Characterization, Genomic Analysis and Application of Five Lytic Phages Against Carbapenem-Resistant Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Sample Sources and Processing
2.3. Phage Isolation and Purification
2.4. Phage Lysis Rate
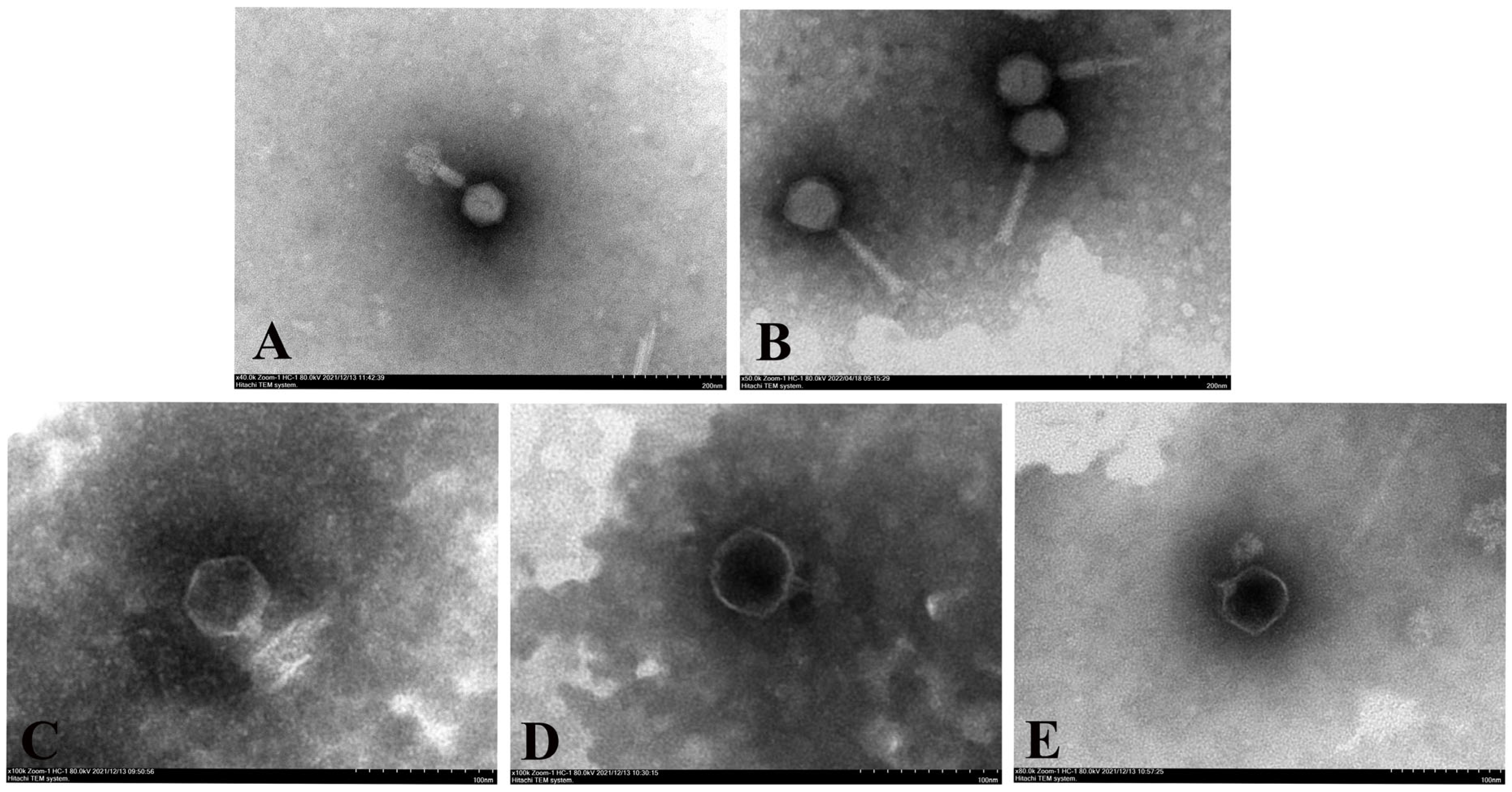
2.5. Morphological Characterization
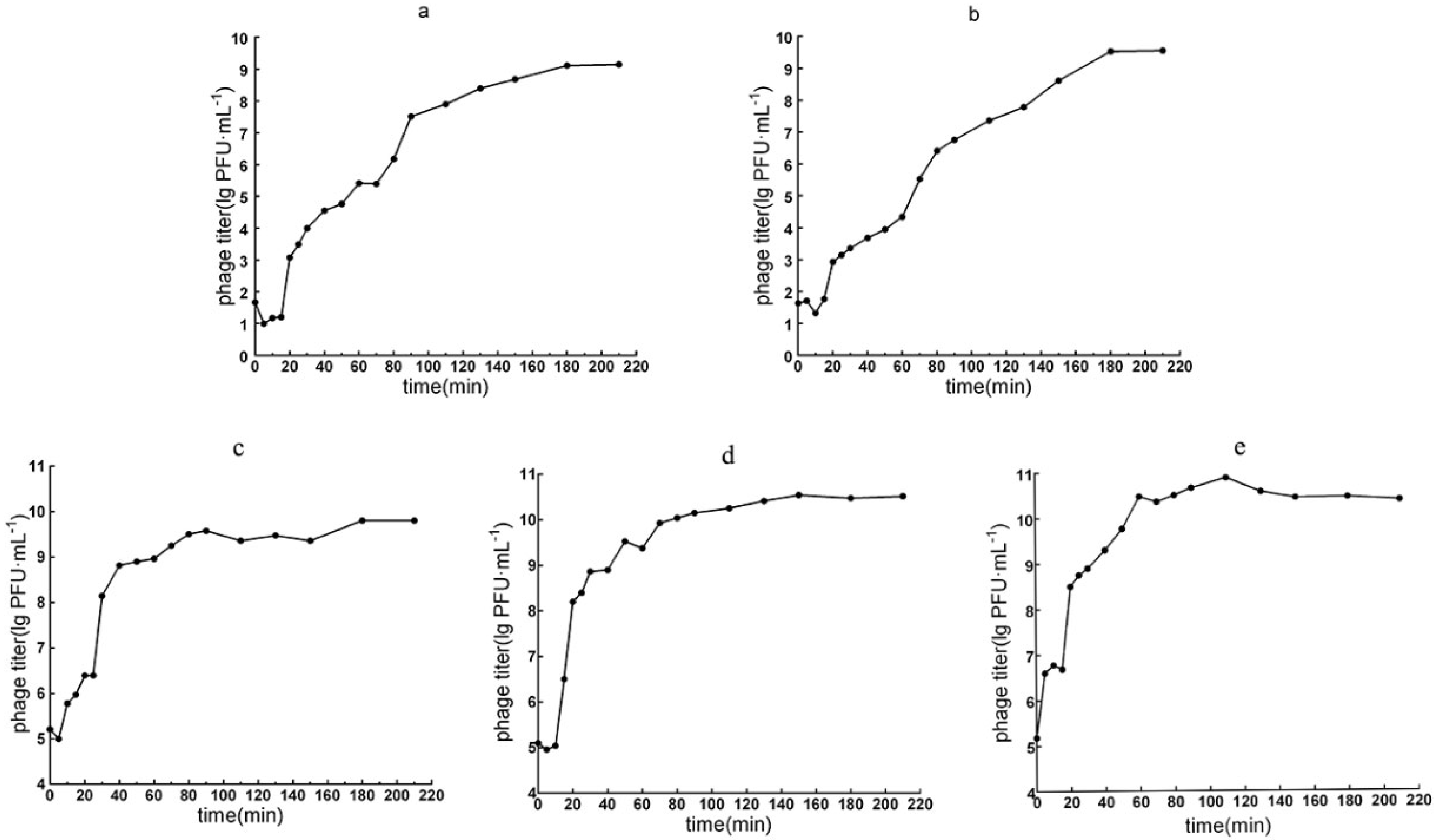
2.6. Optimal Multiplicity of Infection (MOI) and One-Step Growth Curve
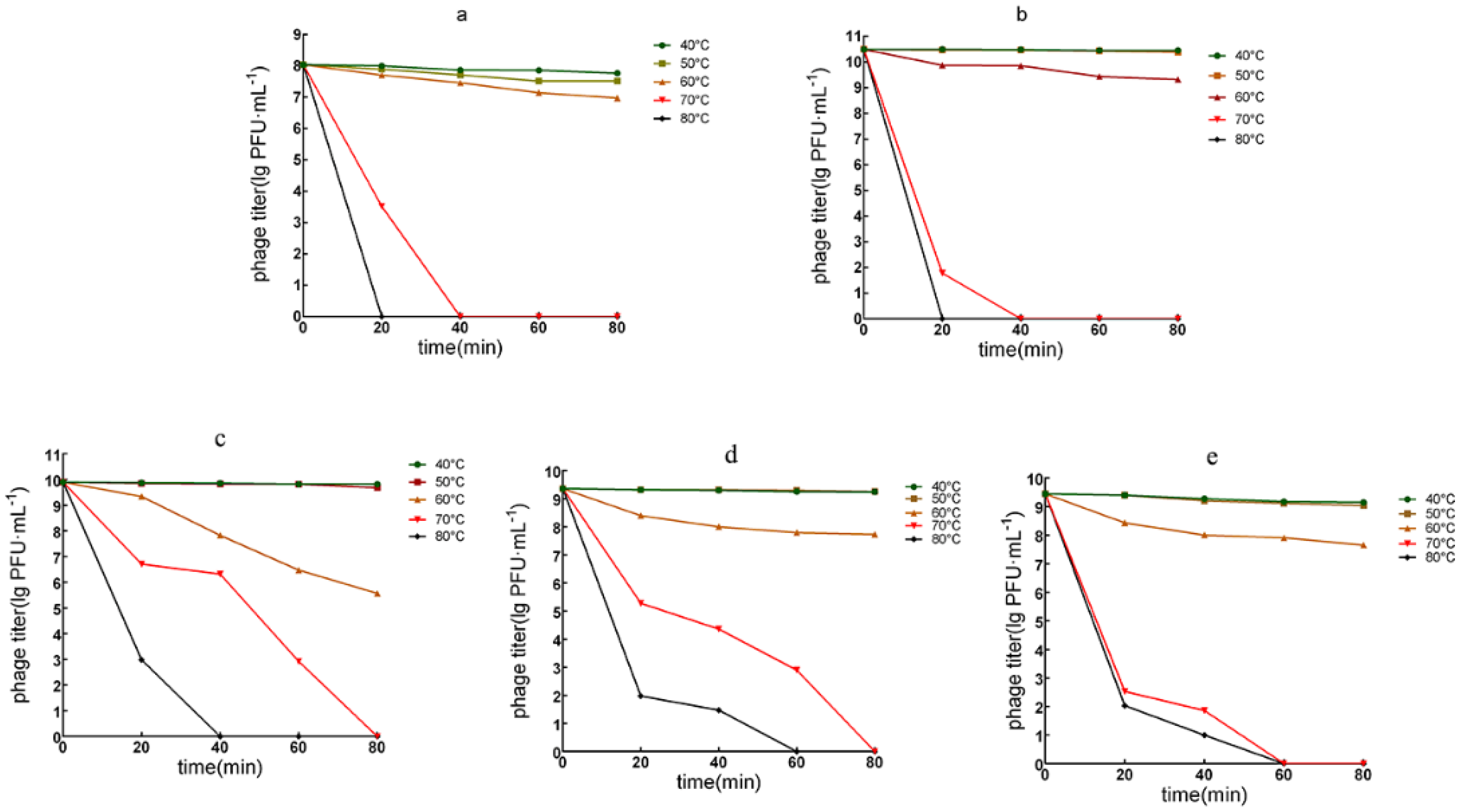
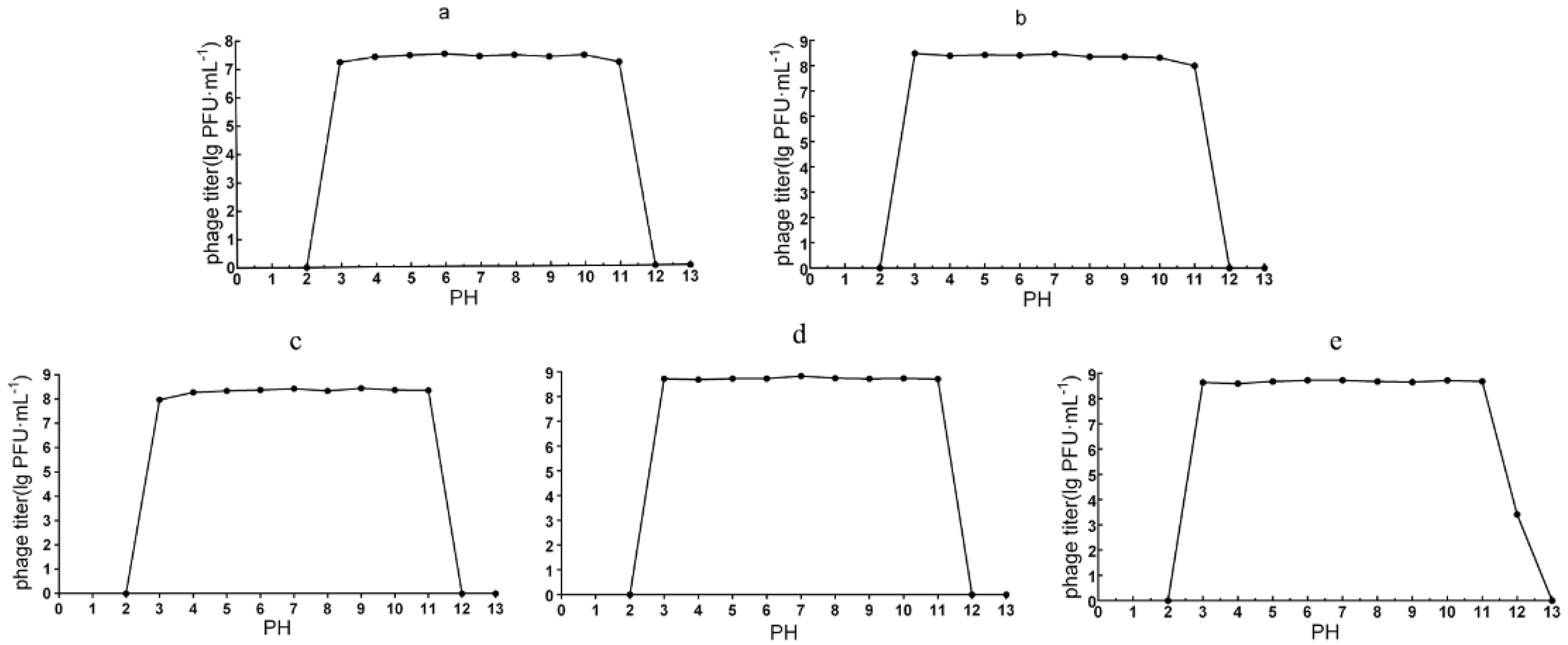
2.7. Thermal Stability and pH Tolerance
2.8. Sequencing and Genome Analysis
2.9. Cocktail Spray Disinfection Effect
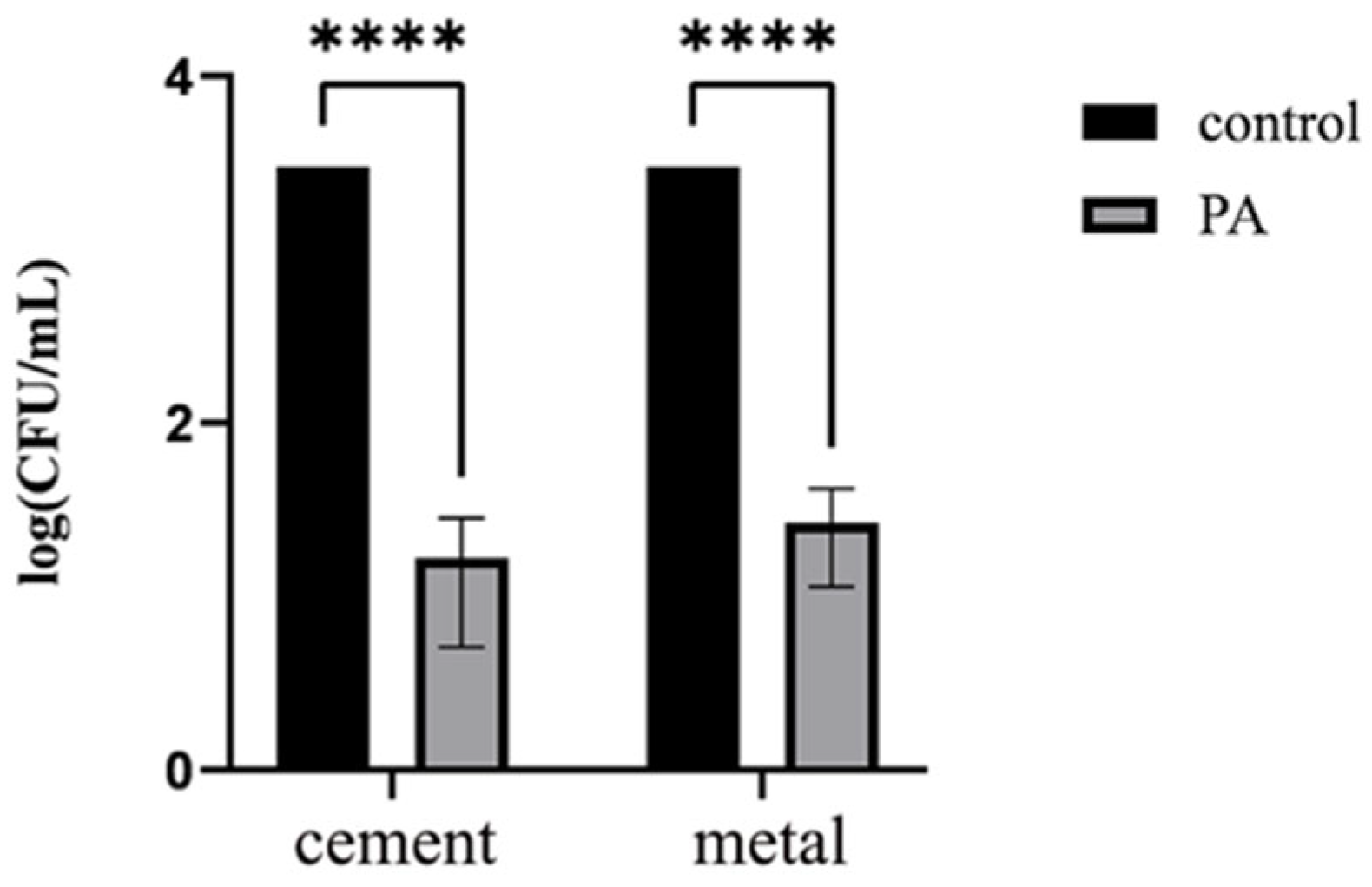
2.10. Disinfection Effect on Cement and Metal Surfaces
2.11. Statistical Analysis
2.12. Nucleotide Sequence Accession Numbers
3. Results
3.1. Phage Isolation and Identification
3.2. Phage Electron Microscopy
3.3. Phage Biological Characteristics
3.3.1. Optimal MOI and the One-Step Growth Curve
3.3.2. Phage Temperature Tolerance
3.3.3. pH Stability
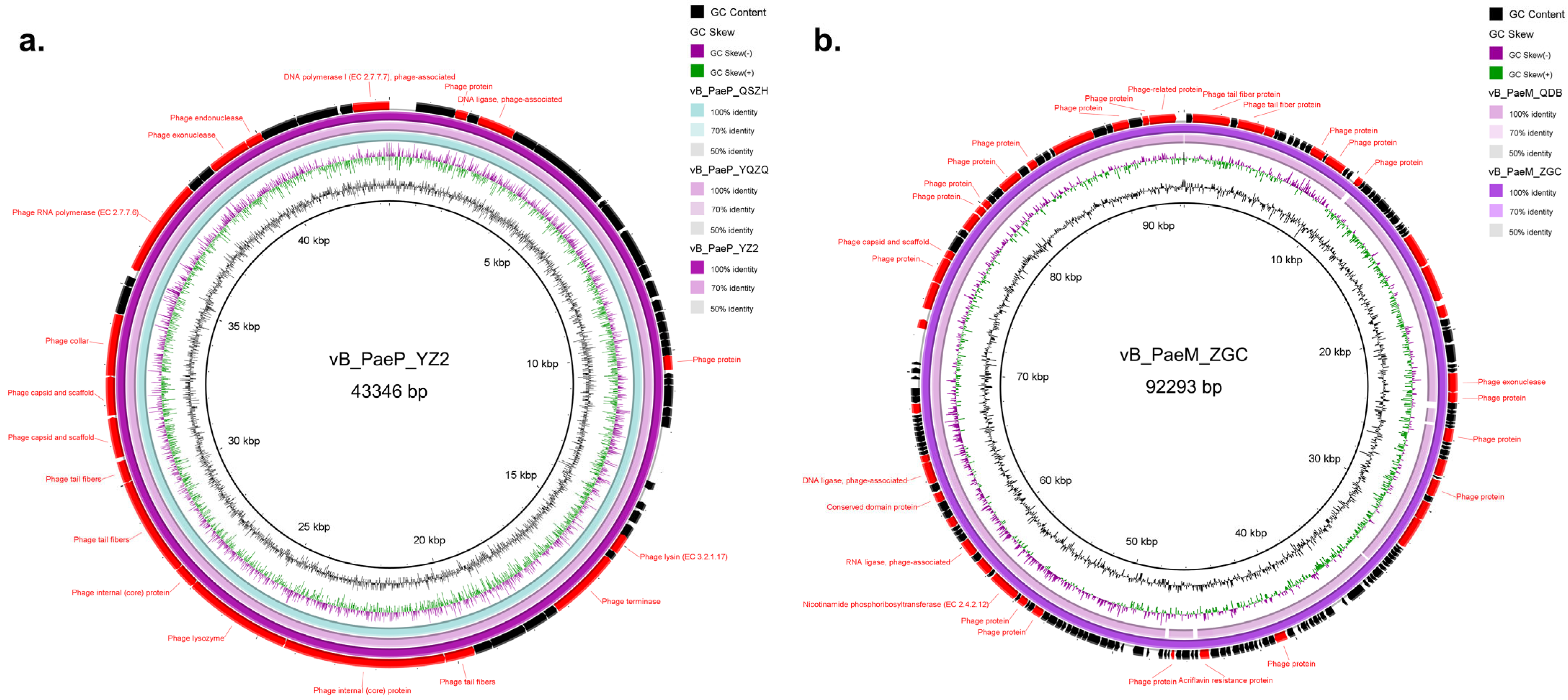
3.4. Genomic Characterization
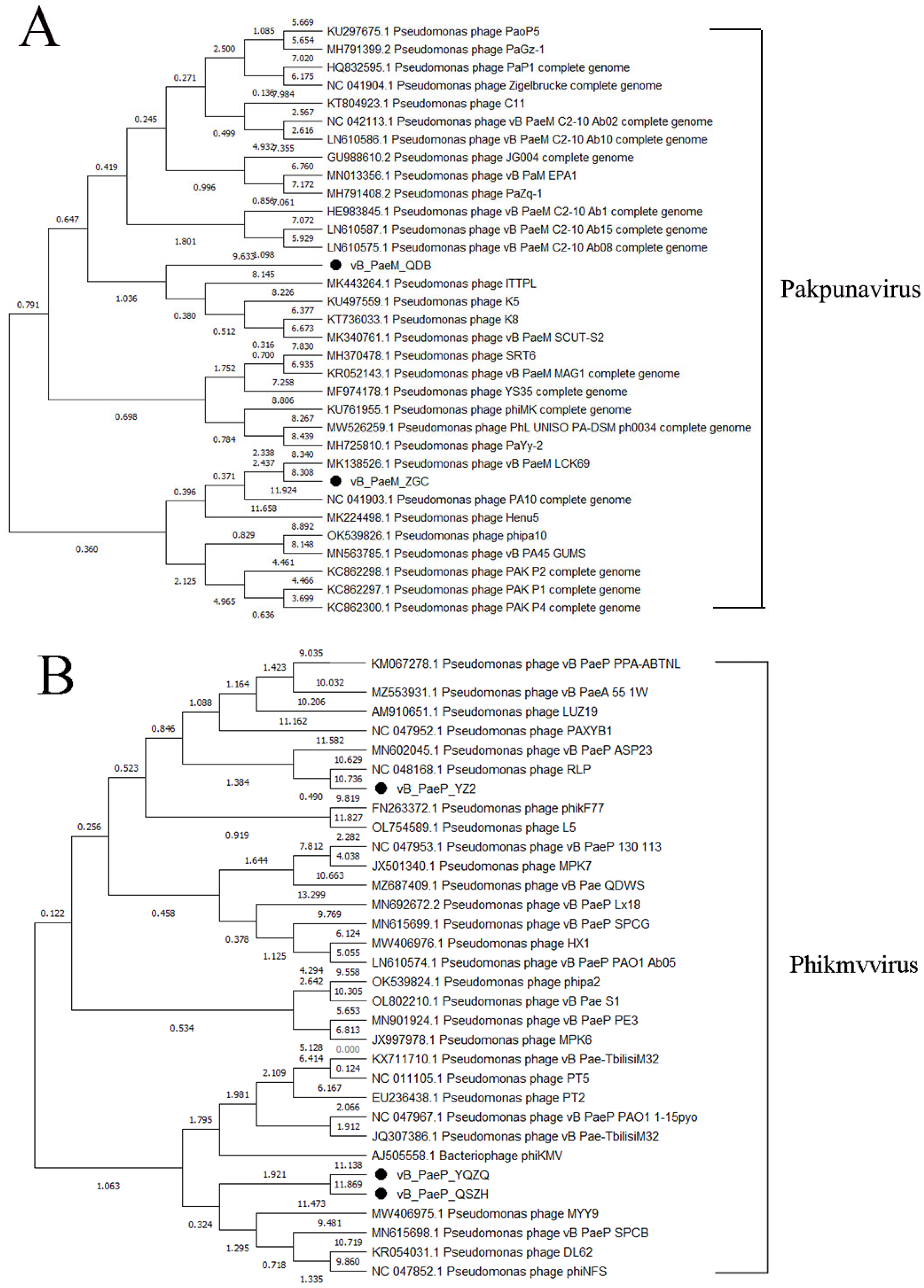
3.5. Phylogenetic Tree Analysis
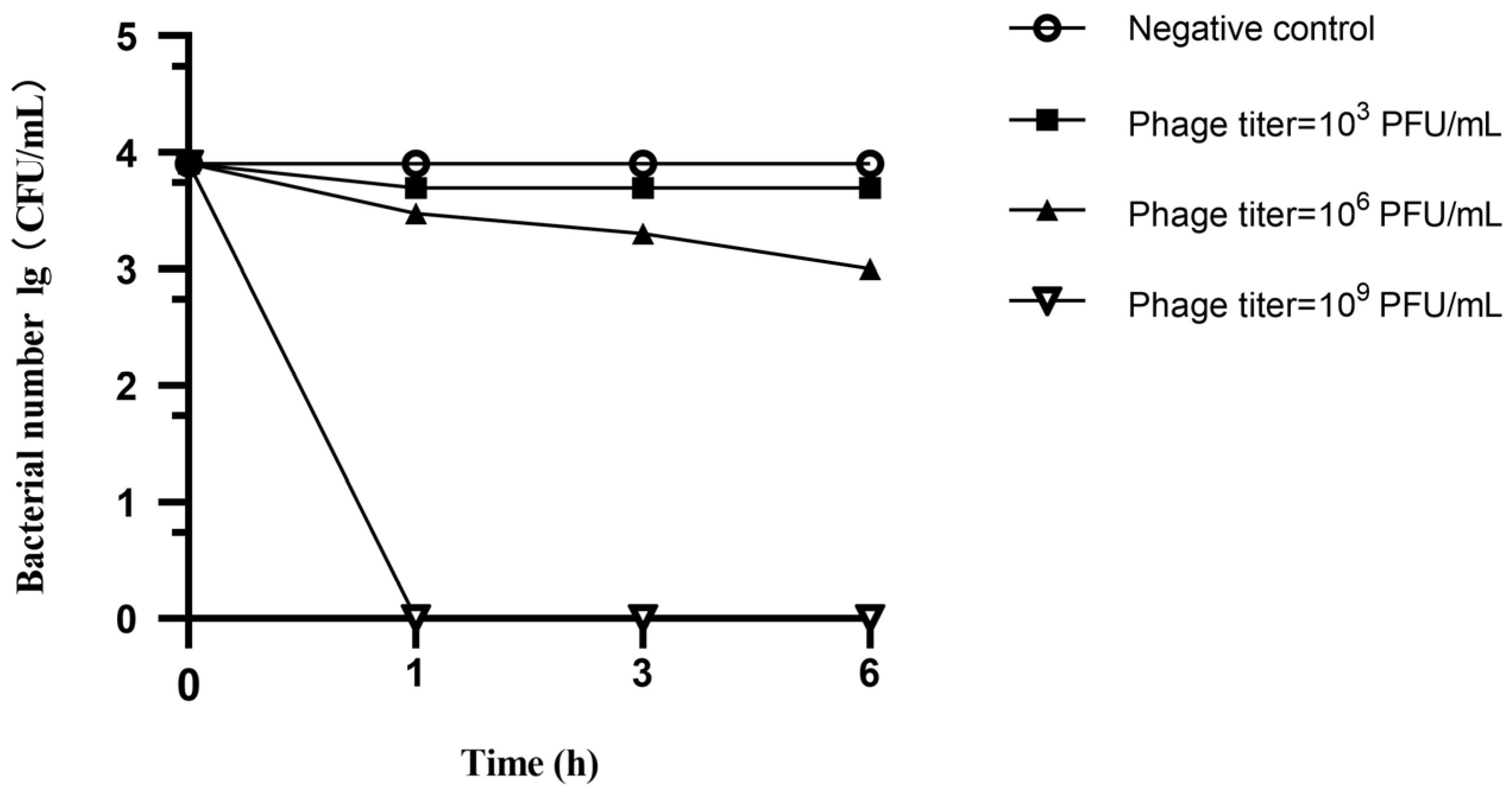
3.6. Spray Disinfection Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handley, J.A.; Park, S.H.; Kim, S.A.; Ricke, S.C. Microbiome Profiles of Commercial Broilers Through Evisceration and Immersion Chilling During Poultry Slaughter and the Identification of Potential Indicator Microorganisms. Front. Microbiol. 2018, 9, 345. [Google Scholar] [CrossRef]
- Gomila, A.; Carratalà, J.; Badia, J.M.; Camprubí, D.; Piriz, M.; Shaw, E.; Diaz-Brito, V.; Espejo, E.; Nicolás, C.; Brugués, M.; et al. Preoperative oral antibiotic prophylaxis reduces Pseudomonas aeruginosa surgical site infections after elective colorectal surgery: A multicenter prospective cohort study. BMC Infect. Dis. 2018, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.H.A.; Rossato, L.; Brito, G.T.; Bet, G.; Simionatto, S. Carbapenem-resistant Pseudomonas aeruginosa strains: A worrying health problem in intensive care units. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e71. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Lee, Y.L.; Liu, P.Y.; Lu, M.C.; Ko, W.C.; Hsueh, P.R. Multicenter surveillance of antimicrobial susceptibilities and resistance mechanisms among Enterobacterales species and non-fermenting Gram-negative bacteria from different infection sources in Taiwan from 2016 to 2018. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi 2022, 55, 463–473. [Google Scholar] [CrossRef]
- Martins, W.; Narciso, A.C.; Cayô, R.; Santos, S.V.; Fehlberg, L.C.C.; Ramos, P.L.; da Cruz, J.B.; Gales, A.C. SPM-1-producing Pseudomonas aeruginosa ST277 clone recovered from microbiota of migratory birds. Diagn. Microbiol. Infect. Dis. 2018, 90, 221–227. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Cohan, F.M.; Zandi, M.; Turner, P.E. Broadscale phage therapy is unlikely to select for widespread evolution of bacterial resistance to virus infection. Virus Evol. 2020, 6, veaa060. [Google Scholar] [CrossRef]
- Holloway, B.W.; Egan, J.B.; Monk, M. Lysogeny in Pseudomonas aeruginosa. Aust. J. Exp. Biol. Med. Sci. 1960, 38, 321–329. [Google Scholar] [CrossRef]
- Jaiswal, A.; Koley, H.; Ghosh, A.; Palit, A.; Sarkar, B. Efficacy of cocktail phage therapy in treating Vibrio cholerae infection in rabbit model. Microbes Infect. 2013, 15, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.R.; De Vos, D.; Friman, V.P.; Pirnay, J.P.; Buckling, A. Effects of sequential and simultaneous applications of bacteriophages on populations of Pseudomonas aeruginosa in vitro and in wax moth larvae. Appl. Environ. Microbiol. 2012, 78, 5646–5652. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.; Anggård, E.; Harper, D. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. Activity of Bacteriophages in Removing Biofilms of Pseudomonas aeruginosa Isolates from Chronic Rhinosinusitis Patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef]
- Pei, R.; Lamas-Samanamud, G. Inhibition of biofilm formation by T7 bacteriophages producing Quorum-Quenching enzymes. Appl. Environ. Microbiol. 2014, 80, 5340–5348. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Chun, H.S.; Park, C.; Nho, D.; Lee, R.; Cho, S.Y.; Kim, C.J.; Lee, D.G. Effect of Antimicrobial Wipes on Hospital-Associated Bacterial and Fungal Strains. Infect. Chemother. 2024, 56, 522–533. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Pseudomonas aeruginosa infection of avian origin: Zoonosis and one health implications. Vet. World 2021, 14, 2155–2159. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Ma, W.; Ding, Y.; Zhang, Y.; Yang, Q.; Wang, J.; Wang, X. Characterization and Application of a Lytic Phage D10 against Multidrug-Resistant Salmonella. Viruses 2021, 13, 1626. [Google Scholar] [CrossRef]
- Cong, C.; Wei, B.; Cui, H.; Li, X.; Yuan, Y.; Wang, L.; Li, S.; Xu, Y. Isolation, characterization and comparison of lytic Epseptimavirus phages targeting Salmonella. Food Res. Int. 2021, 147, 110480. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [CrossRef]
- Marei, E.M. Isolation and Characterization of Pseudomonas aeruginosa and its Virulent Bacteriophages. Pak. J. Biol. Sci. 2020, 23, 491–500. [Google Scholar] [CrossRef]
- Shmidov, E.; Lebenthal-Loinger, I.; Roth, S.; Karako-Lampert, S.; Zander, I.; Shoshani, S.; Danielli, A.; Banin, E. PrrT/A, a Pseudomonas aeruginosa Bacterial Encoded Toxin-Antitoxin System Involved in Prophage Regulation and Biofilm Formation. Microbiol. Spectr. 2022, 10, e0118222. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, Y.P.; Lin, N.T.; Yang, H.H.; Teh, S.H.; Lin, L.C. Therapeutic effect and anti-biofilm ability assessment of a novel phage, phiPA1-3, against carbapenem-resistant Pseudomonas aeruginosa. Virus Res. 2023, 335, 199178. [Google Scholar] [CrossRef]
- Sun, W.J.; Liu, C.F.; Yu, L.; Cui, F.J.; Zhou, Q.; Yu, S.L.; Sun, L. A novel bacteriophage KSL-1 of 2-Keto-gluconic acid producer Pseudomonas fluorescens K1005: Isolation, characterization and its remedial action. BMC Microbiol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Dang, W.; Xie, Z.; Zhang, F.; Hao, X.; Sun, S.; Liu, X.; Luo, Y.; Li, M.; et al. Isolation and Characterization of Three Pseudomonas aeruginosa Viruses with Therapeutic Potential. Microbiol. Spectr. 2023, 11, e0463622. [Google Scholar] [CrossRef]
- Rihtman, B.; Meaden, S.; Clokie, M.R.; Koskella, B.; Millard, A.D. Assessing Illumina technology for the high-throughput sequencing of bacteriophage genomes. PeerJ 2016, 4, e2055. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, N.; Wang, X.; Ren, H.; Zhang, C.; Zou, L.; Han, L.; Guo, L.; Liu, W. Characterization of a lytic Escherichia coli phage CE1 and its potential use in therapy against avian pathogenic Escherichia coli infections. Front. Microbiol. 2023, 14, 1091442. [Google Scholar] [CrossRef]
- Jean-Paul, P.; Blasdel, B.G.; Laurent, B.; Angus, B.; Nina, C.; Clark, J.R.; Sofia, C.-R.; Laurent, D.; Alain, D.; Daniel, D.V.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar]
- Victor, K.; Olga, S.; Elena, P.; Maria, B.; Sergey, K.; Alla, K.; Elena, C.; Leonid, K.; Damian, M.; Olga, P. Modular Approach to Select Bacteriophages Targeting Pseudomonas aeruginosa for Their Application to Children Suffering with Cystic Fibrosis. Front. Microbiol. 2016, 7, 1631. [Google Scholar]
- Zschach, H.; Joensen, K.G.; Lindhard, B.; Lund, O.; Goderdzishvili, M.; Chkonia, I.; Jgenti, G.; Kvatadze, N.; Alavidze, Z.; Kutter, E.M.; et al. What Can We Learn from a Metagenomic Analysis of a Georgian Bacteriophage Cocktail? Viruses 2015, 7, 6570–6589. [Google Scholar] [CrossRef]
- Haniyeh, K.; Narges, T.; Reza, S.A.; Reza, K.M.; Mohammad, S.; Zargham, S. Isolation, characterization, and genomic analysis of vBPaePTUMSP121, a new lytic bacteriophage infecting Pseudomonas aeruginosa. Arch. Virol. 2022, 168, 8. [Google Scholar]
- Khashayar, S.; Mohadeseh, B.; Lili, Z.; Abolghasem, H.; Tao, H.; Hongduo, B.; Mojtaba, M.; Maoda, P.; Heye, W.; Ruicheng, W.; et al. Biodiversity of New Lytic Bacteriophages Infecting Shigella spp. in Freshwater Environment. Front. Microbiol. 2021, 12, 619323. [Google Scholar]
- Bae, J.Y.; Wu, J.; Lee, H.J.; Jo, E.J.; Murugaiyan, S.; Chung, E.; Lee, S.W. Biocontrol potential of a lytic bacteriophage PE204 against bacterial wilt of tomato. J. Microbiol. Biotechnol. 2012, 22, 1613–1620. [Google Scholar] [CrossRef]
- Tan, C.W.; Rukayadi, Y.; Hasan, H.; Abdul-Mutalib, N.A.; Jambari, N.N.; Hara, H.; Thung, T.Y.; Lee, E.; Radu, S. Isolation and Characterization of Six Vibrio parahaemolyticus Lytic Bacteriophages From Seafood Samples. Front. Microbiol. 2021, 12, 616548. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.F.; Reidy, T.G.; Armbruster, C.R.; Sun, M.; Van Tyne, D.; Turner, P.E.; Koff, J.L.; Bomberger, J.M. Lytic bacteriophages induce the secretion of antiviral and proinflammatory cytokines from human respiratory epithelial cells. PLoS Biol. 2024, 22, e3002566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, L.; Rong, J.; Ren, H.; Liu, W.; Zhang, C. Endolysins of bacteriophage vB_Sal-S-S10 can naturally lyse Salmonella enteritidis. BMC Vet. Res. 2022, 18, 410. [Google Scholar] [CrossRef]
- Amgarten, D.; Martins, L.F.; Lombardi, K.C.; Antunes, L.P.; de Souza, A.P.S.; Nicastro, G.G.; Kitajima, E.W.; Quaggio, R.B.; Upton, C.; Setubal, J.C.; et al. Three novel Pseudomonas phages isolated from composting provide insights into the evolution and diversity of tailed phages. BMC Genom. 2017, 18, 346. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Taati Moghadam, M.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 45. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Laurent, F.; Leboucher, G.; Merabischvilli, M.; Djebara, S.; Gustave, C.A.; Perpoint, T.; Barrey, C.; Pirnay, J.P.; et al. Personalized bacteriophage therapy to treat pandrug-resistant spinal Pseudomonas aeruginosa infection. Nat. Commun. 2022, 13, 4239. [Google Scholar] [CrossRef] [PubMed]
- Fuerte-Stone, J.; Mimee, M. Host happy hour: Phage cocktail targets IBD-associated microbes. Cell Host Microbe 2022, 30, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.J.; Rapp, E.M.; Rankin, W.R.; McKenzie, S.M.; Brasko, B.K.; Hebert, K.E.; Bachert, B.A.; Kick, A.R.; Burpo, F.J.; Barnhill, J.C. Combinations of Bacteriophage Are Efficacious against Multidrug-Resistant Pseudomonas aeruginosa and Enhance Sensitivity to Carbapenem Antibiotics. Viruses 2024, 16, 1000. [Google Scholar] [CrossRef] [PubMed]
| Phages | Number of CRPA Strains from Different Sources Lysed by Phage (%) | Total Number of P. aeruginosa Strains Lysed by Phage | Total Lysis Rates Against 246 Strains (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Duck | Broiler | Laying Hen | Pig | Geese | Human | Total CRPA | |||
| vB_PaeP_QSZH | 3 (50.0) | 7 (63.6) | 15 (71.4) | 4 (80.0) | 6 (85.7) | 11 (47.8) | 46 (63.0) | 178 | 72.4 |
| vB_PaeP_YQZQ | 2 (33.3) | 7 (63.6) | 10 (47.6) | 5 (100) | 6 (85.7) | 12 (52.2) | 42 (57.5) | 176 | 71.5 |
| vB_PaeP_YZ2 | 3 (50.0) | 8 (72.7) | 11 (52.4) | 4 (80.0) | 6 (85.7) | 12 (52.2) | 44 (60.3) | 167 | 67.9 |
| vB_PaeM_QDB | 0 (0.0) | 4 (36.4) | 10 (47.6) | 3 (60.0) | 7 (100) | 10 (43.5) | 34 (46.5) | 141 | 57.3 |
| vB_PaeM_ZGC | 2 (33.3) | 7 (63.6) | 9 (42.9) | 2 (40.0) | 6 (85.7) | 9 (39.1) | 35 (47.9) | 132 | 53.7 |
| vB_PaeP_YZ54 | 1 (16.7) | 5 (45.5) | 10 (47.6) | 3 (60.0) | 5 (71.4) | 9 (39.1) | 33 (45.2) | 164 | 66.7 |
| vB_PaeP_YZ44 | 1 (16.7) | 3 (27.3) | 2 (9.5) | 1 (20.0) | 5 (71.4) | 8 (34.8) | 20 (27.4) | 130 | 52.8 |
| vB_PaeP_DZSCG | 1 (16.7) | 1 (9.1) | 5 (23.8) | 1 (20.0) | 5 (71.4) | 8 (34.8) | 21 (28.8) | 129 | 52.4 |
| vB_PaeM_HE1 | 0 (0.0) | 1 (9.1) | 4 (19.0) | 1 (20.0) | 7 (100) | 9 (39.1) | 22 (30.1) | 140 | 57.1 |
| vB_PaeM_YZ5 | 1 (16.7) | 1 (9.1) | 5 (23.8) | 1 (20.0) | 6 (85.7) | 8 (34.8) | 22 (30.1) | 134 | 54.4 |
| vB_PaeM_YYZ2 | 1 (16.7) | 1 (9.1) | 3 (14.3) | 2 (40.0) | 6 (85.7) | 7 (30.4) | 20 (27.4) | 136 | 55.3 |
| vB_PaeP_SWH | 1 (16.7) | 0 (0.0) | 2 (9.5) | 0 (0.0) | 5 (71.4) | 9 (39.1) | 17 (23.3) | 130 | 52.5 |
| vB_PaeP_XHC | 0 (0.0) | 1 (9.1) | 6 (28.6) | 1 (20.0) | 4 (57.1) | 8 (34.8) | 20 (27.4) | 133 | 53.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-P.; Li, C.-A.; Zhao, Y.; Wang, Z.; Wang, J.; Song, F.-J.; Liu, B.-T. Characterization, Genomic Analysis and Application of Five Lytic Phages Against Carbapenem-Resistant Pseudomonas aeruginosa. Microorganisms 2025, 13, 1587. https://doi.org/10.3390/microorganisms13071587
Zhang L-P, Li C-A, Zhao Y, Wang Z, Wang J, Song F-J, Liu B-T. Characterization, Genomic Analysis and Application of Five Lytic Phages Against Carbapenem-Resistant Pseudomonas aeruginosa. Microorganisms. 2025; 13(7):1587. https://doi.org/10.3390/microorganisms13071587
Chicago/Turabian StyleZhang, Li-Ping, Chang-An Li, Yongda Zhao, Zeqing Wang, Junjie Wang, Feng-Jing Song, and Bao-Tao Liu. 2025. "Characterization, Genomic Analysis and Application of Five Lytic Phages Against Carbapenem-Resistant Pseudomonas aeruginosa" Microorganisms 13, no. 7: 1587. https://doi.org/10.3390/microorganisms13071587
APA StyleZhang, L.-P., Li, C.-A., Zhao, Y., Wang, Z., Wang, J., Song, F.-J., & Liu, B.-T. (2025). Characterization, Genomic Analysis and Application of Five Lytic Phages Against Carbapenem-Resistant Pseudomonas aeruginosa. Microorganisms, 13(7), 1587. https://doi.org/10.3390/microorganisms13071587






