Co-Application of Seaweed Extract (Solieria filiformis) and Silicon: Effect on Sporulation, Mycorrhizal Colonization, and Initial Growth of Mimosa caesalpiniaefolia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Soil
2.2. Seaweed Type and Production Process
2.3. Process and Experimental Design
2.4. Plant Growth Parameters
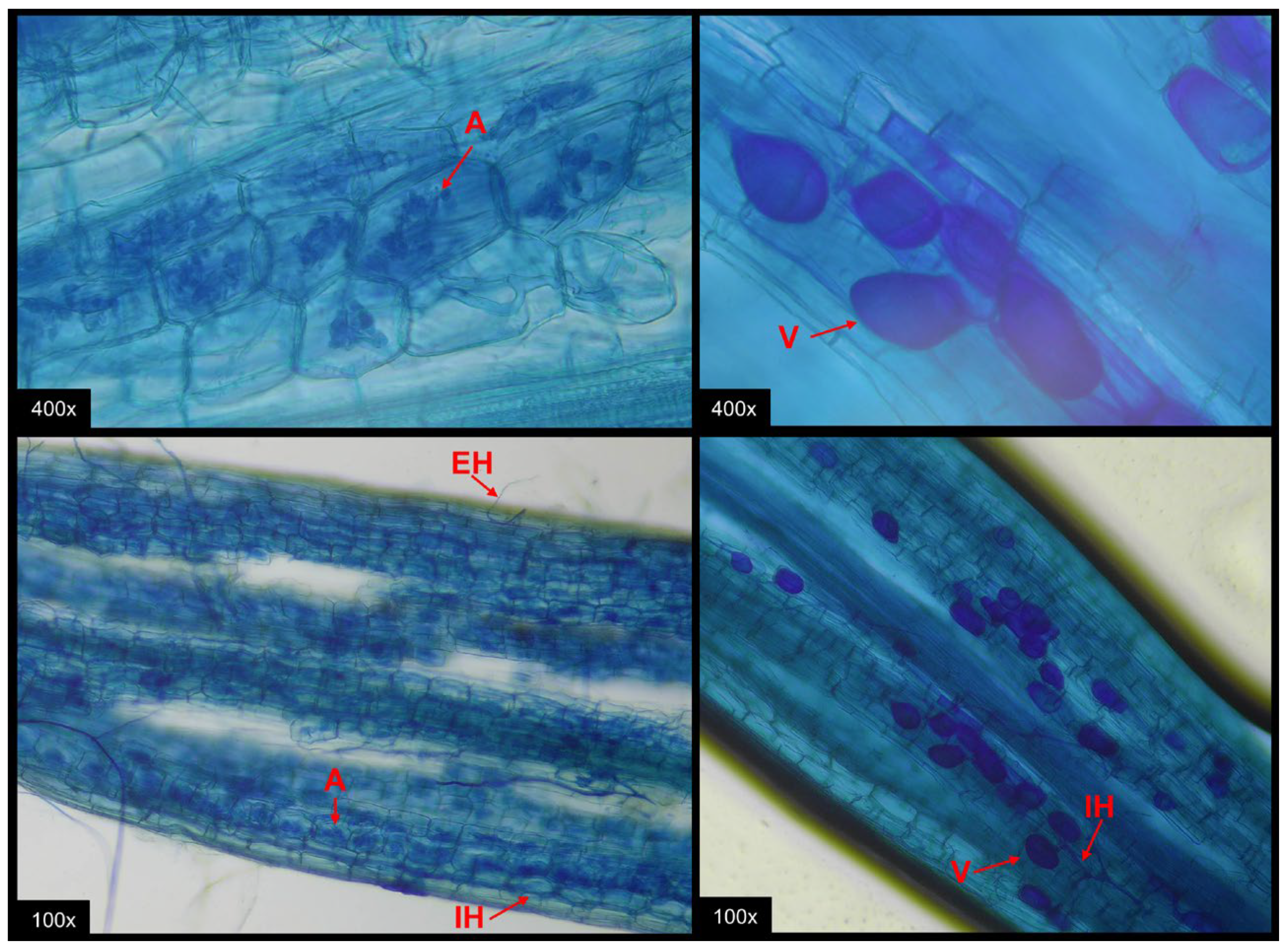
2.5. Quantification of Mycorrhizal Colonization and Spore Abundance
2.6. Silicon in Shoots and Roots and Phosphorus, Sodium, and Potassium in Shoots
2.7. Soil Chemical Analysis
2.8. Statistical Analysis
3. Results
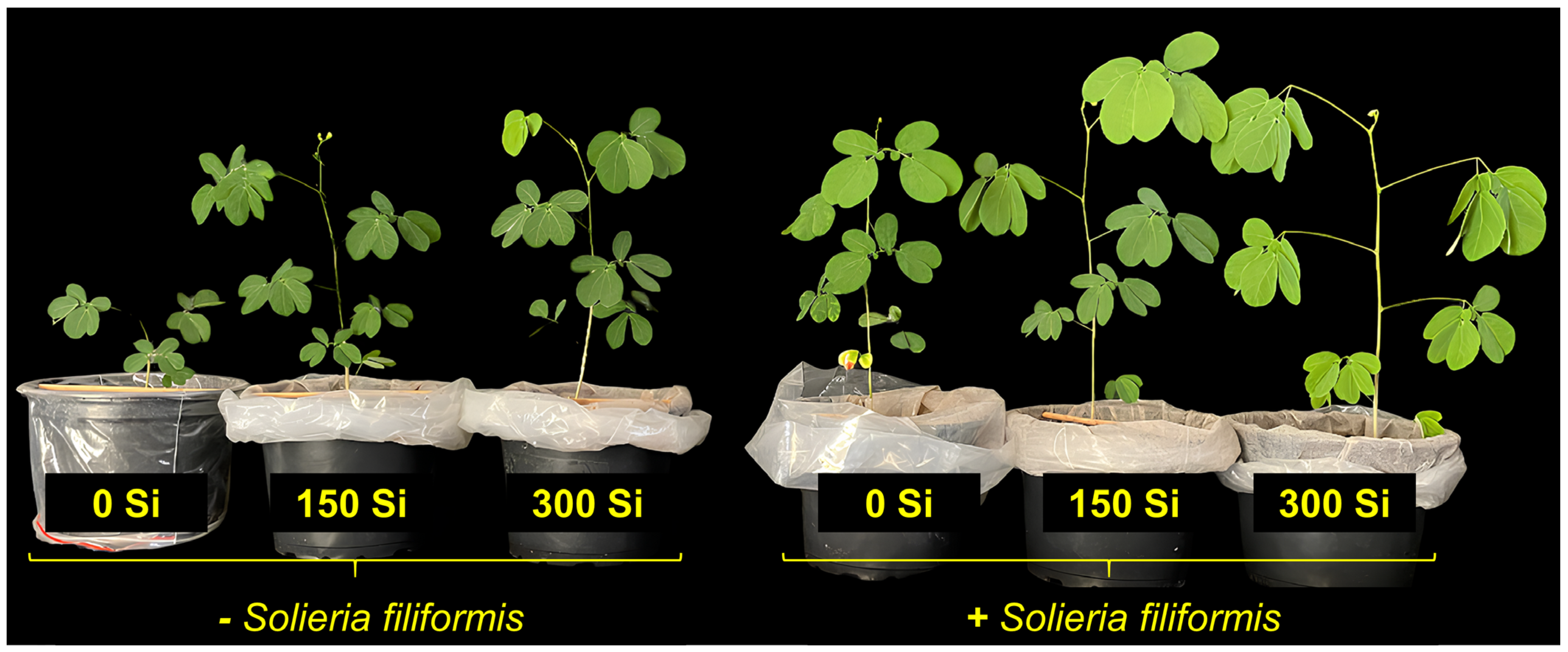
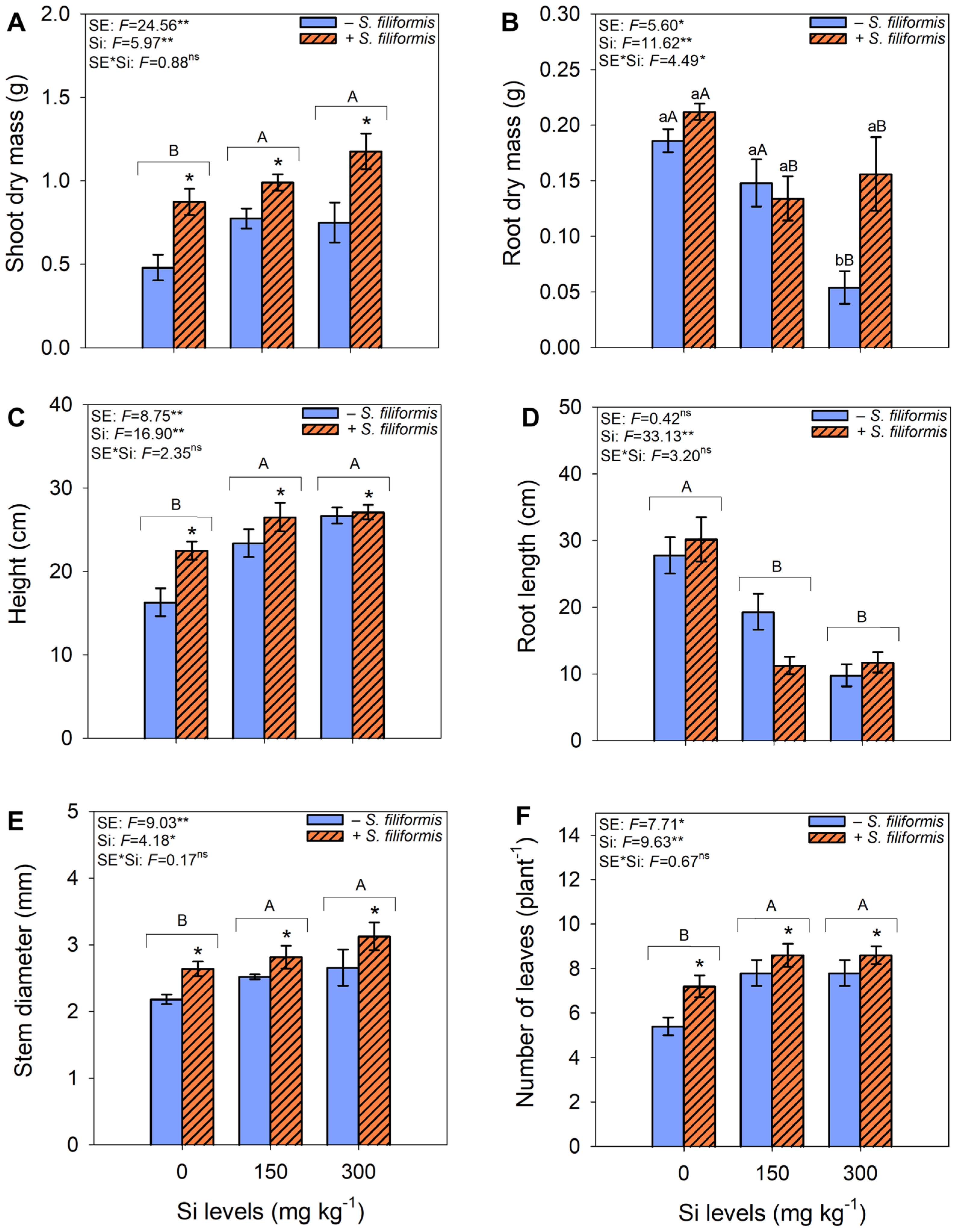
3.1. Plant Growth
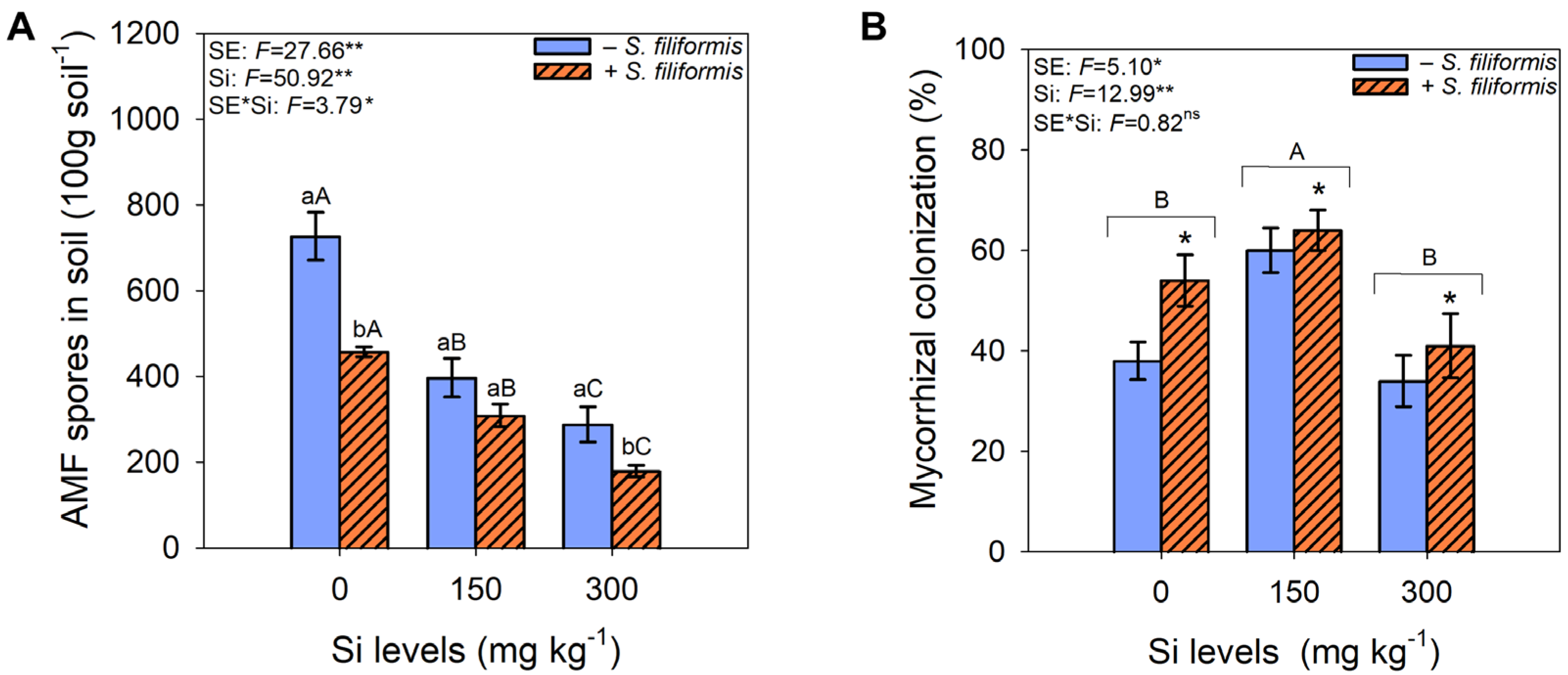
3.2. Abundance of AMF Spores in the Soil and Mycorrhizal Colonization
3.3. Silicon in Shoots and Roots and Phosphorus, Sodium, and Potassium in Shoots
3.4. Soil Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engel, D.C.H.; Feltrim, D.; Rodrigues, M.; Baptistella, J.L.C.; Mazzafera, P. Algae extract increases seed production of soybean plants and alters nitrogen metabolism. Agriculture 2023, 13, 1296. [Google Scholar] [CrossRef]
- Ramos, G.; Russi, P.M.; da Nóbrega Souza, G.; Alves, V.M.; Boas, B.G.V.; Derner, R.B.; Owatari, M.S. Effects of crude extract from the macroalgae Kappaphycus alvarezii on the common bean (Phaseolus vulgaris) crops. Braz. J. Anim. Environ. Res. 2025, 8, e76538. [Google Scholar] [CrossRef]
- Jmaili, K.; Asbai, Z.; Waddi, K.; Bahlaouan, B.; Silkina, A.; Boutaleb, N. Non-Microbial Biostimulants for Plant Growth and Abiotic Stress Mitigation: A Review of Recent Scientific Innovations. Int. J. Environ. Stud. 2025, 74, 401–431. [Google Scholar] [CrossRef]
- Oliveira, A.C.V. Efeitos da Aplicação do Extrato Bruto da Alga Vermelha Gracilaria birdiae em Cultura de Alface (Lactuca sativa); Trabalho de Conclusão de Curso, Universidade Federal do Ceará: Fortaleza, Brazil, 2017; p. 80. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Castro, G.M.C.D.; Benevides, N.M.B.; Cabral, M.C.; Miranda, R.D.S.; Gomes Filho, E.; Rocha, M.V.P.; Araújo, M.L.H. Optimized acid hydrolysis of the polysaccharides from the seaweed Solieria filiformis (Kützing) PW Gabrielson for bioethanol production. Acta Sci. Biol. Sci. 2017, 39, 423. [Google Scholar] [CrossRef]
- Ribeiro, E.E.; Nobre, I.G.; Silva, D.R.; da Silva, W.M.; Sousa, S.K.; Holanda, T.B.; Matos, W.O. Profile of Inorganic Elements of Seaweed from the Brazilian Northeast Coast. Mar. Pollut. Bull. 2024, 202, 116413. [Google Scholar] [CrossRef]
- Rasouli, F.; Nasiri, Y.; Hassanpouraghdam, M.B.; Asadi, M.; Qaderi, T.; Trifa, A.; Szczepanek, M. Seaweed extract and arbuscular mycorrhiza co-application affect the growth responses and essential oil composition of Foeniculum vulgare L. Sci. Rep. 2023, 13, 11902. [Google Scholar] [CrossRef]
- Hines, S.; van der Zwan, T.; Shiell, K.; Shotton, K.; Prithiviraj, B. Alkaline extract of the seaweed Ascophyllum nodosum stimulates arbuscular mycorrhizal fungi and their endomycorrhization of plant roots. Sci. Rep. 2021, 11, 13491. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, M.; Qu, L.; Biere, A. Effects of Arbuscular Mycorrhizal Fungi on Plant Growth and Herbivore Infestation Depend on Availability of Soil Water and Nutrients. Front. Plant Sci. 2023, 14, 1101932. [Google Scholar] [CrossRef]
- Garcia, K.G.V.; Mendes Filho, P.F.; Pinheiro, J.I.; do Carmo, J.F.; de Araújo Pereira, A.P.; Martins, C.M.; de Abreu, M.G.P.; de Souza Oliveira Filho, J. Attenuation of Manganese-Induced Toxicity in Leucaena leucocephala Colonized by Arbuscular Mycorrhizae. Water Air Soil. Pollut. 2020, 231, 22. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular Mycorrhizal Fungi in Conferring Tolerance to Biotic Stresses in Plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Boyno, G.; Rezaee Danesh, Y.; Demir, S.; Teniz, N.; Mulet, J.M.; Porcel, R. The Complex Interplay Between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Synergies, Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 16774. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y. Silicon as a Smart Fertilizer for Sustainability and Crop Improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of Silicon in Plant Stress Tolerance: Opportunities to Achieve a Sustainable Cropping System. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Attia, A.; Tisarum, R.; Cha-um, S.; Datta, A. Interactive Effects of Ascophyllum nodosum Seaweed Extract and Silicon on Growth, Fruit Yield and Quality, and Water Productivity of Tomato under Water Stress. Silicon 2023, 15, 2263–2278. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of Silicon in Plant Drought Stress Responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Etesami, H.; Schaller, J. Improving Phosphorus Availability to Rice through Silicon Management in Paddy Soils: A Review of the Role of Silicate-Solubilizing Bacteria. Rhizosphere 2023, 27, 100749. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, D.; Singh, S.; Ramamurthy, P.C.; Verma, T.; Pujari, M.; Prasad, R. Physiological and Molecular Insights into the Role of Silicon in Improving Plant Performance under Abiotic Stresses. Plant Soil 2023, 486, 25–43. [Google Scholar] [CrossRef]
- Moradtalab, N.; Hajiboland, R.; Aliasgharzad, N.; Hartmann, T.E.; Neumann, G. Silicon and the Association with an Arbuscular-Mycorrhizal Fungus (Rhizophagus clarus) Mitigate the Adverse Effects of Drought Stress on Strawberry. Agronomy 2019, 9, 41. [Google Scholar] [CrossRef]
- Carmo, J.F.; Garcia, K.G.V.; Mendes Filho, P.F.; de Araújo Pereira, A.P.; Pinheiro, J.I. Silicon Application and Mycorrhiza Inoculation Promoted Leucaena leucocephala Growth in a Soil Highly Contaminated by Manganese. Nativa 2022, 10, 410–416. [Google Scholar] [CrossRef]
- Garcia, K.G.V.; Gomes, V.F.F.; Mendes Filho, P.F.; Martins, C.M.; da Silva Júnior, J.M.T.; Cunha, C.S.M.; Pinheiro, J.I. Arbuscular Mycorrhizal Fungi in the Phytostabilization of Soil Degraded by Manganese Mining. J. Agric. Sci. 2018, 10, 192–202. [Google Scholar] [CrossRef][Green Version]
- Maia, E.P.V.; Garcia, K.G.V.; de Souza Oliveira Filho, J.; Pinheiro, J.I.; Mendes Filho, P.F. Co-Inoculation of Rhizobium and Arbuscular Mycorrhiza Increases Mimosa caesalpiniaefolia Growth in Soil Degraded by Manganese Mining. Water Air Soil Pollut. 2023, 234, 289. [Google Scholar] [CrossRef]
- Silva Borges, M.P.; Silva, D.V.; de Freitas Souza, M.; Silva, T.S.; da Silva Teófilo, T.M.; da Silva, C.C.; Dos Santos, J.B. Glyphosate Effects on Tree Species Natives from Cerrado and Caatinga Brazilian Biome: Assessing Sensitivity to Two Ways of Contamination. Sci. Total Environ. 2021, 769, 144113. [Google Scholar] [CrossRef]
- Paiva, L.L.D.; Azevedo, T.K.B.D.; Pimenta, A.S.; Canto, J.L.D.; Souza, M.J.C.D.; Ucella Filho, J.G.M. Effect of thinning on volumes of biomass and bark tannins content of Mimosa caesalpiniifolia Benth. trees. Rev. Árvore 2023, 47, e4728. [Google Scholar] [CrossRef]
- Pinheiro, E.S.; Alves, A.R.; Holanda, A.C.D.; Silveira, G.V.D.S.; Loiola, Â.T.; Silva, P.C.D.; Silva, K.E.D. Effects of planting density on characteristics of sabiá wood. Rev. Caatinga 2024, 37, e11927. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Silva, F.C.; Dasilva, F.C. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2009; 573p. [Google Scholar]
- Liang, Y.; Wong, J.W.C.; Wei, L. Silicon-Mediated Enhancement of Cadmium Tolerance in Maize (Zea mays L.) Grown in Cadmium Contaminated Soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Jupri, A.; Fanani, R.A.; Syafitri, S.M.; Mayshara, S.; Nurijawati.; Pebriani, S.A.; Sunarpi, H. Growth and Yield of Rice Plants Sprayed with Sargassum polycystum Extracted with Different of Concentration. AIP Conf. Proc. 2019, 2199, 70009. [Google Scholar] [CrossRef]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A Review of Seaweed Extract’s Potential as a Biostimulant to Enhance Growth and Mitigate Stress in Horticulture Crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Hungria, M.; Araujo, R.S. Manual de Métodos Empregados em Estudos de Microbiologia Agrícola, 1st ed.; Embrapa: Brasilia, Brazil, 1994. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements, 1st ed.; FAO: Rome, Italy, 1998. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Elliott, C.L.; Snyder, G.H. Autoclave-Induced Digestion for the Colorimetric Determination of Silicon in Rice Straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Pereira, H.; Nolla, A. Análise de Silício: Solo, Planta e Fertilizante; Embrapa Soja (CNPSo): Londrina, Brazil, 2004; No. 2; p. 34. [Google Scholar]
- Silva, I.B.; Fujii, M.T.; Scaff, M.F. Diversidade de Algas Marinhas: Programa de Pós-Graduação em Biodiversidade Vegetal e Meio Ambiente, Programa de Capacitação de Monitores e Educadores; Instituto de Botânica—Jardim Botânico de São Paulo: São Paulo, Brazil, 2010; Available online: https://smastr16.blob.core.windows.net/pgibt/sites/242/2023/10/diversidade-algas-marinhas-ingrid-balesteros.pdf (accessed on 24 February 2025).
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon Nanoparticles Decrease Arsenic Translocation and Mitigate Phytotoxicity in Tomato Plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Wang, D.; Hou, L.; Zhang, L.; Liu, P. The Mechanisms of Silicon on Maintaining Water Balance under Water Deficit Stress. Physiol. Plant 2021, 173, 1253–1262. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Silva, L.D.; Ramos, R.A.; Andrade, C.A.D.; Zambrosi, F.C.B.; Pereira, S.P. High Soil Silicon Concentrations Inhibit Coffee Root Growth without Affecting Leaf Gas Exchange. Rev. Bras. Ciênc. Solo 2011, 35, 939–948. [Google Scholar] [CrossRef]
- Cassel, J.L.; Gysi, T.; Rother, G.M.; Pimenta, B.D.; Ludwig, R.L.; dos Santos, D.B. Benefícios da Aplicação de Silício em Plantas. Braz. J. Anim. Environ. Res. 2021, 4, 6601–6615. [Google Scholar] [CrossRef]
- Pires, G.C.; de Lima, M.E.; Zanchi, C.S.; de Freitas, C.M.; de Souza, J.M.A.; de Camargo, T.A.; de Souza, E.D. Arbuscular Mycorrhizal Fungi in the Rhizosphere of Soybean in Integrated Crop Livestock Systems with Intercropping in the Pasture Phase. Rhizosphere 2021, 17, 100270. [Google Scholar] [CrossRef]
- Hajiboland, R.; Cheraghvareh, L.; Poschenrieder, C. Improvement of Drought Tolerance in Tobacco (Nicotiana rustica L.) Plants by Silicon. J. Plant Nutr. 2017, 40, 1661–1676. [Google Scholar] [CrossRef]
- Etesami, H.; Li, Z.; Maathuis, F.J.; Cooke, J. The Combined Use of Silicon and Arbuscular Mycorrhizas to Mitigate Salinity and Drought Stress in Rice. Environ. Exp. Bot. 2022, 201, 104955. [Google Scholar] [CrossRef]
- Fu, F.F.; Akagi, T.; Yabuki, S.; Iwaki, M.; Ogura, N. Distribution of Rare Earth Elements in Seaweed: Implication of Two Different Sources of Rare Earth Elements and Silicon in Seaweed. J. Phycol. 2000, 36, 62–70. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and Future Prospects on the Action Mechanisms in Alleviating Biotic and Abiotic Stresses in Plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Sobral, L.F.; Barreto, M.D.V.; Da Silva, A.J.; Dos Anjos, J.L.; Barretto, M.C.D.V.; Airon, J.D.S.; Joezio, L.D.A. Guia Prático para Interpretação de Resultados de Análises de Solo; Embrapa Tabuleiros Costeiros (Cpatc): Aracaju, Brazil, 2015; 13p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/142260/1/Doc-206.pdf (accessed on 4 March 2025).
- Inocencio, M.F.; Carvalho, J.G.D.; Furtini Neto, A.E. Potássio, sódio e crescimento inicial de espécies florestais sob substituição de potássio por sódio. Rev. Árvore 2014, 38, 113–123. [Google Scholar] [CrossRef]
- Tejasree, A.; Mirza, A. Synergistic effects of silicon and seaweed extract on growth and leaf nutrient content of papaya cv Red Lady. J. Appl. Nat. Sci. 2024, 16, 1250–1255. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Ali, A.M.; Bijay-Singh. Silicon: A Crucial Element for Enhancing Plant Resilience in Challenging Environments. J. Plant Nutr. 2025, 48, 486–521. [Google Scholar] [CrossRef]
- Santos, H.C.; Oliveira, F.H.T.; Souza, A.P.; Salcedo, I.H.; Silva, V.D.M. Phosphorus Availability as a Function of Its Time of Contact with Different Soils. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 996–1001. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Lyons, G.; English, P.; Guppy, C.N. Silicon Nutrition of Rice Is Affected by Soil PH, Weathering and Silicon Fertilisation. J. Plant Nutr. Soil Sci. 2011, 174, 437–446. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, Y.; Zhang, L.; Zhang, J.; Zhou, Y.; Huang, H.; Luo, S.; Luo, L. Silicon-based additive on heavy metal remediation in soils: Toxicological effects, remediation techniques, and perspectives. Environ. Res. 2022, 205, 112244. [Google Scholar] [CrossRef]
- Kumar, A.; Hart, P.; Thakur, V.K. Seaweed Based Hydrogels: Extraction, Gelling Characteristics, and Applications in the Agriculture Sector. ACS Sustain. Resour. Manag. 2024, 1, 1876–1905. [Google Scholar] [CrossRef]

| pH | EC | Mg2+ | Na+ | K+ | H + Al | Al3+ | BS | AS | ESP |
| (H2O) | (dS/m) | (cmolc/kg) | (%) | (%) | (%) | ||||
| 5.0 | 0.9 | 0.4 | 0.6 | 0.18 | 4.46 | 0.5 | 41 | 14 | 1 |
| N | OM | P | Bulk Density | Coarse Sand | Fine Sand | Silt | Clay | Natural Clay | Textural Class |
| (g/kg) | (g/kg) | (mg/kg) | g/cm3 | (g/kg) | - | ||||
| 1.36 | 21.39 | 37 | 1.6 | 586 | 282 | 95 | 37 | 4 | Loamy Sand |
| Treatments | pH | EC | Si | Na | K | P | |
|---|---|---|---|---|---|---|---|
| SE | Si (mg kg−1) | (H2O) | (µS cm−1) | (mg kg−1) | (cmolc kg−1) | (cmolc kg−1) | (mg kg−1) |
| −S. filiformis | 0 | 4.60 ± 0.12 C | 204.60 ± 16.54 B | 2.49 ± 0.08 aC | 0.15 ± 0.003 C | 0.04 ± 0.001 aA | 20.95 ± 1.29 aA |
| 150 | 5.55 ± 0.07 B | 227.92 ± 17.77 A | 3.96 ± 0.19 aB | 0.54 ± 0.008 B | 0.03 ± 0.0009 bB | 21.31 ± 1.12 aA | |
| 300 | 6.21 ± 0.05 A | 255.60 ± 7.89 A | 7.87 ± 0.64 aA | 0.87 ± 0.031 A | 0.01 ± 0.001 bC | 16.36 ± 0.79 bB | |
| +S. filiformis | 0 | 4.56 ± 0.02 C | 210.56 ± 8.18 B | 2.58 ± 0.17 aB | 0.16 ± 0.003 C | 0.03 ± 0.001 bA | 20.95 ± 1.00 aA |
| 150 | 5.68 ± 0.07 B | 263.00 ± 11.15 A | 3.45 ± 0.13 aB | 0.55 ± 0.005 B | 0.04 ± 0.001 aA | 22.67 ± 0.42 aA | |
| 300 | 6.31 ± 0.08 A | 240.20 ± 8.12 A | 6.01 ± 0.17 bA | 0.86 ± 0.011 A | 0.03 ± 0.001 aB | 21.48 ± 1.10 aA | |
| F test | |||||||
| SE | 0.95 ns | 0.72 ns | 9.70 ** | 0.21 ns | 20.04 ** | 7.00 * | |
| Si | 226.60 ** | 6.74 ** | 116.93 ** | 1213.11 ** | 63.10 ** | 4.86 * | |
| SE*Si | 0.65 ns | 2.12 ns | 5.56 * | 0.45 ns | 33.53 ** | 2.52 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, I.A.; de Andrade, J.L.S.; Barbosa, F.L.A.; Almeida, M.d.S.; Araújo, M.L.H.; de Souza, A.J.; Araujo, A.S.F.; Pereira, A.P.d.A.; Garcia, K.G.V. Co-Application of Seaweed Extract (Solieria filiformis) and Silicon: Effect on Sporulation, Mycorrhizal Colonization, and Initial Growth of Mimosa caesalpiniaefolia. Microorganisms 2025, 13, 1581. https://doi.org/10.3390/microorganisms13071581
da Silva IA, de Andrade JLS, Barbosa FLA, Almeida MdS, Araújo MLH, de Souza AJ, Araujo ASF, Pereira APdA, Garcia KGV. Co-Application of Seaweed Extract (Solieria filiformis) and Silicon: Effect on Sporulation, Mycorrhizal Colonization, and Initial Growth of Mimosa caesalpiniaefolia. Microorganisms. 2025; 13(7):1581. https://doi.org/10.3390/microorganisms13071581
Chicago/Turabian Styleda Silva, Isaac Alves, José Lucas Sousa de Andrade, Francisco Luan Almeida Barbosa, Murilo de Sousa Almeida, Marjory Lima Holanda Araújo, Adijailton Jose de Souza, Ademir Sergio Ferreira Araujo, Arthur Prudêncio de Araujo Pereira, and Kaio Gráculo Vieira Garcia. 2025. "Co-Application of Seaweed Extract (Solieria filiformis) and Silicon: Effect on Sporulation, Mycorrhizal Colonization, and Initial Growth of Mimosa caesalpiniaefolia" Microorganisms 13, no. 7: 1581. https://doi.org/10.3390/microorganisms13071581
APA Styleda Silva, I. A., de Andrade, J. L. S., Barbosa, F. L. A., Almeida, M. d. S., Araújo, M. L. H., de Souza, A. J., Araujo, A. S. F., Pereira, A. P. d. A., & Garcia, K. G. V. (2025). Co-Application of Seaweed Extract (Solieria filiformis) and Silicon: Effect on Sporulation, Mycorrhizal Colonization, and Initial Growth of Mimosa caesalpiniaefolia. Microorganisms, 13(7), 1581. https://doi.org/10.3390/microorganisms13071581








