Comparative Effects of the Single and Binary Fermentations of Latilactobacillus sakei and Staphylococcus carnosus on the Growth and Metabolomic Profiles of Fermented Beef Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Sampling of Fermented Beef Sausages
2.3. Enumeration of LAB and Staphylococci
2.4. pH Analysis
2.5. Sample Preparation
2.6. LC-MS Detection
2.7. Metabolite Identification
2.8. Statistical Analysis
3. Results and Discussion
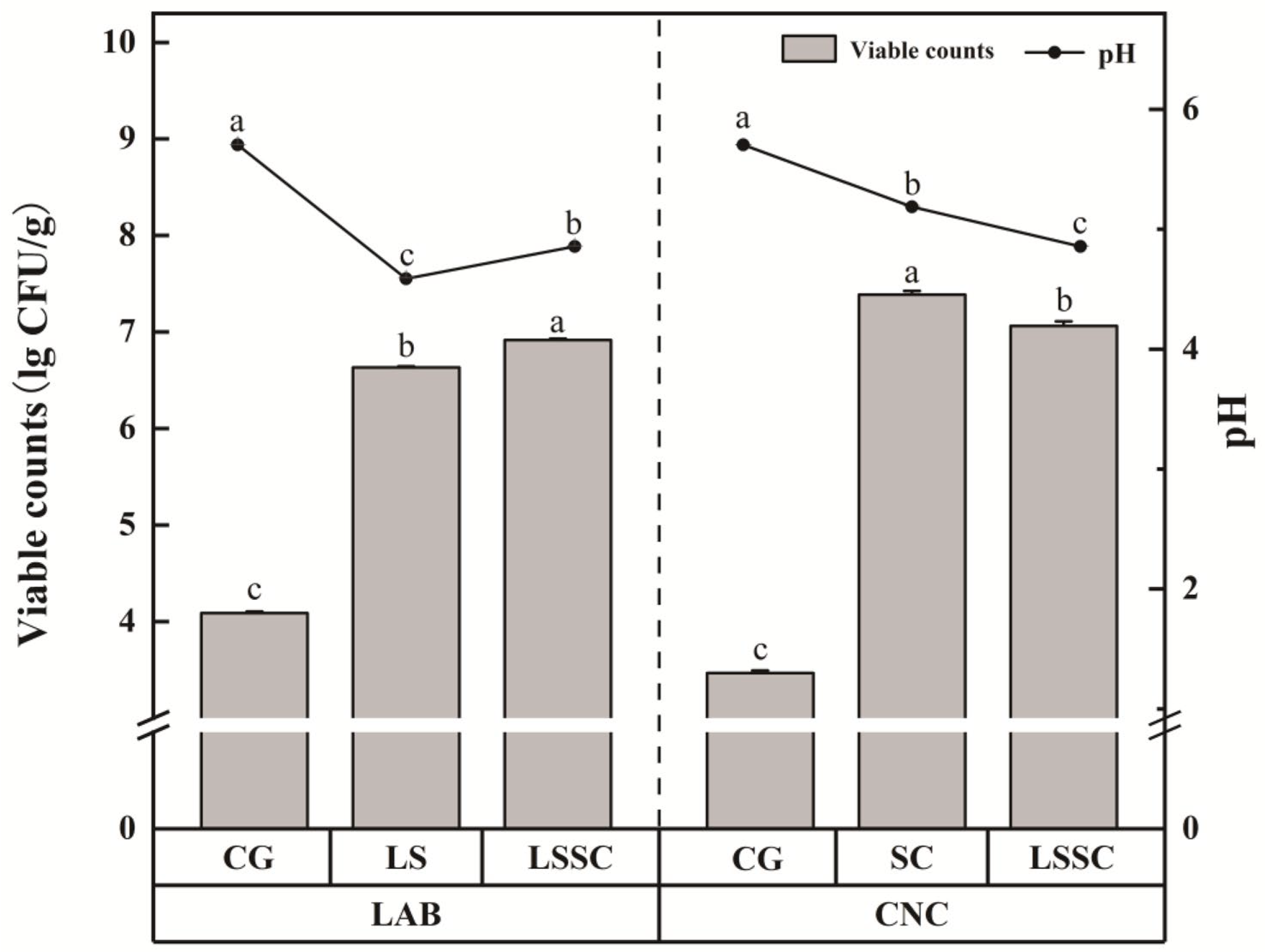
3.1. The Effect of Single and Co-Fermentation of L. sakei and S. carnosus on the pH Value and Viable Cell Count of Fermented Meat Products
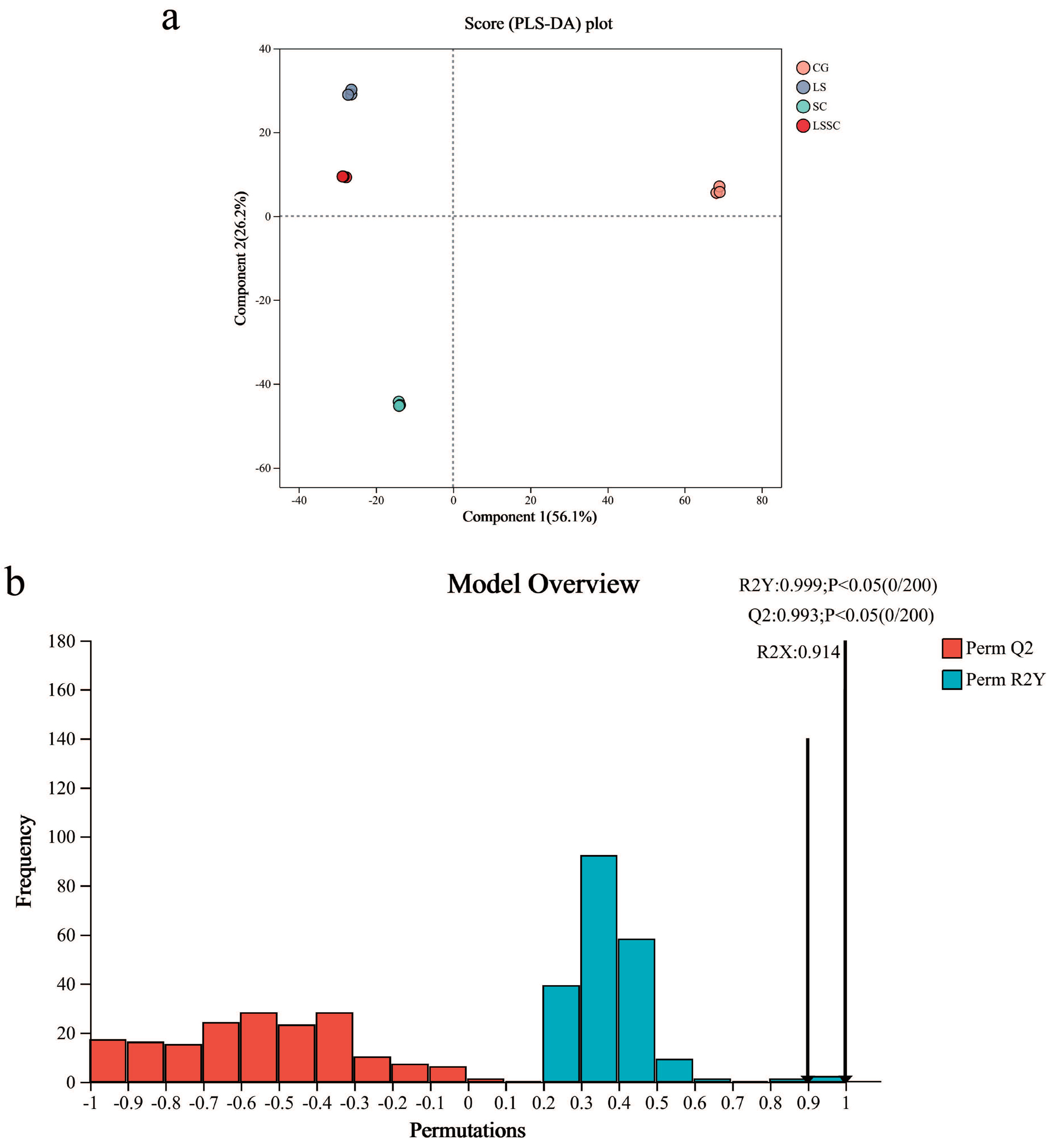
3.2. The Effect of Single and Co-Fermentation of L. sakei and S. carnosus on the Metabolomics of Fermented Meat Products
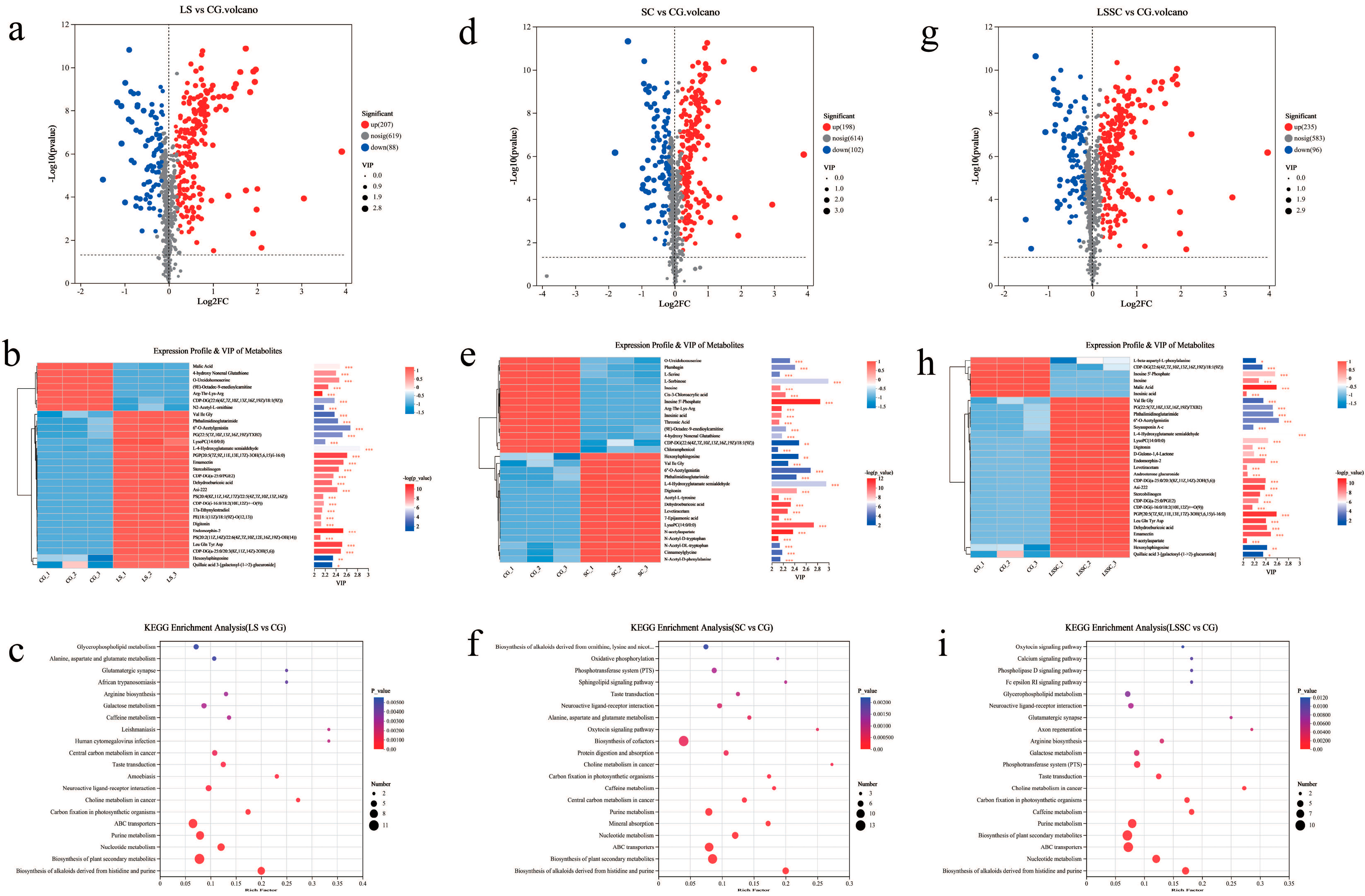
3.3. The Changes in Differential Metabolites During Single and Co-Fermentation of L. sakei and S. carnosus
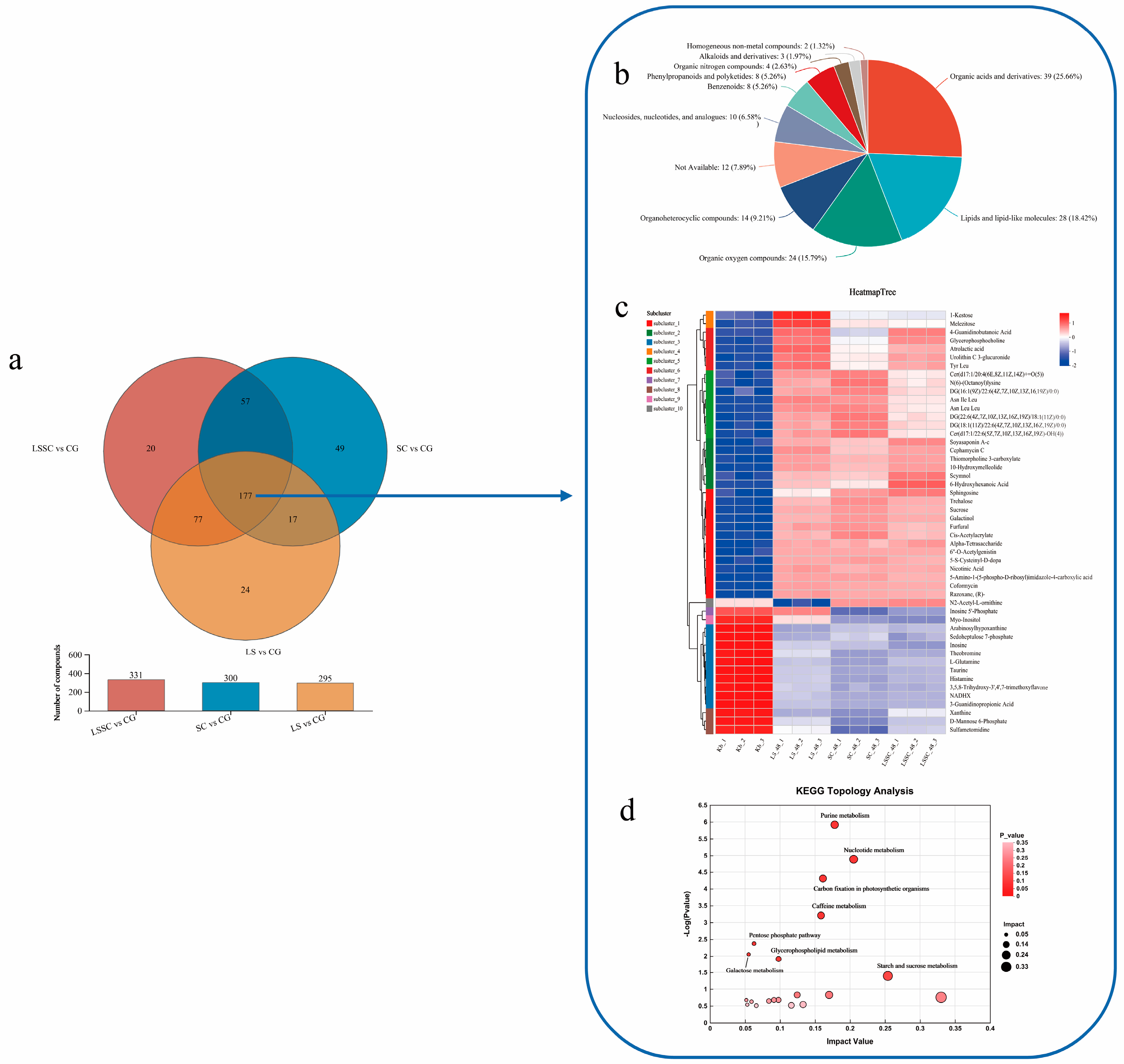
3.4. Consistent Differential Metabolites Identified After the Single and Co-Fermentation of L. sakei and S. carnosus
3.5. Metabolic Pathway Analysis of the Single and Co-Fermentation of L. sakei and S. carnosus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbieri, F.; Tabanelli, G.; Comas-Basté, O.; Latorre-Moratalla, M.; Angelucci, C.; Gardini, F.; Montanari, C.; García-López, J.D.; Baños, A. Improvement of the safety of artisanal Spanish fermented sausages: Spotlight on the role of bacteriocinogenic Lactiplantibacillus paraplantarum against a Companilactobacillus alimentarius histaminogenic strain. Food Control 2025, 168, 110962. [Google Scholar] [CrossRef]
- Gu, Y.; Qiao, R.; Jin, B.; He, Y.; Tian, J.B. Effect of Limosilactobacillus fermentum 332 on physicochemical characteristics, volatile flavor components, and Quorum sensing in fermented sausage. Sci. Rep. 2023, 13, 3942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Yang, W.; Wang, L.; Huang, X.-H.; Qin, L. Comprehensive insights into the mechanism of flavor formation driven via inoculation with mixed starter cultures in dry-fermented tilapia sausages: Integration of macrogenomics, volatilomics, and lipidomics. Food Chem. 2024, 455, 139950. [Google Scholar] [CrossRef]
- Liu, M.; Luo, H.; Xiao, Q.; Chen, C.; Xu, B.; Li, P. Effect of Latilactobacillus sakei and Staphylococcus xylosus on the textural characteristics of dry fermented sausages. Food Biosci. 2024, 59, 103972. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, C.; Hu, Y.; Yang, L.; Xu, B.-C. Enhancement of fermented sausage quality driven by mixed starter cultures: Elucidating the perspective of flavor profile and microbial communities. Food Res. Int. 2024, 178, 113951. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Rebecchi, A.; Dallolio, M.; Braceschi, G.; Domínguez, R.; Dallolio, G.; Trevisan, M.; Lorenzo, J.M.; Lucini, L. Changes in the chemical and sensory profile of ripened Italian salami following the addition of different microbial starters. Meat Sci. 2021, 180, 108584. [Google Scholar] [CrossRef]
- Suh, J. Critical review: Metabolomics in dairy science—Evaluation of milk and milk product quality. Food Res. Int. 2022, 154, 110984. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Ning, J.; Wang, S.; Nie, Q.; Wang, W.; Zhang, J.; Ji, L. Effect of fermentation by Pediococcus pentosaceus and Staphylococcus carnosus on the metabolite profile of sausages. Food Res. Int. 2022, 162, 112096. [Google Scholar] [CrossRef]
- Wu, X.; Pan, D.; Xia, Q.; Sun, Y.; Geng, F.; Cao, J.; Zhou, C. The combination of high-throughput sequencing and LC-MS/MS reveals the mechanism of Staphylococcus inoculation on bacterial community succession and taste development during the processing of dry-cured bacon. J. Sci. Food Agric. 2023, 103, 7187–7198. [Google Scholar] [CrossRef]
- Shao, X.; Wang, H.; Song, X.; Xu, N.; Sun, J.; Xu, X. Effects of different mixed starter cultures on microbial communities, taste and aroma compounds of traditional Chinese fermented sausages. Food Chem. X 2024, 21, 101225. [Google Scholar] [CrossRef]
- Jiang, L.; Mu, Y.; Su, W.; Tian, H.; Zhao, M.; Su, G.; Zhao, C. Effects of Pediococcus acidilactici and Rhizopus oryzae on microbiota and metabolomic profiling in fermented dry-cure mutton sausages. Food Chem. 2023, 403, 134431. [Google Scholar] [CrossRef]
- Widenmann, A.W.; Schiffer, C.J.; Ehrmann, M.A.; Vogel, R.F. Impact of different sugars and glycosyltransferases on the assertiveness of Latilactobacillus sakei in raw sausage fermentations. Int. J. Food Microbiol. 2022, 366, 109575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, S.; Cui, W.; Zhu, C.; Jiao, Y.; Li, J.; Yin, F.; Han, M. Isolation, identification and fermentation properties of lactic acid bacteria from naturally fermented ham in Yunnan. Food Ferment. Ind. 2020, 46, 56–61. [Google Scholar] [CrossRef]
- Najjari, A.; Boumaiza, M.; Jaballah, S.; Boudabous, A.; Ouzari, H.M. Application of isolated Lactobacillus sakei and Staphylococcus xylosus strains as a probiotic starter culture during the industrial manufacture of Tunisian dry-fermented sausages. Food Sci. Nutr. 2020, 8, 4172–4184. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; Myter, N.; Vuyst, L.D.; Leroy, F. Species diversity and metabolic impact of the microbiota are low in spontaneously acidified Belgian sausages with an added starter culture of Staphylococcus carnosus. Food Microbiol. 2012, 29, 167–177. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Zhou, H.; Xu, B. Influence of curing on the metabolite profile of water-boiled salted duck. Food Chem. 2022, 397, 133752. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, Y.; Tan, L.; Yang, L.; Wang, C.; Xu, B. Innovative insights into dry fermented sausages flavor: Unraveling the impact of varied lactobacillus genera-driven fermentation. Food Biosci. 2024, 60, 104331. [Google Scholar] [CrossRef]
- Tremonte, P.; Reale, A.; Renzo, T.D.; Tipaldi, L.; Succi, M. Interactions between Lactobacillus sakei and CNC (Staphylococcus xylosus and Kocuria varians) and their influence on proteolytic activity. Lett. Appl. Microbiol. 2010, 51, 586–594. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Brezesinski, G.; Geue, T.; Fontaine, P. On the Interaction between Digitonin and Cholesterol in Langmuir Monolayers. Langmuir 2016, 32, 9064–9073. [Google Scholar] [CrossRef]
- Mbuyane, L.L.; Bauer, F.F.; Divol, B. The metabolism of lipids in yeasts and applications in oenology. Food Res. Int. 2021, 141, 110142. [Google Scholar] [CrossRef]
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Lei, H. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar] [CrossRef]
- Tseng, L.F. Endomorphin-1 and Endomorphin-2: Involvement of Endogenous μ-Opioid Receptor Ligands in Analgesia, Antinociceptive Tolerance, Antianalgesia, and Hyperalgesia. J. Exp. Clin. Med. 2014, 6, 151–156. [Google Scholar] [CrossRef]
- Zadina, J.E. Isolation and distribution of endomorphins in the central nervous system. Jpn J. Pharmacol. 2002, 89, 203–208. [Google Scholar] [CrossRef]
- Haustein, K.O. Therapeutic range of cardiac glycosides. Basic Res. Cardiol. 1984, 79, 147–153. [Google Scholar] [CrossRef]
- Sirianni, A.C.; Jiang, J.; Zeng, J.; Mao, L.L.; Zhou, S.; Sugarbaker, P.; Zhang, X.; Li, W.; Friedlander, R.M.; Wang, X. N-acetyl-l-tryptophan, but not N-acetyl-d-tryptophan, rescues neuronal cell death in models of amyotrophic lateral sclerosis. J. Neurochem. 2015, 134, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, L.; Klomp, L.W.; Berger, R.; de Koning, T.J. L-Serine synthesis in the central nervous system: A review on serine deficiency disorders. Mol. Genet. Metab. 2010, 99, 256–262. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, X.; Xiao, W.; Shi, J.; Xu, Z. Production of L-serine and its derivative L-cysteine from renewable feedstocks using Corynebacterium glutamicum: Advances and perspectives. Crit. Rev. Biotechnol. 2024, 44, 448–461. [Google Scholar] [CrossRef]
- Li, C.Y.; Basit, A.; Gupta, A.; Gáborik, Z.; Kis, E.; Prasad, B. Major glucuronide metabolites of testosterone are primarily transported by MRP2 and MRP3 in human liver, intestine and kidney. J. Steroid Biochem. Mol. Biol 2019, 191, 105350. [Google Scholar] [CrossRef]
- Hajkó, J.; Lipták, A.; Pozsgay, V. Synthesis of L-glucose from D-gulono-1,4-lactone. Carbohydr. Res. 1999, 321, 116–120. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Kano, H.S.C.; Yamada, N.; Fukuuchi, T.; Yamaoka, N.; Kaneko, K.; Asami, Y. Species-dependent patterns of incorporation of purine mononucleotides and nucleosides by lactic acid bacteria. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Chang, J.Y.; Lee, J.H. Arginine metabolism and the role of arginine deiminase-producing microorganisms in kimchi fermentation. Heliyon 2022, 8, 11802. [Google Scholar] [CrossRef]
- Chen, S.; Inui, S.; Aisyah, R.; Nakashima, R.; Kawaguchi, T.; Hinomoto, M.; Nakagawa, Y.; Sakuma, T.; Sotomaru, Y.; Ohshima, N.; et al. Role of Gpcpd1 in intestinal alpha-glycerophosphocholine metabolism and trimethylamine N-oxide production. J. Biol. Chem. 2024, 300, 107965. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.-I.; Bott, M. Microbial synthesis of health-promoting inositols. Curr. Opin. Biotechnol. 2024, 87, 103114. [Google Scholar] [CrossRef]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef]
- Zhan, Q.; Thakur, K.; Feng, J.Y.; Zhu, Y.Y.; Zhang, J.G.; Wei, Z.J. LC–MS based metabolomics analysis of okara fermented by Bacillus subtilis DC-15: Insights into nutritional and functional profile. Food Chem. 2023, 413, 135656. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zheng, Y.; Cui, W.; Bai, X.; Zhu, C.; Zhao, G. Comparative Effects of the Single and Binary Fermentations of Latilactobacillus sakei and Staphylococcus carnosus on the Growth and Metabolomic Profiles of Fermented Beef Sausages. Microorganisms 2025, 13, 1523. https://doi.org/10.3390/microorganisms13071523
Li X, Zheng Y, Cui W, Bai X, Zhu C, Zhao G. Comparative Effects of the Single and Binary Fermentations of Latilactobacillus sakei and Staphylococcus carnosus on the Growth and Metabolomic Profiles of Fermented Beef Sausages. Microorganisms. 2025; 13(7):1523. https://doi.org/10.3390/microorganisms13071523
Chicago/Turabian StyleLi, Xuan, Yangyi Zheng, Wenming Cui, Xueyuan Bai, Chaozhi Zhu, and Gaiming Zhao. 2025. "Comparative Effects of the Single and Binary Fermentations of Latilactobacillus sakei and Staphylococcus carnosus on the Growth and Metabolomic Profiles of Fermented Beef Sausages" Microorganisms 13, no. 7: 1523. https://doi.org/10.3390/microorganisms13071523
APA StyleLi, X., Zheng, Y., Cui, W., Bai, X., Zhu, C., & Zhao, G. (2025). Comparative Effects of the Single and Binary Fermentations of Latilactobacillus sakei and Staphylococcus carnosus on the Growth and Metabolomic Profiles of Fermented Beef Sausages. Microorganisms, 13(7), 1523. https://doi.org/10.3390/microorganisms13071523





