Regime Shifts in Microbial and Water Quality Dynamics in Red Tilapia Ponds
Abstract
1. Introduction
2. Materials and Methods
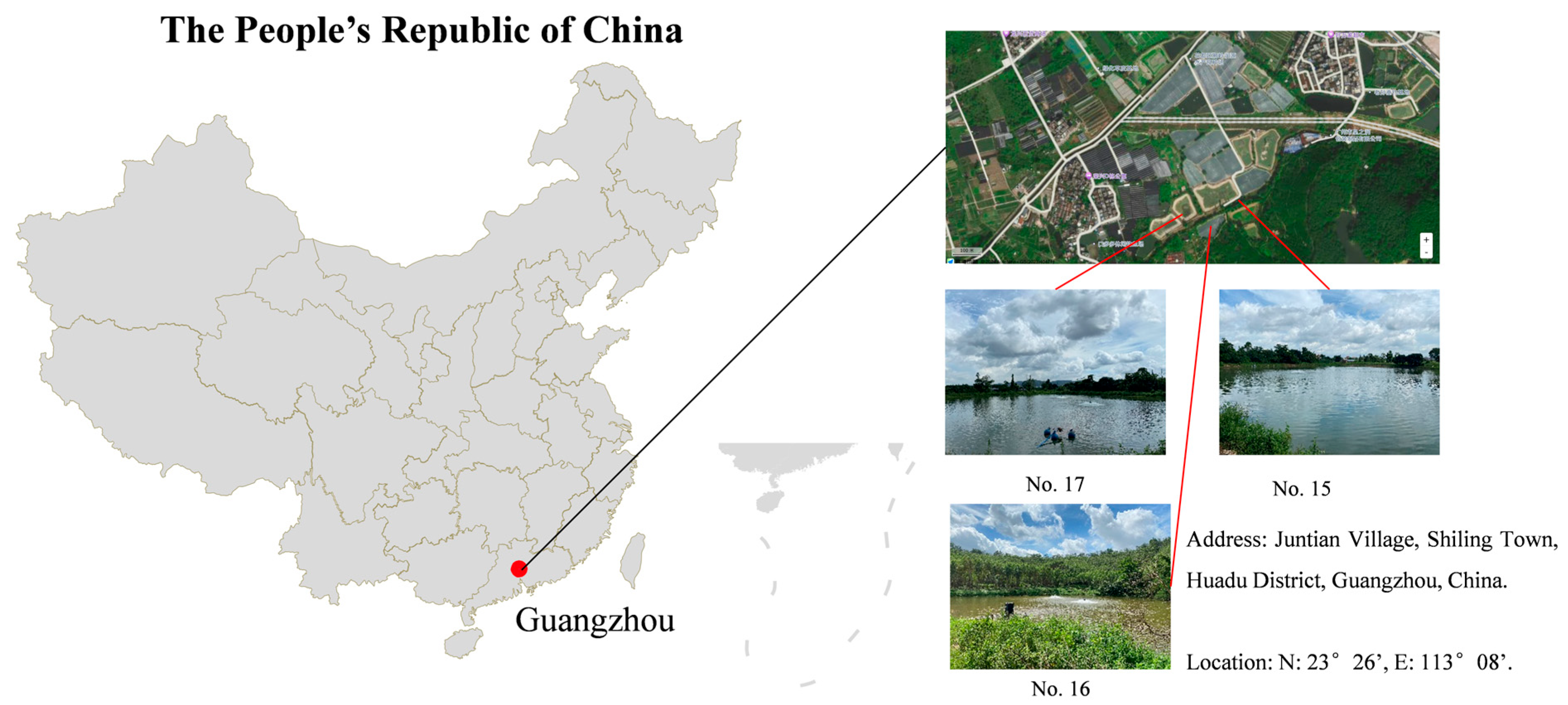
2.1. Farming Management
2.2. Water Sampling and Characterization
2.3. DNA Extraction and High-Throughput Sequencing
2.4. Statistical Analysis
3. Results
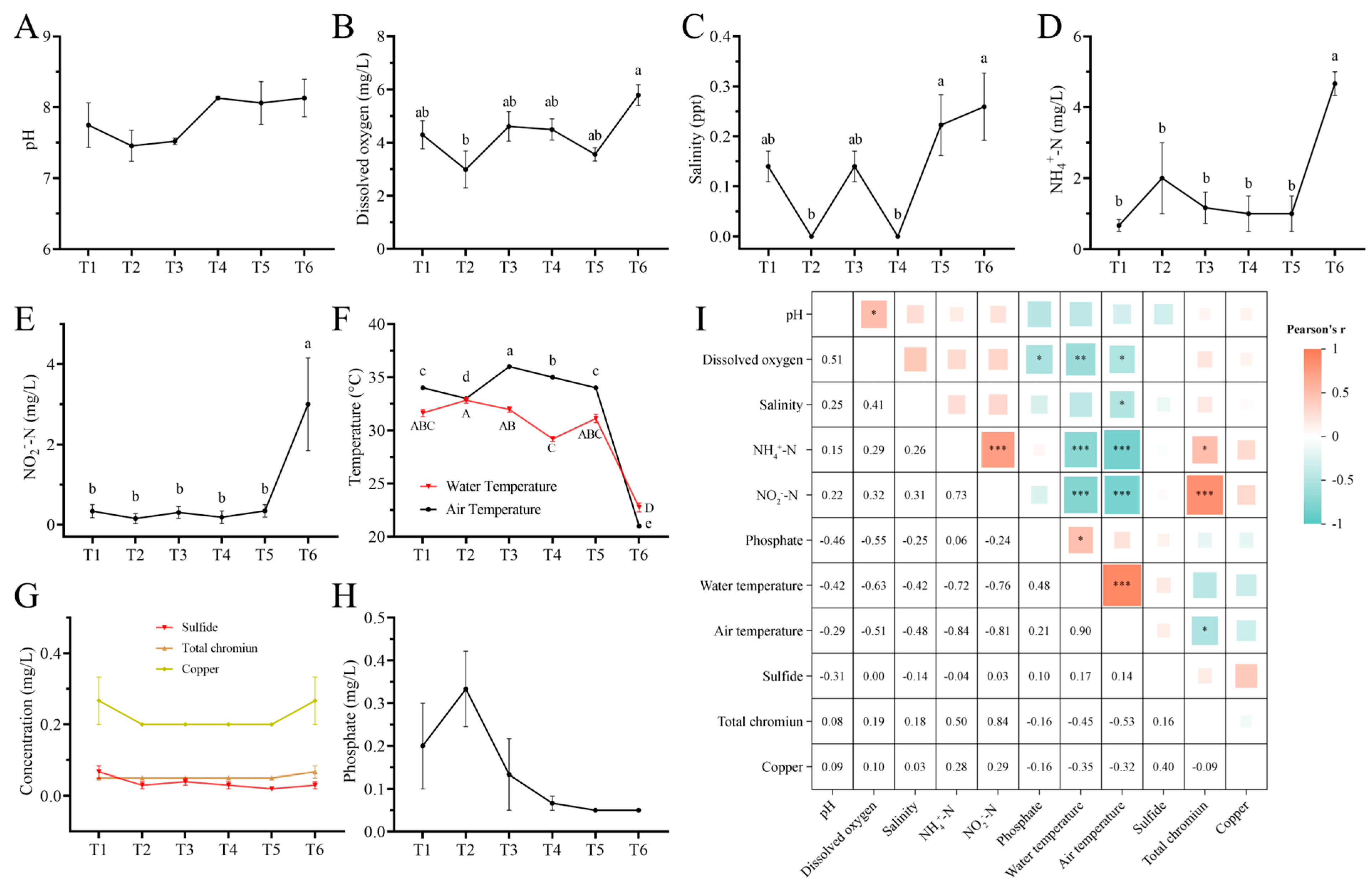
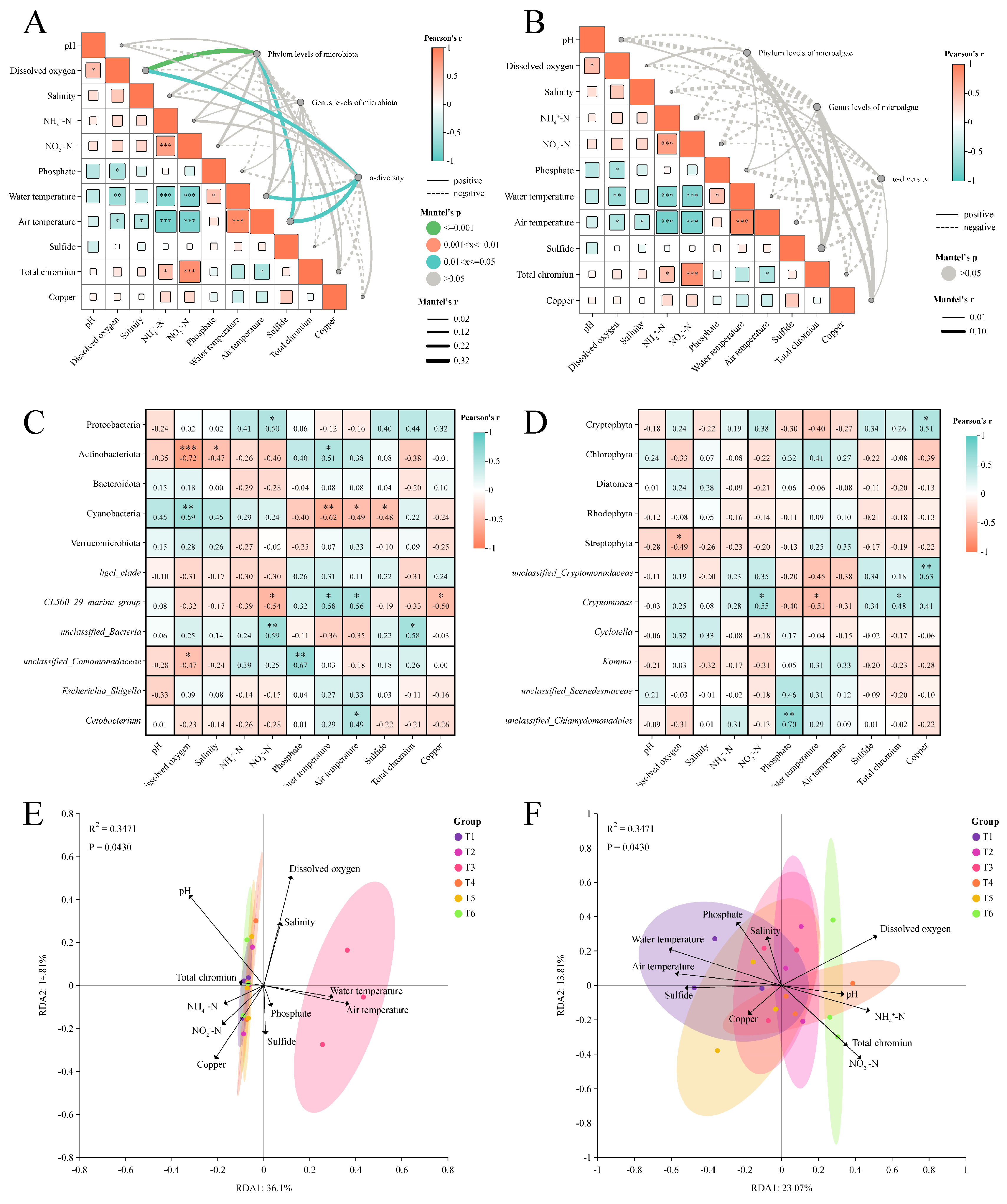
3.1. Water Quality Indicators and Correlation
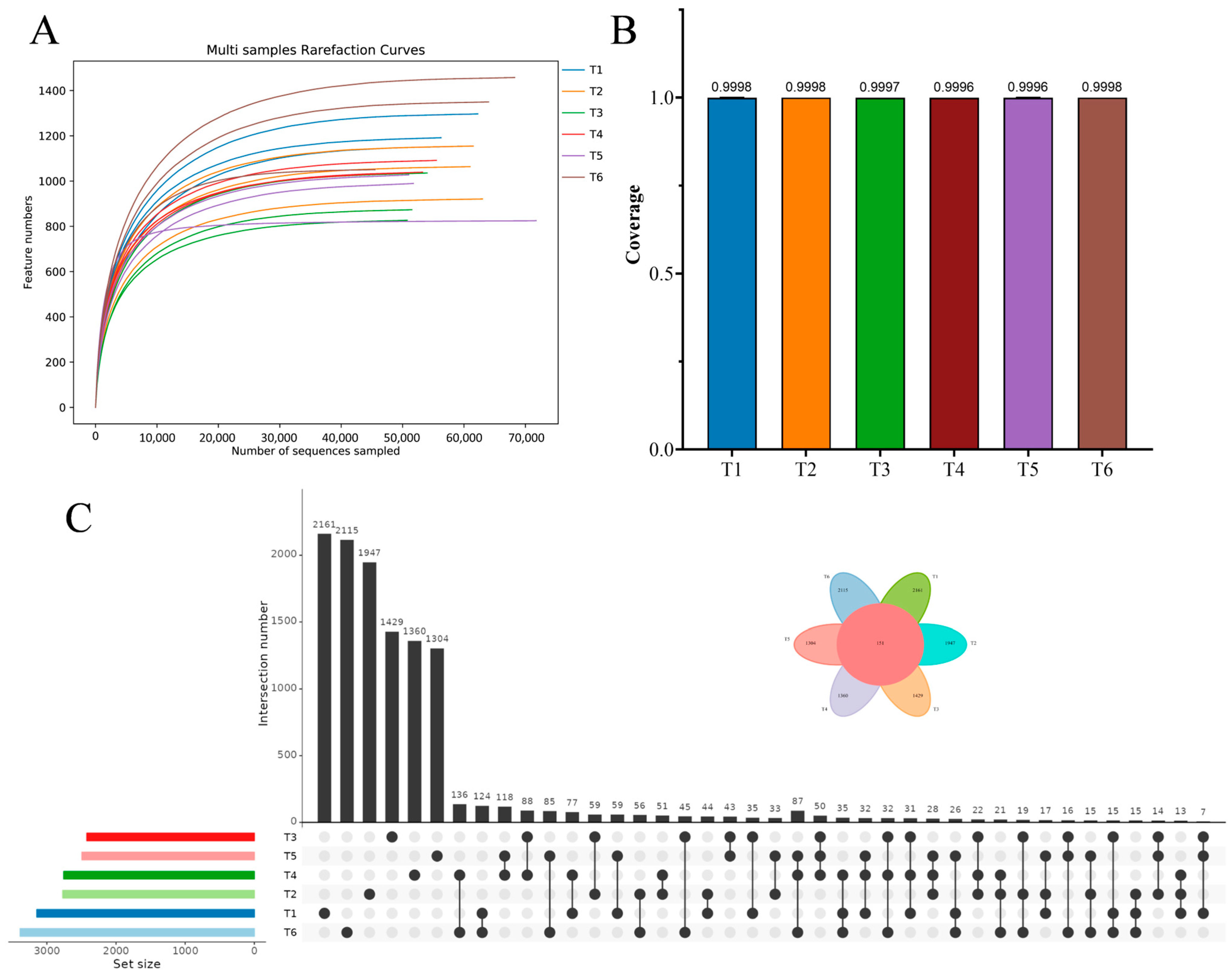
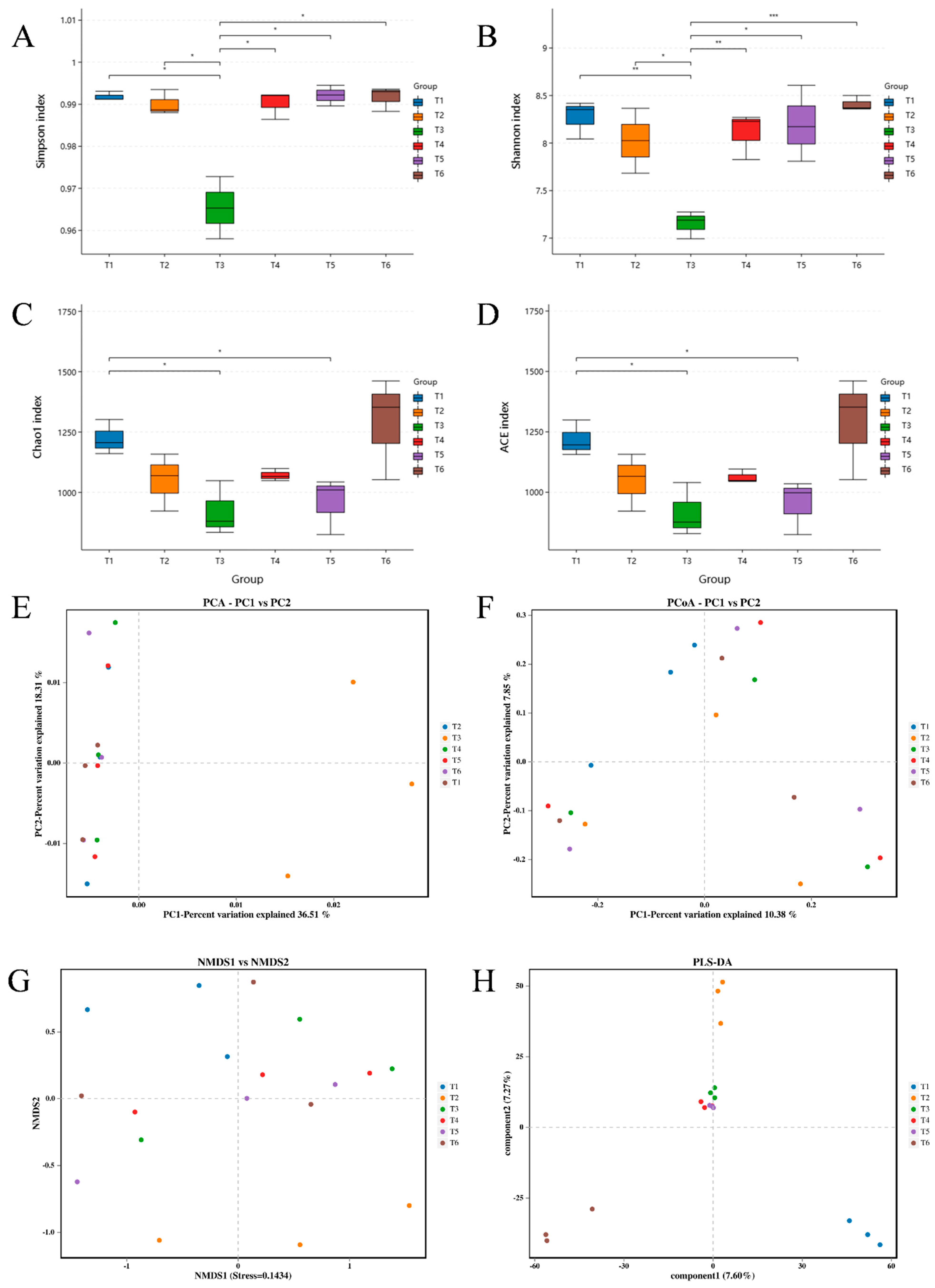
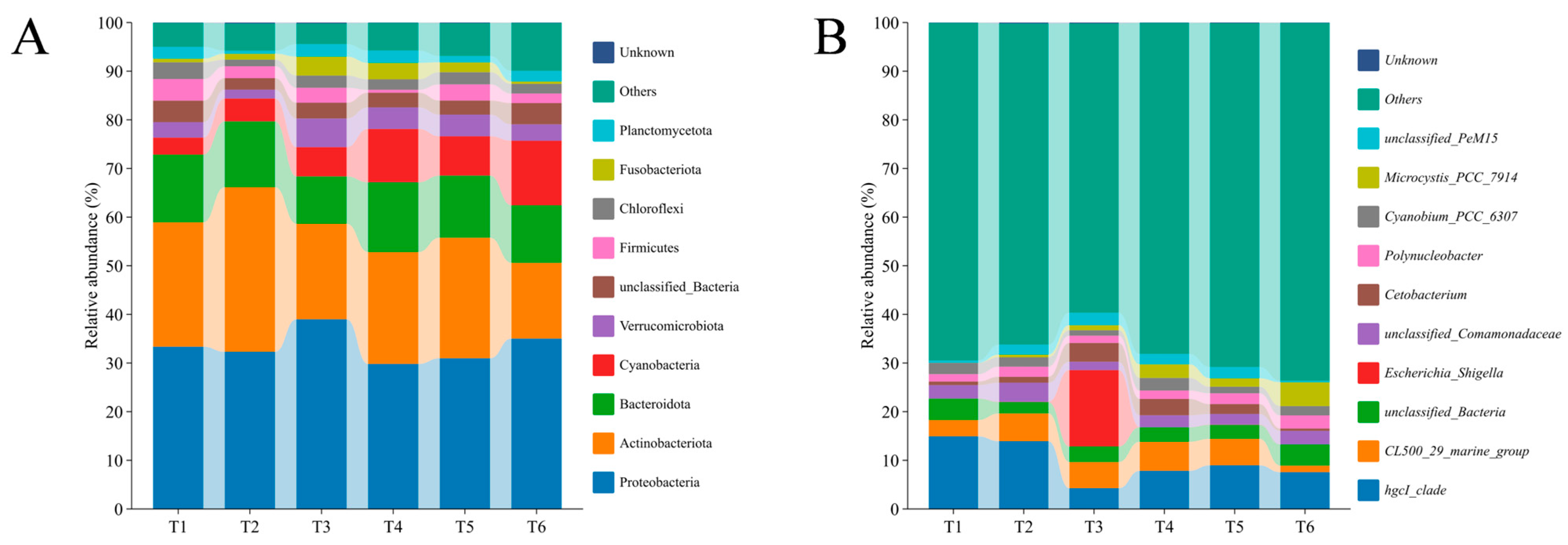
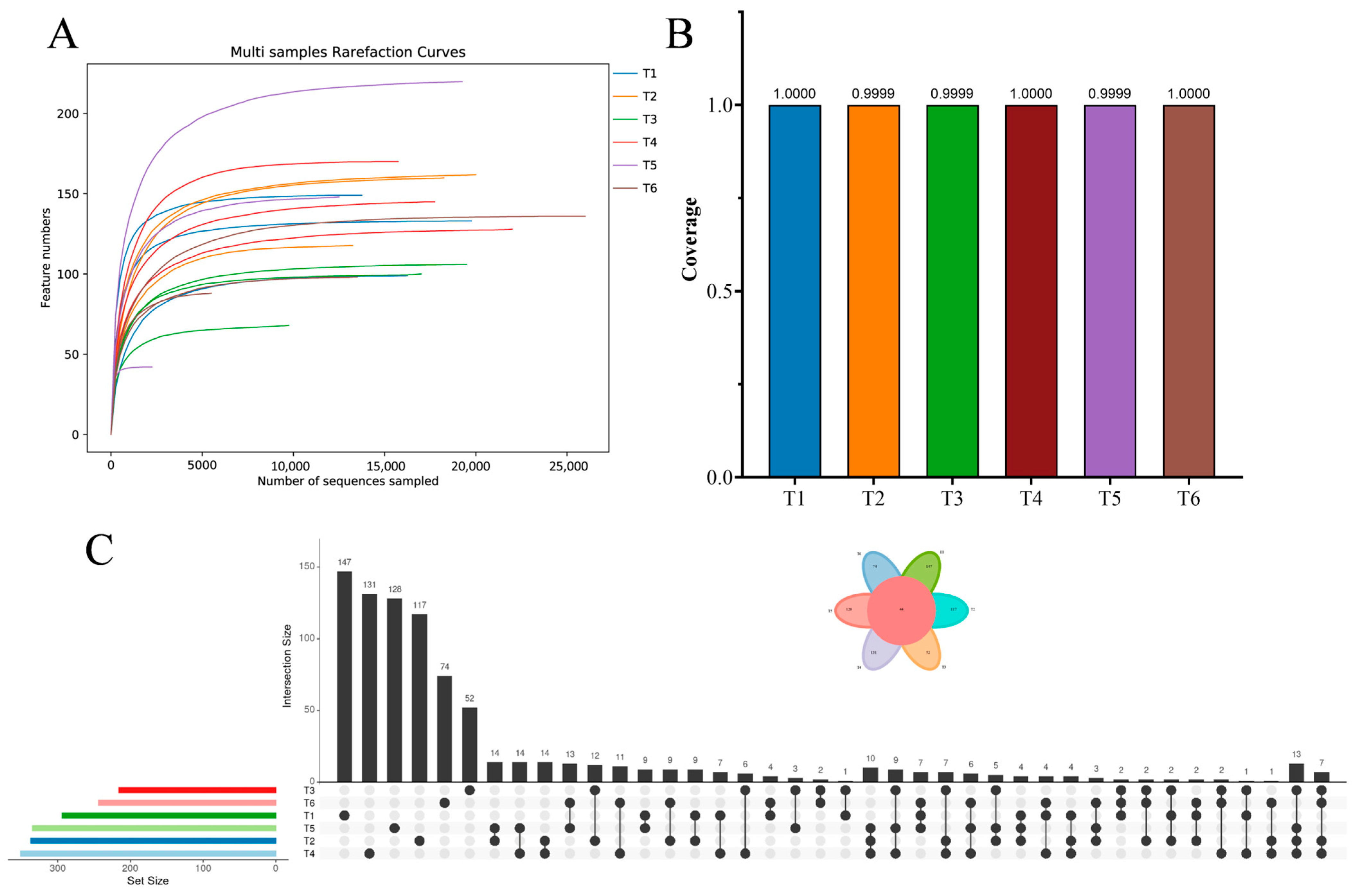
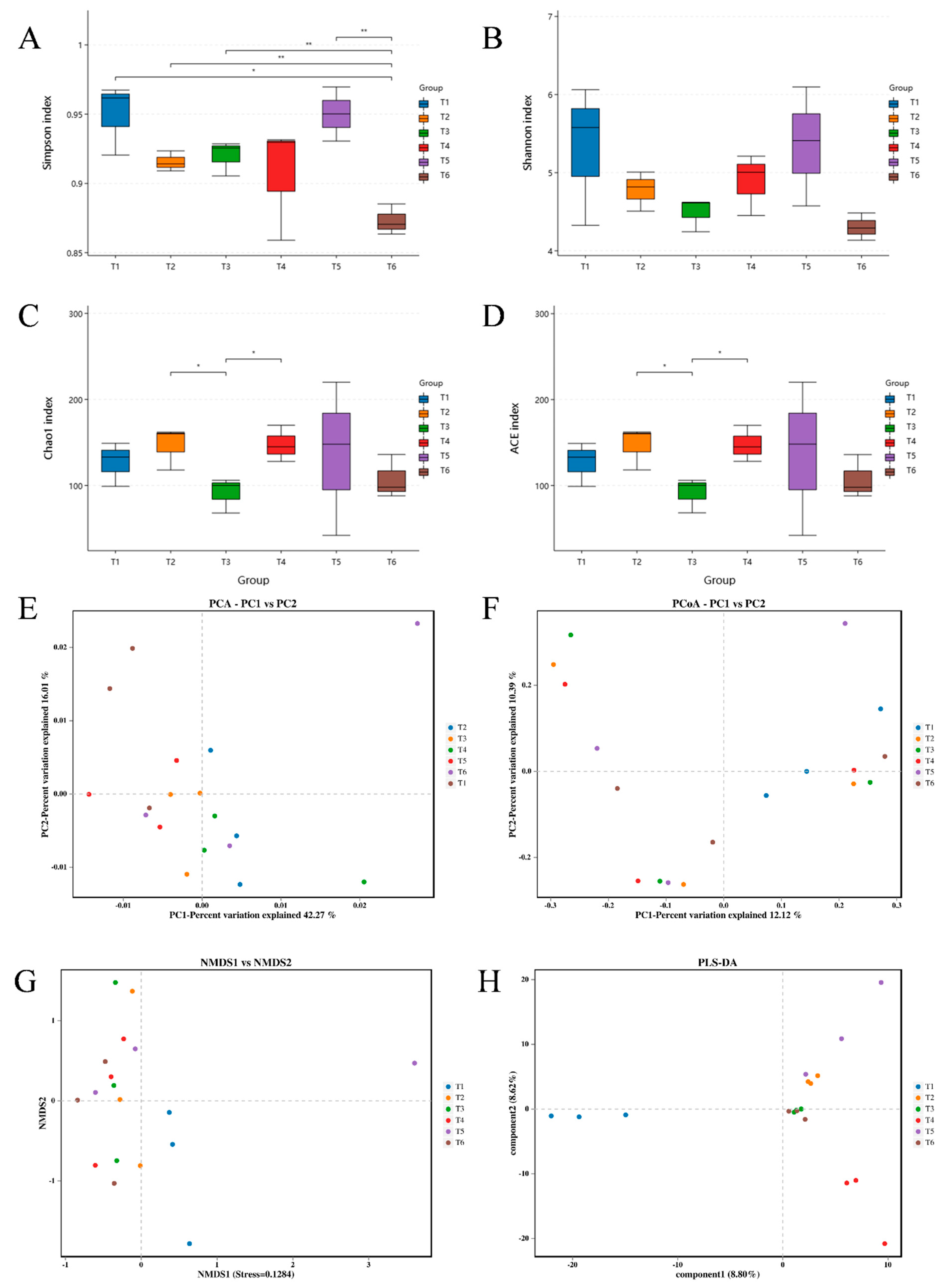
3.2. Bacterial Community Dynamics
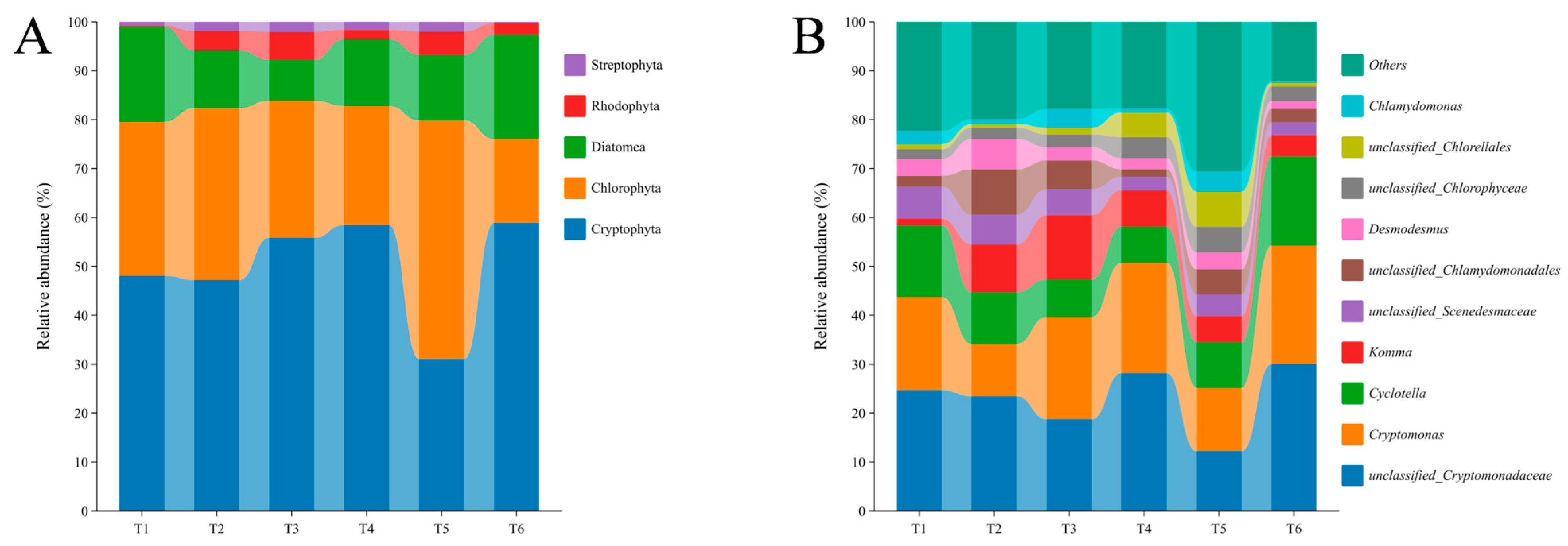
3.3. Microalgal Community Dynamics
3.4. Correlation Analysis Between Water Quality Indicators and Microalgal–Bacterial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Mramba, R.P.; Kahindi, E.J. Pond water quality and its relation to fish yield and disease occurrence in small-scale aquaculture in arid areas. Heliyon 2023, 9, e16753. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Vo, T.S.; Tran-Nguyen, P.L.; Nguyen, M.N.; Pham, V.H.; Matsuhashi, R.; Kim, K.; Vo, T. A comprehensive review of aeration and wastewater treatment. Aquaculture 2024, 591, 741113. [Google Scholar] [CrossRef]
- Herath, S.S.; Satoh, S. 15—Environmental impact of phosphorus and nitrogen from aquaculture. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 369–386. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Thompson, C.C.; Wasielesky, W., Jr.; Landuci, F.; Lima, M.S.; Bacha, L.; Perazzolo, L.; Lourenço-Marques, C.; Soares, F.; Pousao-Ferreira, P.; Hanson, L.; et al. Understanding the role of microbes in health and disease of farmed aquatic organisms. Mar. Life Sci. Technol. 2024, 6, 579–609. [Google Scholar] [CrossRef]
- Parvathy, A.J.; Das, B.C.; Jifiriya, M.J.; Varghese, T.; Pillai, D.; Kumar, V.J.R. Ammonia induced toxico-physiological responses in fish and management interventions. Rev. Aquac. 2023, 15, 452–479. [Google Scholar] [CrossRef]
- Neissi, A.; Rafiee, G.; Rahimi, S.; Farahmand, H.; Pandit, S.; Mijakovic, I. Enriched microbial communities for ammonium and nitrite removal from recirculating aquaculture systems. Chemosphere 2022, 295, 133811. [Google Scholar] [CrossRef]
- Cheung, K.C.; Poon, B.H.T.; Lan, C.Y.; Wong, M.H. Assessment of metal and nutrient concentrations in river water and sediment collected from the cities in the Pearl River Delta, South China. Chemosphere 2003, 52, 1431–1440. [Google Scholar] [CrossRef]
- Banihashemi, E.S.; Khara, H.; Pajand, Z.; Rahnandeh, M. Histopathological study of gill, kidney and liver of Persian Sturgeon (Acipenser persicus Borodin, 1897) and Stellate (Acipenser stellatus Pallas, 1811) exposed to sublethal concentration of un-ionised ammonia UAN. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2016, 40, 1443–1450. [Google Scholar] [CrossRef]
- Qi, C.L.; Han, F.L.; Wang, X.D.; Xu, C.; Huang, Z.P.; Li, E.C.; Qin, J.G.; Chen, L.Q. High protein diet alleviates the high pH stress in Chinese mitten crab Eriocheir sinensis. Aquaculture 2020, 516, 734523. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Laiz-Carrión, R.; Ortega, A.; De la Gándara, F.; Quintanilla, J.M.; Mancera, J.M. Survival of Atlantic bluefin tuna (Thunnus thynnus) larvae hatched at different salinity and pH conditions. Aquaculture 2022, 560, 738457. [Google Scholar] [CrossRef]
- Bruno, A.; Cafiso, A.; Sandionigi, A.; Galimberti, A.; Magnani, D.; Manfrin, A.; Petroni, G.; Casiraghi, M.; Bazzocchi, C. Red mark syndrome: Is the aquaculture water microbiome a keystone for understanding the disease aetiology? Front. Microbiol. 2023, 14, 1059127. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.M.; Gao, P.C.; Zhou, K.; Yao, Z.L.; Lai, Q.F. Indicative role of bacterial community structure in aquaculture water on the health of Litopenaeus vannamei. Mar. Fish. 2023, 45, 594–604. [Google Scholar] [CrossRef]
- Zhang, D.M.; Wang, X.; Xiong, J.B.; Zhu, J.L.; Wang, Y.N.; Zhao, Q.F.; Chen, H.P.; Guo, A.N.; Wu, J.F.; Dai, H.P. Bacterioplankton assemblages as biological indicators of shrimp health status. Ecol. Indic. 2014, 38, 218–224. [Google Scholar] [CrossRef]
- Onishchenko, N.A.; Gorbach, V.V.; Shustov, Y.A. Effect of an aquaculture freshwater body on the behavior and growth of European perch. Russ. J. Ecol. 2020, 51, 260–265. [Google Scholar] [CrossRef]
- Huang, Q.; Li, M.; Xu, S.; Li, C.W. Temporal dynamics of microbial communities in the water of polyculture pond system for Chinese swimming crab Portunus trituberculatus. J. Exp. Mar. Biol. Ecol. 2024, 579, 152047. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Pan, L.Q.; Huang, F.; Gao, S.; Su, C.; Zhang, M.Z.; He, Z.Y. Metagenomic analysis of composition, function and cycling processes of microbial community in water, sediment and effluent of Litopenaeus vannamei farming environments under different culture modes. Aquaculture 2019, 506, 280–293. [Google Scholar] [CrossRef]
- Sun, R.B.; Wang, F.H.; Hu, C.S.; Liu, B.B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Calatrava, V.; Sanz-Luque, E.; Tejada-Jiménez, M.; Llamas, A.; Plouviez, M.; Guieysse, B.; Fernández, E.; Galván, A. Chlamydomonas reinhardtii, an Algal Model in the Nitrogen Cycle. Plants 2020, 9, 903. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Jiang, F.J.; Hu, Z.L.; Zheng, Y.H. Performance of Platymonas and microbial community analysis under different C/N ratio in biofloc technology aquaculture system. J. Water Process. Eng. 2021, 43, 102257. [Google Scholar] [CrossRef]
- Wang, C.; Pan, L.Q.; Zhang, K.Q.; Xu, W.J.; Zhao, D.H.; Mei, L. Effects of different carbon sources addition on nutrition composition and extracellular enzymes activity of bioflocs, and digestive enzymes activity and growth performance of Litopenaeus vannamei in zero-exchange culture tanks. Aquac. Res. 2016, 47, 3307–3318. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Bolívar, N.C.; Pereira, S.A.; Guertler, C.; Vieira, F.D.; Mouriño, J.L.P.; Seiffert, W.Q. Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei. Aquaculture 2015, 448, 273–279. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Kim, S.K.; Song, J.H.; Rajeev, M.; Kim, S.K.; Kang, I.L.; Jang, I.K.; Cho, J.C. Exploring bacterioplankton communities and their temporal dynamics in the rearing water of a biofloc-based shrimp (Litopenaeus vannamei) aquaculture system. Front. Microbiol. 2022, 13, 995699. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, K.Y.; Liu, M.; Wang, B.J.; Wang, L. Microbial community dynamics and nitrogen transformation associated with turbid-turned culture stage of Pacific white shrimp, Litopenaeus vannamei in small greenhouse farms. Aquaculture 2024, 593, 741285. [Google Scholar] [CrossRef]
- Chen, J.L.; Fan, Z.; Tan, D.J.; Jiang, D.N.; Wang, D.S. A review of genetic advances related to sex control and manipulation in Tilapia. J. World Aquac. Soc. 2018, 49, 277–291. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Kitiyodom, S.; Kamble, M.T.; Supchukun, K.; Saengkrit, N.; Sukkarun, P.; Medhe, S.V.; Thompson, K.D.; Boonrungsiman, S.; Temisak, S.; et al. Immersion of nanostructured lipid carriers loaded with 17-alpha methyltestosterone for masculinization of red tilapia (Oreochromis sp.). Aquaculture 2024, 586, 740780. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Biggs, R.; Peterson, G.D.; Rocha, J.C. The Regime Shifts Database: A framework for analyzing regime shifts in social-ecological systems. Ecol. Soc. 2018, 23, 25. [Google Scholar] [CrossRef]
- HJ 535-2009; Water Quality―Determination of Ammonia Nitrogen―Nessler’s Reagent Spectrophotometry. The Ministry of Ecology and Environment: Beijing, China, 2009.
- GB 7493-87; Water Quality-Determination of Nitrogen (Nitrite)-Spectrophotometric Method. The Ministry of Ecology and Environment: Beijing, China, 1987.
- HJ 1226-2021; Water Quality—Determination of Sulfide—Methylene Blue Spectrophotometric Method. The Ministry of Ecology and Environment: Beijing, China, 2021.
- GB 7466-87; Water Quality-Determination of Total Chromiun. The Ministry of Ecology and Environment: Beijing, China, 1987.
- HJ 485-2009; Water Quality―Determination of Copper―Sodium Diethydlthiocabamate Spectrophotometric Method. The Ministry of Ecology and Environment: Beijing, China, 2009.
- HJ 669-2013; Water Quality-Determination of Phosphate-Ion Chromatography. The Ministry of Ecology and Environment: Beijing, China, 2013.
- Lie, A.A.Y.; Liu, Z.F.; Hu, S.K.; Jones, A.C.; Kim, D.Y.; Countway, P.D.; Amaral-Zettler, L.A.; Cary, S.C.; Sherr, E.B.; Sherr, B.F.; et al. Investigating Microbial Eukaryotic Diversity from a Global Census: Insights from a Comparison of Pyrotag and Full-Length Sequences of 18S rRNA Genes. Appl. Environ. Microbiol. 2014, 80, 4363–4373. [Google Scholar] [CrossRef]
- Tarigan, N.B.; Amal, M., Jr.; Ekasari, J.; Keesman, K.J.; Verdegem, M. Nitrogen, phosphorus, and carbon dynamics in biofloc system of Nile tilapia fed with high non-starch polysaccharides diet. Aquaculture 2025, 596, 741714. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.L.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.X.; Zhou, A.G. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Xiao, R.C.; Wei, Y.G.; An, D.; Li, D.L.; Ta, X.X.; Wu, Y.H.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Azevedo, A.C.B.; Bozza, D.A.; Doria, H.B.; Osorio, F.H.T.; Corcini, C.D.; Pereira, F.A.; Varela, A.S.; Esquivel, L.; Silva, C.P.; Campos, S.X.; et al. Low levels of inorganic copper impair reproduction parameters in Oreochromis niloticus after chronic exposure. Aquaculture 2021, 545, 737186. [Google Scholar] [CrossRef]
- Velma, V.; Tchounwou, P.B. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, carassius auratus. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 698, 43–51. [Google Scholar] [CrossRef]
- Suchana, S.A.; Ahmed, M.S.; Islam, S.M.M.; Rahman, M.L.; Rohani, M.F.; Ferdusi, T.; Ahmmad, A.K.S.; Fatema, M.K.; Badruzzaman, M.; Shahjahan, M. Chromium Exposure Causes Structural Aberrations of Erythrocytes, Gills, Liver, Kidney, and Genetic Damage in Striped Catfish Pangasianodon hypophthalmus. Biol. Trace Elem. Res. 2021, 199, 3869–3885. [Google Scholar] [CrossRef]
- Chew, S.F.; Ip, Y.K. Excretory nitrogen metabolism and defence against ammonia toxicity in air-breathing fishes. J. Fish Biol. 2014, 84, 603–638. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.J.; Jiang, C.C.; Peng, X.W.; Zhou, X.D.; Sun, Q.; Zhu, L.F.; Xie, X.M.; Zhuang, X.L. Improvement of the growth performance, intestinal health, and water quality in juvenile crucian carp (Carassius auratus gibelio) biofortified system with the bacteria-microalgae association. Aquaculture 2023, 562, 738848. [Google Scholar] [CrossRef]
- Luo, G.Z.; Xu, J.X.; Meng, H.Y. Nitrate accumulation in biofloc aquaculture systems. Aquaculture 2020, 520, 734675. [Google Scholar] [CrossRef]
- Azhar, M.H.; Suciyono, S.; Budi, D.S.; Ulkhaq, M.F.; Anugrahwati, M.; Ekasari, J. Biofloc-based co-culture systems of Nile tilapia (Oreochromis niloticus) and redclaw crayfish (Cherax quadricarinatus) with different carbon-nitrogen ratios. Aquac. Int. 2020, 28, 1293–1304. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Ecology—Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Dee, L.E.; Ferraro, P.J.; Severen, C.N.; Kimmel, K.A.; Borer, E.T.; Byrnes, J.E.K.; Clark, A.T.; Hautier, Y.; Hector, A.; Raynaud, X.; et al. Clarifying the effect of biodiversity on productivity in natural ecosystems with longitudinal data and methods for causal inference. Nat. Commun. 2023, 14, 12. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Powell, J.T.; Banack, S.A.; Cox, P.A.; Ladjimi, M.; Sultan, A.A.; Chemaitelly, H.; Richer, R.A. Cyanotoxin accumulation and growth patterns of biocrust communities under variable environmental conditions. Toxicon-X 2024, 23, 100199. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Li, S.G.; Gao, Y.C.; Chen, Y.Y.; Zhan, A.B. Environment-driven geographical distribution of bacterial communities and identification of indicator taxa in Songhua River. Ecol. Indic. 2019, 101, 62–70. [Google Scholar] [CrossRef]
- Li, J.; Luo, H.B.; Lai, J.D.; Zhang, R. Effects of Biodiversity and Its Interactions on Ecosystem Multifunctionality. Forests 2024, 15, 1701. [Google Scholar] [CrossRef]
- Zhou, M.; Wan, Q.Y.; Babu, V.S.; Qiu, Q.J.; Kou, H.Y.; Lin, C.; Zhao, L.J.; Yang, L.; Li, J.; Huang, Y.M.; et al. Bacterial features in tilapia (Oreochromis niloticus) and environments in a goose-tilapia polyculture model. Aquaculture 2018, 497, 313–319. [Google Scholar] [CrossRef]
- Fan, L.M.; Barry, K.; Shi, L.L.; Song, C.; Meng, S.L.; Qiu, L.P.; Hu, G.D.; Zheng, Y.; Li, F.J.; Chen, J.Z.; et al. Archaeal community compositions in tilapia pond systems and their influencing factors. World J. Microbiol. Biotechnol. 2018, 34, 43. [Google Scholar] [CrossRef]
- Lian, Y.L.; Zheng, X.F.; Xie, S.Q.; Dan, A.; Wang, J.; Tang, J.Y.; Zhu, X.; Shi, B.J. Microbiota composition and correlations with environmental factors in grass carp (Ctenopharyngodon idella) culture ponds in South China. PeerJ 2023, 11, e15892. [Google Scholar] [CrossRef]
- Ruprecht, J.E.; Birrer, S.C.; Dafforn, K.A.; Mitrovic, S.M.; Crane, S.L.; Johnston, E.L.; Wemheuer, F.; Navarro, A.; Harrison, A.J.; Turner, I.L.; et al. Wastewater effluents cause microbial community shifts and change trophic status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef]
- Hugoni, M.; Vellet, A.; Debroas, D. Unique and highly variable bacterial communities inhabiting the surface microlayer of an oligotrophic lake. Aquat. Microb. Ecol. 2017, 79, 115–125. [Google Scholar] [CrossRef]
- Reis, M.P.; Suhadolnik, M.L.S.; Dias, M.F.; Avila, M.P.; Motta, A.M.; Barbosa, F.A.R.; Nascimento, A.M.A. Characterizing a riverine microbiome impacted by extreme disturbance caused by a mining sludge tsunami. Chemosphere 2020, 253, 126584. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, M.M.; Li, X.C.; Lu, W.X.; Li, J. River bacterial community structure and co-occurrence patterns under the influence of different domestic sewage types. J. Environ. Manag. 2020, 266, 110590. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Sayed, A.E.H.; El-Sayed, Y.S.; El-Far, A.H. Hepatoprotective efficacy of Spirulina platensis against lead-induced oxidative stress and genotoxicity in catfish; Clarias gariepinus. Ecotoxicol. Environ. Saf. 2017, 143, 344–350. [Google Scholar] [CrossRef]
- Toughan, H.; Khalil, S.R.; El-Ghoneimy, A.A.; Awad, A.; Seddek, A.S. Effect of dietary supplementation with Spirulina platensis on Atrazine-induced oxidative stress-mediated hepatic damage and inflammation in the common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2018, 149, 135–142. [Google Scholar] [CrossRef]
- Qu, F.; Wang, Y.T.; Yu, D.; Chen, N.W. High-frequency monitoring reveals phytoplankton succession patterns and the role of cryptophyte in a subtropical river reservoir. Algal Res. 2024, 82, 103680. [Google Scholar] [CrossRef]
- Du, C.L.; Li, G.W.; Xia, R.; Li, C.L.; Zhu, Q.H.; Li, X.G.; Li, J.X.; Zhao, C.; Tian, Z.J.; Zhang, L.Y. New insights into cyanobacterial blooms and the response of associated microbial communities in freshwater ecosystems. Environ. Pollut. 2022, 309, 119781. [Google Scholar] [CrossRef]
- Foysal, M.J.; Timms, V.; Neilan, B.A. Dynamics of the benthic and planktic microbiomes in a Planktothrix-dominated toxic cyanobacterial bloom in Australia. Water Res. 2024, 249, 120980. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Lin, J.J.; Jiang, J.A.; Hu, S.S.; Kang, C.K.; Xu, N.J.; Li, Y.H. Environmental history affects the growth and photosynthesis of a green-tide macroalgae Ulva prolifera. Aquac. Res. 2022, 53, 2509–2517. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zheng, M.S.; Jiang, J.A.; Hu, W.; Xu, N.J.; Li, Y.H. Enhancement of growth in Ulva prolifera by diurnal temperature difference combined with nitrogen enrichment. Mar. Environ. Res. 2023, 186, 105905. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Gong, J.C.; Li, C.X.; Liu, T.; Hu, J.W.; Li, P.F.; Liu, C.Y.; Yang, G.P. Regulation of seawater dissolved carbon pools by environmental changes in Ulva prolifera originating sites: A new perspective on the contribution of U. prolifera to the seawater carbon sink function. Environ. Pollut. 2024, 360, 124679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, J.; Luo, L.; Yu, Y.; Yan, J.; Sun, C.; Miao, X.; Li, W. Regime Shifts in Microbial and Water Quality Dynamics in Red Tilapia Ponds. Microorganisms 2025, 13, 1553. https://doi.org/10.3390/microorganisms13071553
Liu Z, Li J, Luo L, Yu Y, Yan J, Sun C, Miao X, Li W. Regime Shifts in Microbial and Water Quality Dynamics in Red Tilapia Ponds. Microorganisms. 2025; 13(7):1553. https://doi.org/10.3390/microorganisms13071553
Chicago/Turabian StyleLiu, Ziyan, Jiaqi Li, Lei Luo, Yang Yu, Jianing Yan, Caiyun Sun, Xiangjun Miao, and Wensheng Li. 2025. "Regime Shifts in Microbial and Water Quality Dynamics in Red Tilapia Ponds" Microorganisms 13, no. 7: 1553. https://doi.org/10.3390/microorganisms13071553
APA StyleLiu, Z., Li, J., Luo, L., Yu, Y., Yan, J., Sun, C., Miao, X., & Li, W. (2025). Regime Shifts in Microbial and Water Quality Dynamics in Red Tilapia Ponds. Microorganisms, 13(7), 1553. https://doi.org/10.3390/microorganisms13071553






