formation, bacterial community and nutrient removal in shrimp ponds. Bioresour. Technol. 2021, 326, 124727. [Google Scholar] [CrossRef]
- Keshavanath, P.; Gangadhar, B.; Ramesh, T.J.; Van Rooij, J.M.; Beveridge, M.C.M.; Baird, D.J.; Verdegem, M.C.J.; Van Dam, A.A. Use of artificial substrates to enhance production of freshwater herbivorous fish in pond culture. Aquac. Res. 2001, 32, 189–197. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jena, J.; Das, P.C. Periphyton based grow-out farming of Indian major carps with Labeo calbasu (Hamilton) and Puntius gonionotus (Bleeker) for better water quality and enhanced fish production. Aquaculture 2021, 533, 736118. [Google Scholar] [CrossRef]
- Setiadi, E.; Taufik, I.; Widyastuti, Y.R.; Ardi, I.; Saputra, A. Different substrate of trickling filter on growth, survival rate, and water quality of common carp (Cyprinus carpio) cultivation by using an intensive recirculation system. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012027. [Google Scholar] [CrossRef]
- Han, X.; Cao, W.; He, C.; Huang, H.; Yuan, C.; Shi, L. Study on treatment of secondary effluent using an air lift and inner loof bioreactor with filamentous bamboo as bio-carriers. Technol. Water Treat. 2014, 40, 112–115. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, Y.J.; Kim, K.I.; Kim, S.K.; Kim, J.-H. Toxic effects of nitrogenous compounds (ammonia, nitrite, and nitrate) on acute toxicity and antioxidant responses of juvenile olive flounder, Paralichthys olivaceus. Environ. Toxicol. Pharmacol. 2019, 67, 73–78. [Google Scholar] [CrossRef]
- He, S. Study on Pond Juanyang Technology of Siniperca chuatsi. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar]
- Yang, K.; Hou, G.; Zhao, X.; Fang, T.; Wang, L. Effects of aquatic animals-plants synergistic purification system on water quality and economic benefit in mandarin fish pond. Acta Agric. Zhejiangensis 2023, 35, 1709–1719. [Google Scholar] [CrossRef]
- Roques, J.A.C.; Schram, E.; Spanings, T.; van Schaik, T.; Abbink, W.; Boerrigter, J.; de Vries, P.; van de Vis, H.; Flik, G. The impact of elevated water nitrite concentration on physiology, growth and feed intake of African catfish Clarias gariepinus (Burchell 1822). Aquac. Res. 2015, 46, 1384–1395. [Google Scholar] [CrossRef]
- Schram, E.; Roques, J.A.C.; Abbink, W.; Spanings, T.; de Vries, P.; Bierman, S.; de Vis Hv Flik, G. The impact of elevated water ammonia concentration on physiology, growth and feed intake of African catfish (Clarias gariepinus). Aquaculture 2010, 306, 108–115. [Google Scholar] [CrossRef]
- Lu, J.; Bo, Y.; Wang, Y.; Yuan, H.; Xu, Y. Effect of water quality ununiformity on production of marine medaka. Aquaculture 2023, 565, 739114. [Google Scholar] [CrossRef]
- Guo, F.H.; Wang, Z.H.; Chen, B.W.; Zhu, K.H. Acute Toxicity of Ammonia and Nitrite to Adult Siniperca chuatsi. Food Sci. 2009, 30, 397–400. [Google Scholar]
- Yang, F. Effects of Ammonia Nitrogen Stress on Antioxidant and Immune Functions of Juvenile Mandarin Fish (Siniperca chuatsi). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2021. [Google Scholar]
- Ortega, V.A.; Renner, K.J.; Bernier, N.J. Appetite-suppressing effects of ammonia exposure in rainbow trout associated with regional and temporal activation of brain monoaminergic and CRF systems. J. Exp. Biol. 2005, 208, 1855–1866. [Google Scholar] [CrossRef]
- Rodrigues, R.V.; Schwarz, M.H.; Delbos, B.C.; Sampaio, L.s.A. Acute toxicity and sublethal effects of ammonia and nitrite for juvenile cobia Rachycentron canadum. Aquaculture 2007, 271, 553–557. [Google Scholar] [CrossRef]
- Roumieh, R.; Barakat, A.; Abdelmeguid, N.E.; Ghanawi, J.; Patrick Saoud, I. Acute and chronic effects of aqueous ammonia on marbled spinefoot rabbitfish, Siganus rivulatus (Forsskål 1775). Aquac. Res. 2012, 44, 1777–1790. [Google Scholar] [CrossRef]
- Bower, C.E.; Bidwell, J.P. Ionization of Ammonia in Seawater: Effects of Temperature, pH, and Salinity. J. Fish. Res. Board Can. 1978, 35, 1012–1016. [Google Scholar] [CrossRef]
- Lemarié, G.; Dosdat, A.; Covès, D.; Dutto, G.; Gasset, E.; Person-Le Ruyet, J. Effect of chronic ammonia exposure on growth of European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2004, 229, 479–491. [Google Scholar] [CrossRef]
- Debbarma, R.; Biswas, P.; Singh, S.K. An integrated biomarker approach to assess the welfare status of Ompok bimaculatus (Pabda) in biofloc system with altered C/N ratio and subjected to acute ammonia stress. Aquaculture 2021, 545, 737184. [Google Scholar] [CrossRef]
- Xing, H.; Li, S.; Wang, Z.; Gao, X.; Xu, S.; Wang, X. Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pestic. Biochem. Physiol. 2012, 103, 74–80. [Google Scholar] [CrossRef]
- Strzyzewska, E.; Szarek, J.; Babinska, I. Morphologic evaluation of the gills as a tool in the diagnostics of pathological conditions in fish and pollution in the aquatic environment: A review. Veterinární Medicína 2016, 61, 123–132. [Google Scholar] [CrossRef]
- Sinha, A.K.; Matey, V.; Giblen, T.; Blust, R.; De Boeck, G. Gill remodeling in three freshwater teleosts in response to high environmental ammonia. Aquat. Toxicol. 2014, 155, 166–180. [Google Scholar] [CrossRef]
- Pan, R.; Guo, Z.; Xu, W.; Li, S.; Zheng, G.; Zou, S. Cooperative adaptation strategies of different tissues in blunt snout bream (Megalobrama amblycephala) juvenile to acute ammonia nitrogen stress. Environ. Sci. Pollut. Res. 2023, 30, 92042–92052. [Google Scholar] [CrossRef]
- Cienfuegos, K.; Hamdan, A.; Dosta, M.D.C.M.; Aguirre, F. Effect of two probiotics on bacterial community composition from biofloc system and their impact on survival and growth of tilapia (Oreochromis niloticus). Int. J. Fish. Aquat. Stud. 2018, 6, 525–533. [Google Scholar]
- Cai, X.; Liu, R.; Cheng, M.; Liu, T.; Zhang, Y.; Li, X.; Zeng, Y.; Long, H.; Ren, W.; Xie, Z.-y. Characterization of a novel Pseudomonas mosselii 9-1 capable of nitrate reduction to ammonium, ammonium assimilation, and denitrification in aerobic conditions. J. Water Process Eng. 2023, 52, 103531. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.-P.; Shen, Y.-Q. A systematic review of advances in intestinal microflora of fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Tan, K.; Gong, P.; Lei, P.; Guo, Z.; Wang, S.; Gao, S.; Zhou, Y.; Shu, Y.; Zhou, X.; et al. Correlation of microbiota in the gut of fish species and water. 3 Biotech 2020, 10, 472. [Google Scholar] [CrossRef]
- Zhang, X.; You, Y.; Peng, F.; Tang, X.; Zhou, Y.; Liu, J.; Lin, D.; Zhou, Y. Interaction of Microbiota between Fish and the Environment of an In-Pond Raceway System in a Lake. Microorganisms 2022, 10, 1143. [Google Scholar] [CrossRef]
- Cardona, E.; Gueguen, Y.; Magre, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol. 2016, 16, 157. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P.; Monroy-Dosta, M.d.C. Variation of the bacterial composition of biofloc and the intestine of Nile tilapia Oreochromis niloticus, cultivated using biofloc technology, supplied different feed rations. Aquac. Res. 2018, 49, 3658–3668. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.; Dong, J.; Li, W.; Tian, Y.; Hu, J.; Ye, X. Comparative Analysis of the Gut Microbiota of Mandarin Fish (Siniperca chuatsi) Feeding on Compound Diets and Live Baits. Front. Genet. 2022, 13, 797420. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, R.; Xiao, R.; Liu, L.; Zhu, S.; Zhao, J.; Ye, Z. Effects of flow velocity on the growth performance, antioxidant activity, immunity and intestinal health of Chinese Perch (Siniperca chuatsi) in recirculating aquaculture systems. Fish Shellfish. Immunol. 2023, 138, 108811. [Google Scholar] [CrossRef]
- Tang, K.Y.; Wang, Z.W.; Wan, Q.H.; Fang, S.G. Metagenomics Reveals Seasonal Functional Adaptation of the Gut Microbiome to Host Feeding and Fasting in the Chinese Alligator. Front. Microbiol. 2019, 10, 2409. [Google Scholar] [CrossRef]
- Stock, I. Plesiomonas shigelloides: An emerging pathogen with unusual properties. Rev. Med. Microbiol. 2004, 15, 129–139. [Google Scholar] [CrossRef]
- Sayeed, S.; Sack, D.A.; Qadri, F. Protection from Shigella sonnei infection by immunisation of rabbits with Plesiornonas shigelloides (SVCO1). J. Med. Microbiol. 1992, 37, 382–384. [Google Scholar] [CrossRef]
- Jong, S.I.d.; Broek, M.A.v.d.; Merkel, A.Y.; Cortes, P.d.l.T.; Kalamorz, F.; Cook, G.M.; Loosdrecht, M.C.M.v.; McMillan, D.G.G. Genomic analysis of Caldalkalibacillus thermarum TA2.A1 reveals aerobic alkaliphilic metabolism and evolutionary hallmarks linking alkaliphilic bacteria and plant life. Extremophiles 2020, 24, 923–935. [Google Scholar] [CrossRef]
- Danesh, A.; Mamo, G.; Mattiasson, B. Production of haloduracin by Bacillus halodurans using solid-state fermentation. Biotechnol. Lett. 2011, 33, 1339–1344. [Google Scholar] [CrossRef]
- McClerren, A.L.; Cooper, L.E.; Quan, C.; Thomas, P.M.; Kelleher, N.L.; Donk, W.A.v.d. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 17243–17248. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Hao, C.; Wang, H.; Liu, Q.H.; Li, X.D. Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by quantitative competitive, P.C.R. J. Environ. Sci. 2009, 21, 1557–1561. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, C.; Gao, T.; Peng, X.; Ma, S.; Sun, Q.; Xia, B.; Xie, X.; Bai, Z.; Xu, S.; et al. Improvement of fish production and water quality in a recirculating aquaculture pond enhanced with bacteria-microalgae association. Aquaculture 2022, 547, 737420. [Google Scholar] [CrossRef]




| RS | BF | p-Value | |
|---|---|---|---|
| IBW | 38.23 ± 0.17 | 38.80 ± 0.18 | 0.082 |
| FBW | 81.14 ± 0.88 | 78.45 ± 2.58 | 0.380 |
| SR | 100.00 ± 0.00 | 93.33 ± 3.85 | 0.158 |
| FI | 1.56 ± 0.02 | 1.76 ± 0.11 | 0.207 |
| WG | 112.21 ± 1.56 | 100.92 ± 6.45 | 0.164 |
| FCR | 1.13 ± 0.02 | 1.40 ± 0.15 | 0.153 |
| SGR | 1.34 ± 0.01 | 1.24 ± 0.06 | 0.166 |
| RS | BF | p-Value | |
|---|---|---|---|
| Water | |||
| OTUs | 732.00 ± 58.28 | 748.67 ± 110.64 | 0.900 |
| Chao 1 | 886.75 ± 61.25 | 949.71 ± 100.57 | 0.621 |
| Shannon | 4.24 ± 0.37 | 3.48 ± 0.75 | 0.417 |
| Intestine | |||
| OTUs | 271.33 ± 108.89 | 369.00 ± 88.09 | 0.524 |
| Chao 1 | 327.49 ± 103.92 | 488.28 ± 96.76 | 0.321 |
| Shannon | 2.21 ± 0.73 | 2.50 ± 0.82 | 0.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Deng, Z.; Chen, S.; Xiong, X.; Zeng, W.; Chen, F.; Tan, H.; Chen, X.; Yang, C.; He, Y.; et al. Evaluation of the Application Effects of Siniperca chuatsi in Biofloc Systems: A Comparative Study on the Use of Bamboo Flour and Rice Straw as Carbon Sources. Microorganisms 2025, 13, 1631. https://doi.org/10.3390/microorganisms13071631
Zhang H, Deng Z, Chen S, Xiong X, Zeng W, Chen F, Tan H, Chen X, Yang C, He Y, et al. Evaluation of the Application Effects of Siniperca chuatsi in Biofloc Systems: A Comparative Study on the Use of Bamboo Flour and Rice Straw as Carbon Sources. Microorganisms. 2025; 13(7):1631. https://doi.org/10.3390/microorganisms13071631
Chicago/Turabian StyleZhang, Huiling, Zhaojie Deng, Shijun Chen, Xi Xiong, Wenhui Zeng, Fang Chen, Huanjiao Tan, Xuran Chen, Canmin Yang, Yuhui He, and et al. 2025. "Evaluation of the Application Effects of Siniperca chuatsi in Biofloc Systems: A Comparative Study on the Use of Bamboo Flour and Rice Straw as Carbon Sources" Microorganisms 13, no. 7: 1631. https://doi.org/10.3390/microorganisms13071631
APA StyleZhang, H., Deng, Z., Chen, S., Xiong, X., Zeng, W., Chen, F., Tan, H., Chen, X., Yang, C., He, Y., Xie, D., & Gan, L. (2025). Evaluation of the Application Effects of Siniperca chuatsi in Biofloc Systems: A Comparative Study on the Use of Bamboo Flour and Rice Straw as Carbon Sources. Microorganisms, 13(7), 1631. https://doi.org/10.3390/microorganisms13071631








