Morphological and Molecular Characterization of Lasiodiplodia theobromae Causing Stem Gummosis Disease in Rubber Trees and Its Chemical Control Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pathogen Isolation
2.2. Koch’s Postulate Test
2.3. Morphological Identification
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Sequence Alignment and Phylogenetic Analysis
2.6. Field Experiment
3. Results
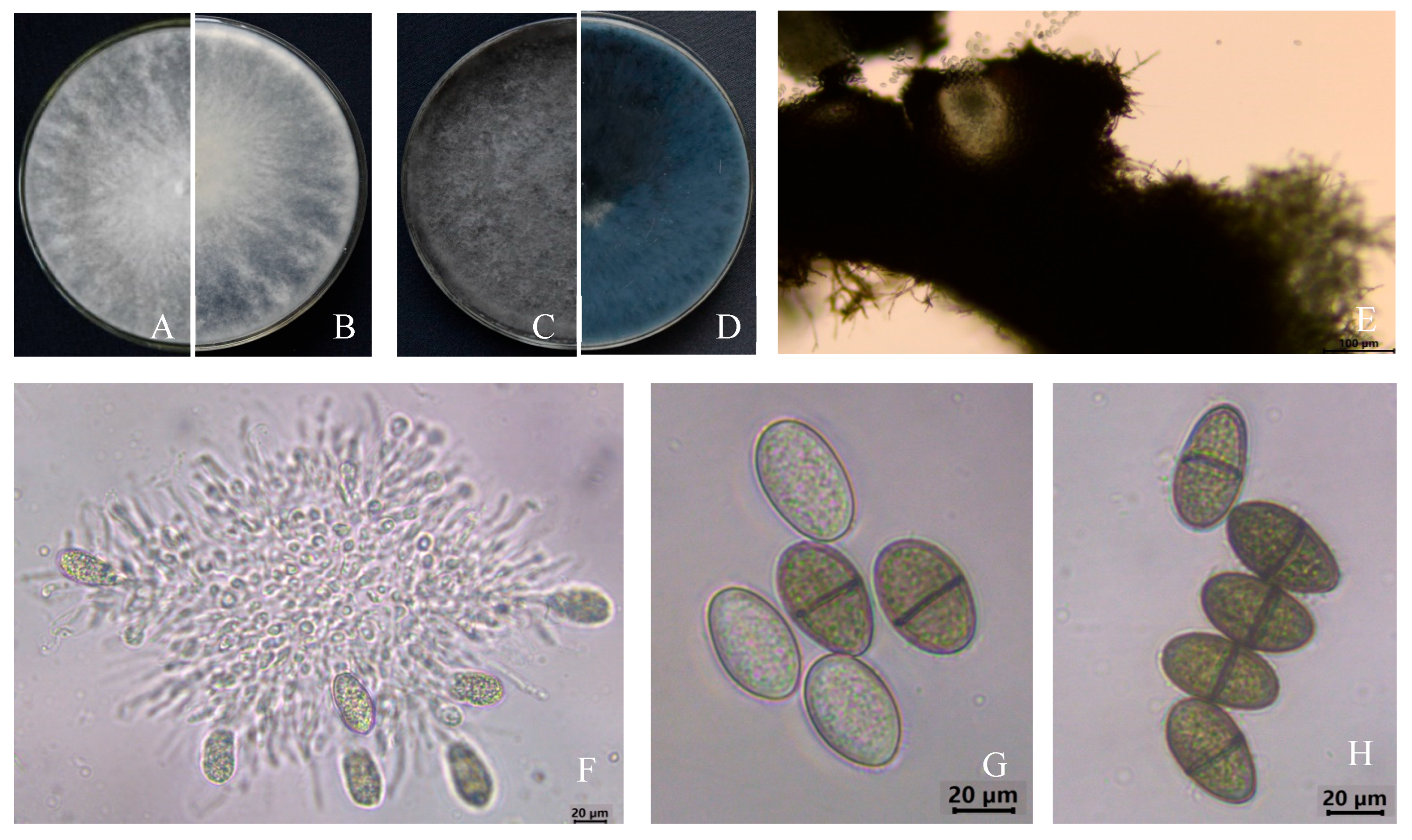
3.1. Disease Symptoms and Pathogen Morphological Identification
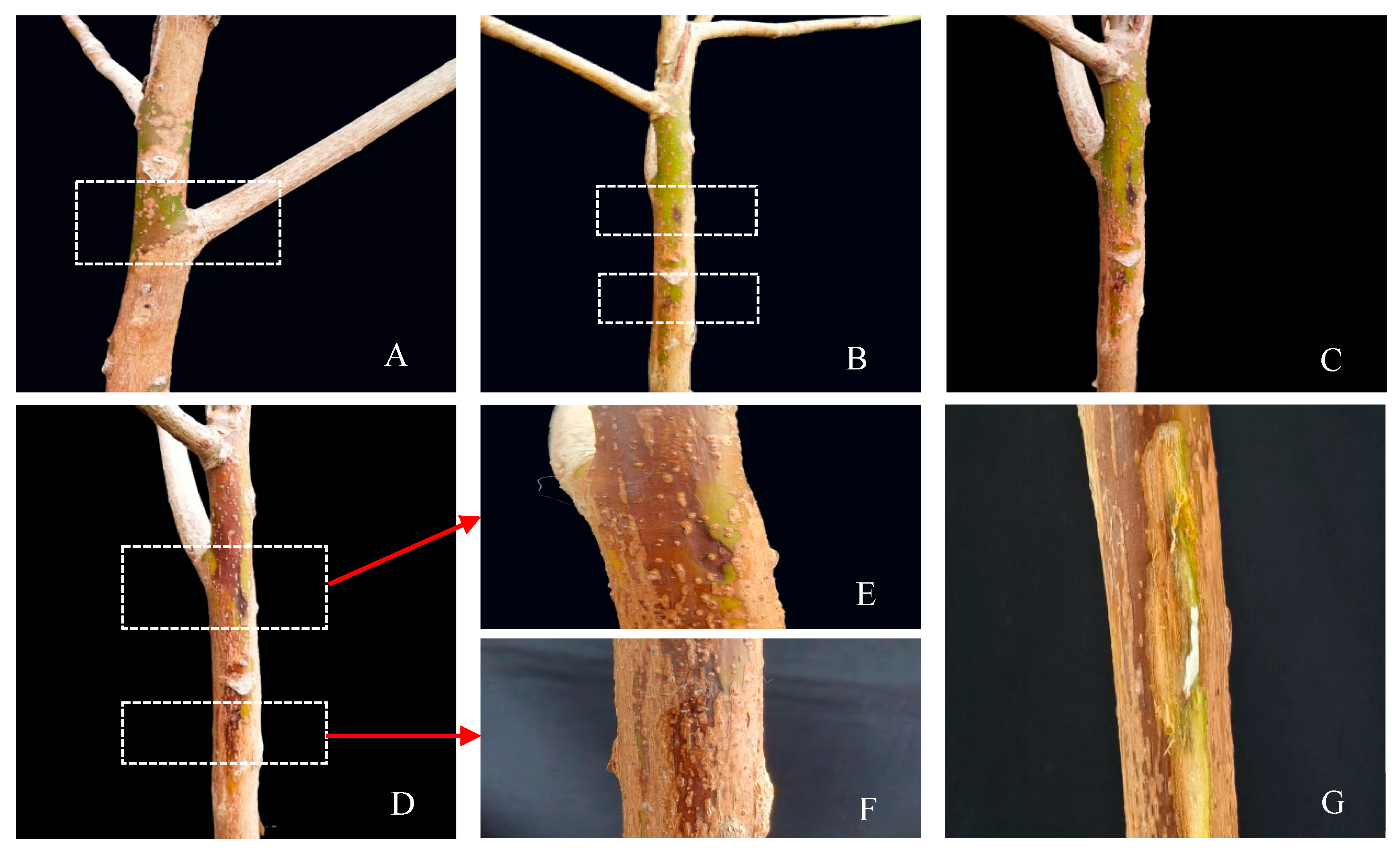
3.2. Pathogenicity Test
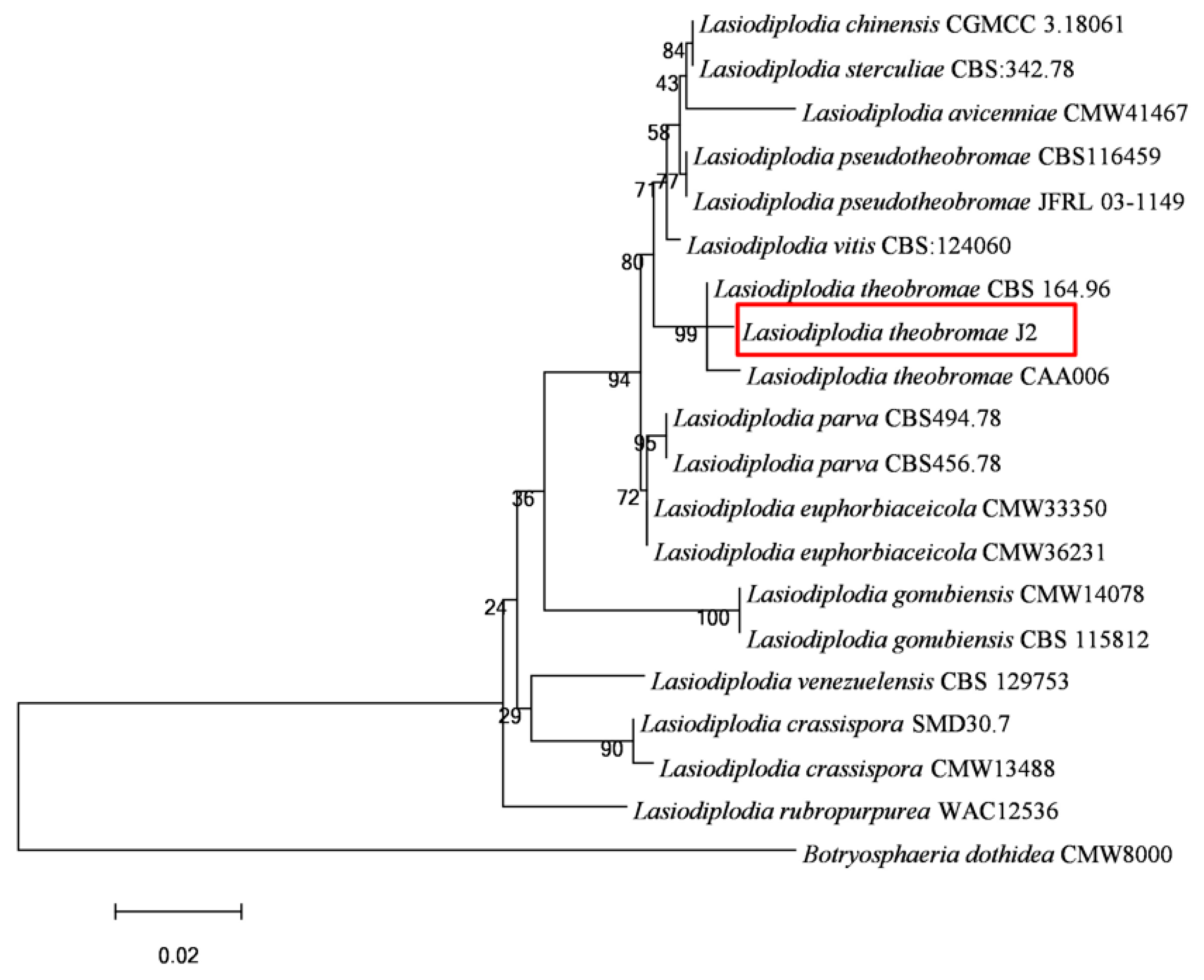
3.3. Sequence, Identification of Pathogen Species, and Phylogenetic Analysis
3.4. Preliminary Fungicide Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Carr, M.K.V. The water relations of rubber (Hevea brasiliensis): A review. Exp. Agric. 2012, 48, 176–193. [Google Scholar] [CrossRef]
- Liu, C.A.; Liang, M.Y.; Tang, J.W.; Jin, Y.Q.; Guo, Z.B.; Siddique, K.H.M. Challenges of the establishment of rubber-based agroforestry systems: Decreases in the diversity and abundance of ground arthropods. J. Environ. Manag. 2021, 292, 112747. [Google Scholar] [CrossRef] [PubMed]
- Limkaisang, S.; Kom-un, S.; Furtado, E.L.; Liew, K.W.; Salleh, B.; Sato, Y. Molecular phylogenetic and morphological analyses of Oidium heveae, a powdery mildew of rubber tree. Mycoscience 2005, 46, 220–226. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhou, G.S.; Fang, S.B. A preliminary study of the northern planting boundary of rubber tree cultivation in China. Acta Ecol. Sin. 2016, 36, 1272–1280. [Google Scholar] [CrossRef]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Chomnunti, P.; Sunpapao, A. Morphological and Molecular Characterization of Calonectria foliicola Associated with Leaf Blight on Rubber Tree (Hevea brasiliensis) in Thailand. J. Fungi 2022, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Huang, Z.D. Cultivation of Rubber Trees in the Northern Tropics; Guangdong Science & Technology Press: Guangzhou, China, 1987; pp. 1–5. [Google Scholar]
- Mahyudin, M.M.; Noran, A.S.; Ismail, M.Z.H.; Ahmad, K. Chapter 18—Diseases of rubber trees: Malaysia as a case study. In Forest Microbiology; Academic Press: Cambridge, MA, USA, 2023; pp. 401–414. [Google Scholar]
- Cesar, M.B.; Borges, F.A.; Bilck, A.P.; Yamashita, F.; Paulino, C.G.; Herculano, R.D. Development and Characterization of Natural Rubber Latex and Polylactic Acid Membranes for Biomedical Application. J. Polym. Environ. 2019, 28, 220–230. [Google Scholar] [CrossRef]
- Baraka, M.A.; Fatma, R.M.; Shaban, W.I.; Arafat, K.H. Efficiency of some plant extracts, natural oils, biofungicides and fungicides against root rot disease of date palm. J. Biol. Chem. Environ. Sci. 2011, 6, 405–429. Available online: https://www.researchgate.net/publication/215643383 (accessed on 12 May 2025).
- Liu, X.B.; Li, B.X.; Cai, J.M.; Zheng, X.L.; Feng, Y.L.; Huang, G.X. Colletotrichum species causing anthracnose of rubber trees in China. Sci. Rep. 2018, 8, 10435. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Zhou, X.M.; Liu, X.B.; Cai, J.M.; Li, B.X. First Report of Rubber Tree Gummosis Disease Caused by Fusarium solani in China. Plant Dis. 2016, 100, 1788. [Google Scholar] [CrossRef]
- Punithalingam, E. Plant Diseases Attributed to Botryodiplodia theobromae Pat; J. Cramer, Vaduz: Liechtenstein, Germany, 1980; Volume 71, pp. 1–123. [Google Scholar]
- Khoo, Y.W.; Hui, T.T.; Khaw, Y.S.; Li, S.; Chong, K.P. First Report of Lasiodiplodia theobromae Causing leaf spot on Aloe vera L. var. chinensis (Haw.) Berg. in Malaysia. Plant Dis. 2022, 106, 2258. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Li, G.H. Characterization of phytotoxin and secreted proteins identifies of Lasiodiplodia theobromae, causes of peach gummosis. Fungal Biol. 2019, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Endes, A.; Kayım, M.; Eskalen, A. First report of Lasiodiplodia theobromae, L. pseudotheobromae, and Diplodia seriata causing bot canker and gummosis of nectarines in Turkey. Plant Dis. 2016, 100, 2321. [Google Scholar] [CrossRef]
- Shah, M.D.; Verma, K.S.; Singh, K.; Kaur, R. Morphological, pathological and molecular variability in Botryodiplodia theobromae (Botryosphaeriaceae) isolates associated with die-back and bark canker of pear trees in Punjab, India. Genet. Mol. Res. 2010, 9, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Wang, M.; Xu, L.X.; Yang, Y. First report of Lasiodiplodia theobromae causing dieback in custard apple (Annona squamosa) tree in China. Plant Dis. 2021, 106, 327. [Google Scholar] [CrossRef]
- Pedraza, J.M.T.; Aguilera, J.A.M.; Díaz, C.N.; Ortiz, D.T.; Monter, Á.V.; Mir, S.G.L. Control of Lasiodiplodia theobromae, the causal agent of dieback of sapote mamey [Pouteria sapota (Jacq.) H. E. Moore and Stearn] Grafts in Mexio. Rev. Fitotec. Mex. Publ. Soc. Mex. Fitogene’tica 2013, 36, 233–238. [Google Scholar] [CrossRef]
- Chen, S.F.; Li, G.Q.; Liu, Q.L.; Li, J.Q.; Liu, F.F. Characteristics of Lasiodiplodia theobromae from Rosa rugosa in South China. Crop Prot. 2016, 79, 51–55. [Google Scholar] [CrossRef]
- Ma, Y.L.; Ahmad, T.; Zheng, Y.Q.; Chengrong, N.; Liu, Y. First Report of Postharvest Stem End Rot of mango fruit (Mangifera indica) caused by Lasiodiplodia theobromae in China. Plant Dis. 2021, 105, 2715. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Shi, Y.P.; Liu, Y.X.; Xiong, B.; Dai, L.M.; Li, L.L.; Li, F.C.; Li, G.H. The pathogen causing Lasiodiplodia leaf spot on rubber tree. J. Yunnan Agric. Univ. 2018, 33, 224–232. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=675375741 (accessed on 14 May 2025).
- Zhao, G.H.; Song, Z.; He, W.L. Stain and mould fungi on rubber wood. Chin. J. Trop. Crops. 1991, 12, 63–67. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=580291&from=Qikan_Search_Index (accessed on 6 May 2025).
- Jiang, G.Z.; Tao, J.X.; Liu, Y.X.; Shi, Y.P.; Cai, Z.Y.; Li, G.H. Identification of the pathogen species causing quick decline diseases of rubber tree. Plant Prot. 2018, 44, 55–60. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=675283854 (accessed on 12 May 2025).
- Hu, W.J.; Li, Z.P.; Wu, R.H.; Ding, J.Y.; Zhang, Y.; Lan, Z.N. Identification and biological characteristics of pathogen causing diebark on rubber trees. Chin. J. Trop. Crops. 2018, 39, 1146–1152. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=675547313 (accessed on 10 May 2025).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 155–160. Available online: http://www.fungaldiversity.org/fdp/sfdp/28-1.pdf (accessed on 12 May 2025).
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Cogley, V.; Atencio, R.; Jaén, M. First report of Lasiodiplodia theobromae causing branch canker and dieback of cashew in Panama. New Dis. Rep. 2022, 45, e12063. [Google Scholar] [CrossRef]
- Sumalatha, N.; Suresh, V.; Kiran, G. A Review on Mango Gummosis Incited by Lasiodiplodia theobromae. Int. J. Environ. Clim. Change 2023, 13, 2317–2330. [Google Scholar] [CrossRef]
- Trai, V.T.N.; Hà, T.T.T.; Hung, N.B. First Report of Lasiodiplodia theobromae Causing Gummosis on Citrus grandis (L.) Osbeck in Vietnam. Res. Plant Dis. 2024, 30, 78–81. [Google Scholar] [CrossRef]
- Asman, A.; Rosmana, A.; Bailey, B.A.; Ali, S.S.; Iwanami, T.; Sjam, S.; Amin, N.; Kuswinanti, T. Pathogenicity of Lasiodiplodia theobromae isolated from cocoa dieback disease in South Sulawesi, Indonesia. J. Phytopathol. 2024, 172, e13352. [Google Scholar] [CrossRef]
- Ghimire, B.; Avin, F.A.; Waliullah, S.; Ali, E.; Baysal-Gurel, F. Real-Time and Rapid Detection of Phytopythium vexans Using Loop-Mediated Isothermal Amplification Assay. Plant Dis. 2023, 107, 3394–3402. [Google Scholar] [CrossRef] [PubMed]
- Bolboli, Z.; Tavakolian, B.; Mostowfizadeh-Ghalamfarsa, R.; Jafari, M.; Cacciola, S.O. Stilbocrea banihashemiana sp. nov. a New Fungal Pathogen Causing Stem Cankers and Twig Dieback of Fruit Trees. J. Fungi 2022, 8, 694. [Google Scholar] [CrossRef]
- Wang, Q.P.; Li, H.T.; Lei, Y.; Su, Y.S.; Long, Y.H. Chitosan as an Adjuvant to Improve Isopyrazam Azoxystrobin against Leaf Spot Disease of Kiwifruit and Enhance Its Photosynthesis, Quality, and Amino Acids. Agriculture 2022, 12, 373. [Google Scholar] [CrossRef]
- Reis, P.; Mondello, V.; Diniz, I.; Alves, A.; Rego, C.; Fontaine, F. Effect of the Combined Treatments with LC2017 and Trichoderma atroviride Strain I-1237 on Disease Development and Defense Responses in Vines Infected by Lasiodiplodia theobromae. Agronomy 2022, 12, 996. [Google Scholar] [CrossRef]
- Goura, K.; Lahlali, R.; Bouchane, O.; Baala, M.; Radouane, N.; Kenfaoui, J.; Ezrari, S.; Hamss, H.E.; Alami, N.E.; Amiri, S.; et al. Identification and Characterization of Fungal Pathogens Causing Trunk and Branch Cankers of Almond Trees in Morocco. Agronomy 2022, 13, 130. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Liang, S.; Luo, D.L.; Ba, L.J. The Inhibitory Mechanism of Eugenol on Lasiodiplodia theobromae and Its Induced Disease Resistance of Passion Fruit. Agronomy 2023, 13, 1408. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wei, J.H.; Chen, X.Y.; Meng, H.; Wang, W.X. Identification of pathogen of Aquilaria sinensis dieback disease. Chin. J. Chin. Mater. Med. 2013, 38, 1707–1711. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=46120806 (accessed on 8 May 2025).
- Xue, Z.N.; Huang, S.L.; Li, X.Z. A study on the pathogenic identification and biological characters of cinnamon dieback. J. Guangxi Agric. Biol. Sci. 2003, 22, 275–279. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=8851159 (accessed on 8 May 2025).
- Netto, M.S.B.; Ferreira, J.H.S.; Souza, G.J.T.; Assunção, I.P.; Assis Câmara Rabelo, F.; Alcântara Neto, F.; Farias, A.R.G.; Melo, M.P. Lasiodiplodia hormozganensis causing leaf blight on Aloe vera in Brazil. Crop Prot. 2024, 179, 106606. [Google Scholar] [CrossRef]
- Shi, G.Y.; Hu, C.J.; Luo, D.A.; Wu, Z.L.; Lu, Y.X.; Fu, G.; Huang, S.L. Identification and biological characteristics of the pathogen causing brown rachis rot of Vitis heyneana. Acta Phytopathol. Sin. 2010, 40, 242–249. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=34395220 (accessed on 14 May 2025).
- Úrbez-Torres, J.R.; Leavitt, G.M.L.; Guerrero, J.; Guevara, J.J.L.; Gubler, W.D. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Maldonado, E.; Alves, A. Identification and pathogenicity of Lasiodiplodia theobromae causing dieback of table grapes in Peru. Eur. J. Plant Pathol. 2014, 141, 477–489. [Google Scholar] [CrossRef]
- Xie, H.H.; Wei, J.G.; Huang, S.P.; Chen, X.H.; Mo, C.Y.; Luo, J.T.; Yang, X.H.; Tan, X.F. Occurrence regularity of Mulberry root rot disease caused by a fungal pathogen and the effects of illumination on pathogen growth. Sci. Seric. 2016, 42, 45–52. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=667902349 (accessed on 12 May 2025).
- Souza, J.F.F.; Silva França, K.R.; Medeiros Ferro, M.M.; Assunção, I.P.; Andrade Lima, G.S.; Farias, A.R.G.; Alcântara Neto, F.; Melo, M.P. Lasiodiplodia theobromae and Lasiodiplodia brasiliense causing dieback and rot fruit of jackfruit tree in Brazil. Crop Prot. 2024, 184, 106763. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Thaochan, N.; Chairin, T.; Sunpapao, A. Morphological and phylogenetic evidences reveal Lasiodiplodia chonburiensis and L. theobromae associated with leaf blight in Hevea brasiliensis in southern Thailand. Diversity. 2023, 15, 961. [Google Scholar] [CrossRef]
- Febbiyanti, T.R.; Wiyono, S.; Yahya, S.; Widodo, W. Lasiodiplodia theobromae fungus causing stem canker disease on rubber tree (Hevea brasiliensis) in Indonesia. J. Agron. 2018, 18, 41–48. [Google Scholar] [CrossRef]
- Encinas, O.; Daniel, G. Degradation of the Gelatinous Layer in Aspen and Rubberwood by the Blue Stain Fungus Lasiodiplodia Theobromae. IAWA J. 1997, 18, 107–115. [Google Scholar] [CrossRef]
- Inyang, P.; Ndifon, A. In vitro efficacy of disparate fungicides against Lasiodiplodia theobromae root rots of orange-fleshed sweet-potato varieties. Indones. J. Agric. Sci. 2022, 23, 56. [Google Scholar] [CrossRef]
- Bhure, S.S.; Patil, P.D.; Pawar, H.D.; Kadam, J.J.; Joshi, M.S.; Mahadik, S.G.; Thorat, S.B. Evaluation of Fungicides, Phytoextracts and Germplasm Screening Against Lasiodiplodia Theobromae Causing Fruit Rot of Jackfruit. J. Plant Dis. Sci. 2023, 18, 26–30. [Google Scholar] [CrossRef]
- Reddy, N.C.; Naika, R.; Shashidhar, K.R.; Mahesh, M.; Gowda, M.; Bai, H.R. Efficacy of selected fungicides against Lasiodiplodia theobromae causing root rot in mulberry. Int. J. Adv. Biochem. Res. 2024, 8, 845–850. [Google Scholar] [CrossRef]
- Bhadra, M.; Khair, A.; Hossain, A.; Sikder, M.M. Efficacy of Trichoderma spp. and fungicides against Lasiodiplodia theobromae. Bangladesh J. Sci. Ind. Res. 2015, 49, 125–130. [Google Scholar] [CrossRef]
- Saeed, E.E.; Sham, A.; El-Tarabily, K.; Abu Elsamen, F.; Iratni, R.; AbuQamar, S. Chemical control of black scorch disease on date palm caused by the fungal pathogen, Thielaviopsis punctulata in United Arab Emirates. Plant Dis. 2016, 100, 2370–2376. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]


| Species | Isolate | Location | GenBank Accession Number | ||
|---|---|---|---|---|---|
| ITS | TEF1-α | TUB2 | |||
| Botryosphaeria dothidea | CMW 8000 | South Africa | AY236949 | AY236898 | AY236927 |
| L. avicenniae | CMW 41467 | South Africa | KP860835 | KP860680 | KP860758 |
| L. chinensis | CGMCC 3.18061 | China | KX499889 | KX499927 | KX500002 |
| L. crassispora | SMD 30.7 | South Africa | OL441871 | OL441927 | OL441983 |
| L. crassispora | CMW 13488 | Australia | DQ103552 | DQ103559 | KU887507 |
| L. euphorbiaceicola | CMW 33350 | South Africa | KU887149 | KU887026 | KU887455 |
| L. euphorbiaceicola | CMW 36231 | South Africa | KU887187 | KU887063 | KU887494 |
| L. gonubiensis | CMW 14078 | South Africa | AY639594 | DQ103567 | EU673126 |
| L. gonubiensis | CBS 115812 | South Africa | KF766191 | DQ458877 | KU887512 |
| L. parva | CBS 494.78 | Portugal | EF622084 | EF622064 | EU673114 |
| L. parva | CBS456.78 | Portugal | EF622083 | EF622063 | KU887523 |
| L. pseudotheobromae | CBS 116459 | Portugal | EF622077 | EF622057 | EU673111 |
| L. pseudotheobromae | JFRL 03-1149 | China | OQ804427 | OQ818099 | OQ818102 |
| L. rubropurpurea | WAC 12536 | Australia | DQ103554 | DQ103572 | KP872425 |
| L. sterculiae | CBS 342.78 | Netherlands | KX464140 | KX464634 | KX464908 |
| L. theobromae | CBS 164.96 | Portugal | AY640255 | AY640258 | EU673110 |
| L. theobromae | CAA 006 | Portugal | DQ458891 | DQ458876 | DQ458859 |
| L. venezuelensis | CBS 129753 | USA | JX545100 | JX545120 | JX545140 |
| L. vitis | CBS 124060 | Netherlands | KX464148 | KX464642 | KX464917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Lin, J.; Wu, H.; Zheng, J.; Zhang, Y.; Zhang, Y.; Li, Z.; Liang, Y.; Lu, Y.; Yi, K.; et al. Morphological and Molecular Characterization of Lasiodiplodia theobromae Causing Stem Gummosis Disease in Rubber Trees and Its Chemical Control Strategies. Microorganisms 2025, 13, 1586. https://doi.org/10.3390/microorganisms13071586
He C, Lin J, Wu H, Zheng J, Zhang Y, Zhang Y, Li Z, Liang Y, Lu Y, Yi K, et al. Morphological and Molecular Characterization of Lasiodiplodia theobromae Causing Stem Gummosis Disease in Rubber Trees and Its Chemical Control Strategies. Microorganisms. 2025; 13(7):1586. https://doi.org/10.3390/microorganisms13071586
Chicago/Turabian StyleHe, Chunping, Jinjing Lin, He Wu, Jinlong Zheng, Yong Zhang, Yu Zhang, Zengping Li, Yanqiong Liang, Ying Lu, Kexian Yi, and et al. 2025. "Morphological and Molecular Characterization of Lasiodiplodia theobromae Causing Stem Gummosis Disease in Rubber Trees and Its Chemical Control Strategies" Microorganisms 13, no. 7: 1586. https://doi.org/10.3390/microorganisms13071586
APA StyleHe, C., Lin, J., Wu, H., Zheng, J., Zhang, Y., Zhang, Y., Li, Z., Liang, Y., Lu, Y., Yi, K., & Wu, W. (2025). Morphological and Molecular Characterization of Lasiodiplodia theobromae Causing Stem Gummosis Disease in Rubber Trees and Its Chemical Control Strategies. Microorganisms, 13(7), 1586. https://doi.org/10.3390/microorganisms13071586






