The Effect of Limosilactobacillus fermentum MG4717 on Oral Health and Biosafety
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Preparation of Cell-Free Supernatant (CFS) and Whole-Cell Lysate (WC)
2.3. Inhibitory Effect of L. fermentum Strains Against Oral Pathogens
2.4. Biofilm Formation
2.5. Disk Diffusion Assay
2.6. Analysis of Methionine Gamma-Lyase (mgl) mRNA Expressions
2.7. Cell Culture
2.8. Adhesion Ability on KB Cells
2.9. Whole-Genome Sequencing (WGS)
2.10. Safety
2.10.1. Cytotoxicity to Intestinal Epithelial Cells
2.10.2. Hemolytic Activity
2.10.3. Bile Salt Hydrolase (BSH) Assay
2.10.4. Antibiotic Susceptibility Test
2.11. Probiotic Properties
2.11.1. Scanning Electron Microscopy (SEM)
2.11.2. Hydrogen Peroxide (H2O2) Production
2.11.3. Adhesion Ability on HT-29 Cells
2.11.4. Survival in Simulated Gastrointestinal Tract (GIT) Conditions
2.12. Statistical Analysis
3. Results
3.1. L. fermentum Strains Derived from the Oral Cavity Inhibit the Growth and Biofilm Formation of Oral Pathogens
3.2. Inhibitory Effect of L. fermentum MG4717 on the Growth of P. gingivalis and F. nucleatum, and Downregulation of mgl mRNA Expression of P. gingivalis
3.3. Adhesion Ability of L. fermentum MG4717 on Oral Epithelial Cells
3.4. Genome Analysis of L. fermentum MG4717
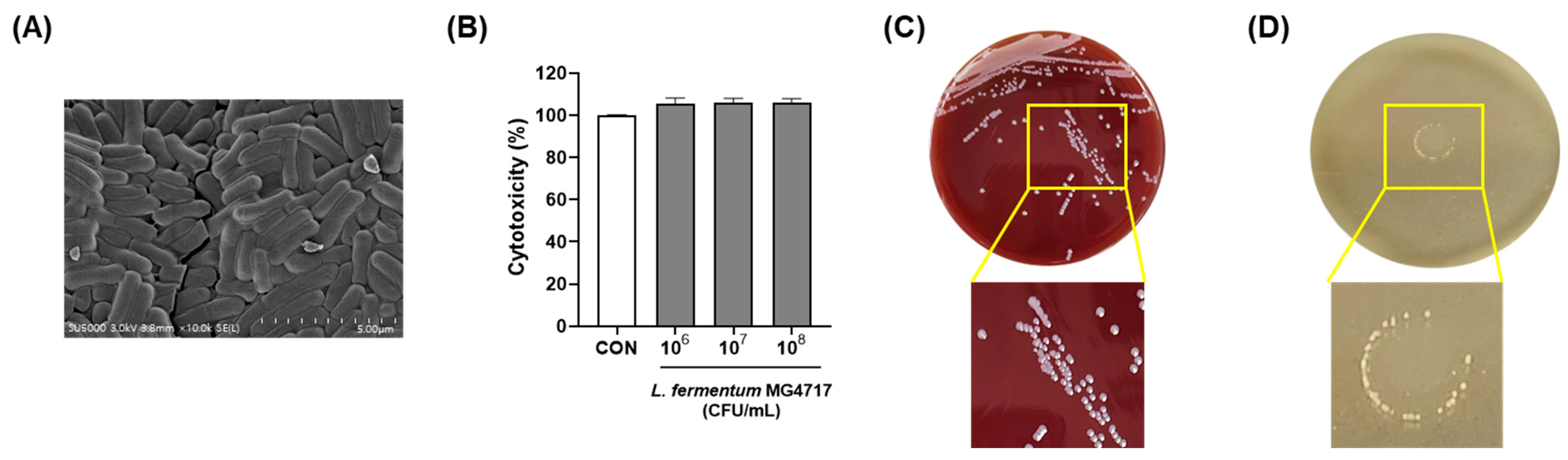
3.5. Morphology and Safety of L. fermentum MG4717
3.6. GIT Stability and Adhesion to HT-29 Cells of the L. fermentum MG4717
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kim, B.-K.; Kim, B.K.; Choi, S.-I. Biosafety characteristics and antibacterial activity of probiotic strains against Streptococcus mutans, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis. Ann. Microbiol. 2025, 75, 2. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Forouzanfar, M.H.; Daoud, F.; Mokdad, A.A.; El Bcheraoui, C.; Moradi-Lakeh, M.; Kyu, H.H.; Barber, R.M.; Wagner, J.; Cercy, K. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016, 387, 2383–2401. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Stingu, C.-S.; Eschrich, K.; Rodloff, A.C.; Schaumann, R.; Jentsch, H. Periodontitis is associated with a loss of colonization by Streptococcus sanguinis. J. Med. Microbiol. 2008, 57, 495–499. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Wolff, A.; Saminsky, M.; Mazor, Y.; Venezia, E.; Bar-Ness Greenstein, R. Effect of Aggregatibacter actinomycetemcomitans from aggressive periodontitis patients on Streptococcus mutans. Oral Dis. 2015, 21, 955–961. [Google Scholar] [CrossRef]
- Shenoy, N.; Shetty, A. Breath Malodor–A Review of the Fundamentals. J. Datta Meghe Inst. Med. Sci. Univ. 2023, 18, 882–888. [Google Scholar] [CrossRef]
- Hara, T.; Sakanaka, A.; Lamont, R.J.; Amano, A.; Kuboniwa, M. Interspecies metabolite transfer fuels the methionine metabolism of Fusobacterium nucleatum to stimulate volatile methyl mercaptan production. Msystems 2024, 9, e00764-23. [Google Scholar] [CrossRef]
- Wu, D.-D.; Ngowi, E.E.; Zhai, Y.-K.; Wang, Y.-Z.; Khan, N.H.; Kombo, A.F.; Khattak, S.; Li, T.; Ji, X.-Y. Role of hydrogen sulfide in oral disease. Oxidative Med. Cell. Longev. 2022, 2022, 1886277. [Google Scholar] [CrossRef]
- Thayumanavan, T.; Harish, B.; Subashkumar, R.; Shanmugapriya, K.; Karthik, V. Streptococcus mutans biofilms in the establishment of dental caries: A review. 3 Biotech 2025, 15, 62. [Google Scholar] [CrossRef]
- Guo, L.; Shokeen, B.; He, X.; Shi, W.; Lux, R. Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum. Mol. Oral Microbiol. 2017, 32, 355–364. [Google Scholar] [CrossRef]
- Morita, M.; Wang, H.L. Association between oral malodor and adult periodontitis: A review. J. Clin. Periodontol. 2001, 28, 813–819. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Fujita, K.; Matsumoto-Nakano, M.; Inagaki, S.; Ooshima, T. Biological functions of glucan-binding protein B of Streptococcus mutans. Oral Microbiol. Immunol. 2007, 22, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Shim, K.M.; Yoo, K.H.; Kim, J.-C.; Kim, S.-H.; Bae, C.-S.; Kim, D.; Park, D.H.; Ryu, J.-W.; Kang, S.S. The effect of cetylpyridinium chloride on halitosis and periodontal disease-related parameters in dogs. Biotechnol. Bioprocess Eng. 2008, 13, 252–255. [Google Scholar] [CrossRef]
- Hotel, A.C.P. Cordoba, Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; In Proceedings of the Report of a Joint FAO/WHO Expert Consultation, Córdoba, Argentina, 1–4 October 2001.
- Meurman, J.H.; Stamatova, I. Probiotics: Contributions to oral health. Oral Dis. 2007, 13, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Stephen, A.S. Use of probiotics and oral health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kim, B.G.; Chung, J.; Lee, H.C.; Oh, J.S. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J. Clin. Periodontol. 2006, 33, 226–232. [Google Scholar] [CrossRef]
- Terai, T.; Okumura, T.; Imai, S.; Nakao, M.; Yamaji, K.; Ito, M.; Nagata, T.; Kaneko, K.; Miyazaki, K.; Okada, A. Screening of probiotic candidates in human oral bacteria for the prevention of dental disease. PLoS ONE 2015, 10, e0128657. [Google Scholar] [CrossRef]
- Arndt, F.; Siems, K.; Walker, S.V.; Bryan, N.C.; Leuko, S.; Moeller, R.; Boschert, A.L. Systematic screening of 42 vancomycin-resistant Enterococcus faecium strains for resistance, biofilm, and desiccation in simulated microgravity. NPJ Microgravity 2024, 10, 103. [Google Scholar] [CrossRef]
- on Additives, E.P.; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. Efsa J. 2018, 16, e05206. [Google Scholar]
- Nguyen, T.H.; Kim, J.-S.; Kwon, H.-J.; Kang, C.-H. The Effect of a glutathione (GSH)-containing cryo-protectant on the viability of probiotic cells using a freeze-drying process. Fermentation 2023, 9, 187. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Limosilactobacillus fermentum MG5091 and Lactococcus lactis MG4668 and MG5474 Suppress Muscle Atrophy by Regulating Apoptosis in C2C12 Cells. Fermentation 2023, 9, 659. [Google Scholar] [CrossRef]
- Shi, T.; Wang, J.; Dong, J.; Hu, P.; Guo, Q. Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and their roles in the progression of respiratory diseases. Pathogens 2023, 12, 1110. [Google Scholar] [CrossRef]
- Abebe, G.M. Oral biofilm and its impact on oral health, psychological and social interaction. Int. J. Oral Dent. Health 2021, 7, 127. [Google Scholar]
- Shahoumi, L.A.; Saleh, M.H.; Meghil, M.M. Virulence factors of the periodontal pathogens: Tools to evade the host immune response and promote carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef]
- Ouhara, K.; Iwasaki, Y.; Kajiya, M.; Savitri, I.; Kitagawa, M.; Tokunaga, N.; Shintani, T.; Ogawa, I.; Hino, T.; Fujita, T. The differential expression of mgl mRNA by Porphyromonas gingivalis affects the production of methyl mercaptan. Oral Dis. 2015, 21, 626–633. [Google Scholar] [CrossRef]
- Stephen, A.S.; Millhouse, E.; Sherry, L.; Aduse-Opoku, J.; Culshaw, S.; Ramage, G.; Bradshaw, D.J.; Burnett, G.R.; Allaker, R.P. In Vitro effect of Porphyromonas gingivalis methionine gamma lyase on biofilm composition and oral inflammatory response. PLoS ONE 2016, 11, e0169157. [Google Scholar] [CrossRef]
- He, X.; Hu, W.; Kaplan, C.W.; Guo, L.; Shi, W.; Lux, R. Adherence to streptococci facilitates Fusobacterium nucleatum integration into an oral microbial community. Microb. Ecol. 2012, 63, 532–542. [Google Scholar] [CrossRef]
- Rosner, O.; Livne, S.; Bsharat, M.; Dviker, S.; Jeffet, U.; Matalon, S.; Sterer, N. Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor. Molecules 2024, 29, 2982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Zhang, S.; Li, Y.; Shi, X.; Liu, D.; Pan, Y. Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of Fusobacterium nucleatum into oral epithelial cells by downregulating FadA and FomA. J. Periodontol. 2022, 93, 515–525. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, L.; Zhang, M.; Zhao, L.; Hao, Y.; Guo, H.; Sang, Y.; Zhang, H.; Ren, F. The adhesion of Lactobacillus salivarius REN to a human intestinal epithelial cell line requires s-layer proteins. Sci. Rep. 2017, 7, 44029. [Google Scholar] [CrossRef]
- Park, D.-Y.; Hwang, J.; Kim, Y.; Lee, D.; Kim, Y.-Y.; Kim, H.-S.; Hwang, I. Antimicrobial activity of Limosilactobacillus fermentum strains isolated from the human oral cavity against Streptococcus mutans. Sci. Rep. 2023, 13, 7969. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Lee, D.-S.; Lee, S.-A.; Kim, M.-S.; Nam, S.-H. Effects of probiotic bacterium Weissella cibaria CMU on periodontal health and microbiota: A randomised, double-blind, placebo-controlled trial. BMC Oral Health 2020, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Park, M.S.; Johnston, T.V.; Ji, G.E.; Hwang, K.T.; Ku, S. Isolation, characterization and biosafety evaluation of Lactobacillus fermentum OK with potential oral probiotic properties. Probiotics Antimicrob. Proteins 2021, 13, 1363–1386. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, J.; Wang, B.; Huo, D.; Wang, Z.; Zhang, J.; Shao, Y. Whole-genome sequencing reveals the mechanisms for evolution of streptomycin resistance in Lactobacillus plantarum. J. Dairy Sci. 2018, 101, 2867–2874. [Google Scholar] [CrossRef]
- Mustapha, M.M.; Srinivasa, V.R.; Griffith, M.P.; Cho, S.-T.; Evans, D.R.; Waggle, K.; Ezeonwuka, C.; Snyder, D.J.; Marsh, J.W.; Harrison, L.H. Genomic diversity of hospital-acquired infections revealed through prospective whole-genome sequencing-based surveillance. Msystems 2022, 7, e01384-21. [Google Scholar] [CrossRef]
- Aryantini, N.P.D.; Yamasaki, E.; Kurazono, H.; Sujaya, I.N.; Urashima, T.; Fukuda, K. In vitro safety assessments and antimicrobial activities of Lactobacillus rhamnosus strains isolated from a fermented mare’s milk. Anim. Sci. J. 2017, 88, 517–525. [Google Scholar] [CrossRef]
- McDermott, P.; Zhao, S.; Wagner, D.; Simjee, S.; Walker, R.; White, D. The food safety perspective of antibiotic resistance. Anim. Biotechnol. 2002, 13, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.L.; Gladney, L.M.; Huang, A.D.; Griswold, T.; Katz, L.S.; Dinsmore, B.A.; Im, M.S.; Kucerova, Z.; Smith, P.A.; Lane, C. Rapid identification of enteric bacteria from whole genome sequences using average nucleotide identity metrics. Front. Microbiol. 2023, 14, 1225207. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Effect of different matrices on probiotic resistance to in vitro simulated gastrointestinal conditions. Int. J. Dairy Technol. 2015, 68, 595–601. [Google Scholar] [CrossRef]
- Saito, V.; Dos Santos, T.; Vinderola, C.; Romano, C.; Nicoli, J.; Araújo, L.; Costa, M.; Andrioli, J.; Uetanabaro, A. Viability and resistance of lactobacilli isolated from cocoa fermentation to simulated gastrointestinal digestive steps in soy yogurt. J. Food Sci. 2014, 79, M208–M213. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.; Jeong, Y.; Kang, H. Anti-inflammatory response in TNFα/IFNγ-induced HaCaT keratinocytes and probiotic properties of Lacticaseibacillus rhamnosus MG4644, Lacticaseibacillus paracasei MG4693, and Lactococcus lactis MG5474. J. Microbiol. Biotechnol. 2023, 33, 1039. [Google Scholar] [CrossRef]



| Bacteria | Strain | NCBI Accession Number | Origin |
|---|---|---|---|
| Limosilactobacillus fermentum | MG4681 | OP035515 | Human (oral) |
| MG4684 | OP035518 | ||
| MG4697 | OP077100 | ||
| MG4700 | OP077103 | ||
| MG4712 | OP077115 | ||
| MG4717 | OP035525 | ||
| MG4737 | OP035543 |
| Strain | No. of Adhered Strains Per One Epithelial Cell | Adhesion Rate (%) |
|---|---|---|
| L. fermentum MG4717 | 10.56 ± 0.56 | 84.73 ± 0.33 |
| Species | Strain | ANI (%) |
|---|---|---|
| Limosilactobacillus fermentum | DSM20052 | 98.73 |
| Limosilactobacillus reuteri subsp. rodentium | 100-23 | 80.57 |
| Limosilactobacillus reuteri subsp. reuteri | JCM1112 | 79.90 |
| Limosilactobacillus reuteri subsp. kinnaridis | AP3 | 77.75 |
| Antibiotics | MIC (µL/mL) | Cut-Off Value (µL/mL) 1 |
|---|---|---|
| Ampicillin | 0.19 | 2 |
| Gentamicin | 0.25 | 16 |
| Kanamycin | 6 | 64 |
| Streptomycin | 6 | 64 |
| Tetracycline | 2 | 8 |
| Chloramphenicol | 3 | 4 |
| Erythromycin | 0.94 | 1 |
| Vancomycin | - | n.r. |
| Clindamycin | 0.32 | 4 |
| Experiments | L. fermentum MG4717 | |
|---|---|---|
| GIT (Log CFU/mL) | Initial counts | 8.21 ± 0.01 |
| Gastric fluid | 7.40 ± 0.04 | |
| Gastrointestinal tract | 7.20 ± 0.04 | |
| Adhesion (Log CFU/mL) | Initial | 8.74 ± 0.03 |
| Adherent | 6.55 ± 0.05 | |
| Adhesion rate (%) | 89.0 ± 1.77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kim, B.-K.; Choi, S.-I. The Effect of Limosilactobacillus fermentum MG4717 on Oral Health and Biosafety. Microorganisms 2025, 13, 1600. https://doi.org/10.3390/microorganisms13071600
Park J-Y, Lee JY, Kim Y, Kim B-K, Choi S-I. The Effect of Limosilactobacillus fermentum MG4717 on Oral Health and Biosafety. Microorganisms. 2025; 13(7):1600. https://doi.org/10.3390/microorganisms13071600
Chicago/Turabian StylePark, Jeong-Yong, Ji Yeon Lee, YongGyeong Kim, Byoung-Kook Kim, and Soo-Im Choi. 2025. "The Effect of Limosilactobacillus fermentum MG4717 on Oral Health and Biosafety" Microorganisms 13, no. 7: 1600. https://doi.org/10.3390/microorganisms13071600
APA StylePark, J.-Y., Lee, J. Y., Kim, Y., Kim, B.-K., & Choi, S.-I. (2025). The Effect of Limosilactobacillus fermentum MG4717 on Oral Health and Biosafety. Microorganisms, 13(7), 1600. https://doi.org/10.3390/microorganisms13071600





