Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond
Abstract
1. Introduction
2. Major Tick-Borne Viruses Affecting Africa: CCHFV, NSDV, and ASFV
2.1. Orthonairovirus Haemorrhagiae (CCHFV)
2.2. Orthonairovirus Nairobiense (NSDV)
2.3. African Swine Fever Virus (ASFV)
3. Climate Change and Tick Distribution in Africa
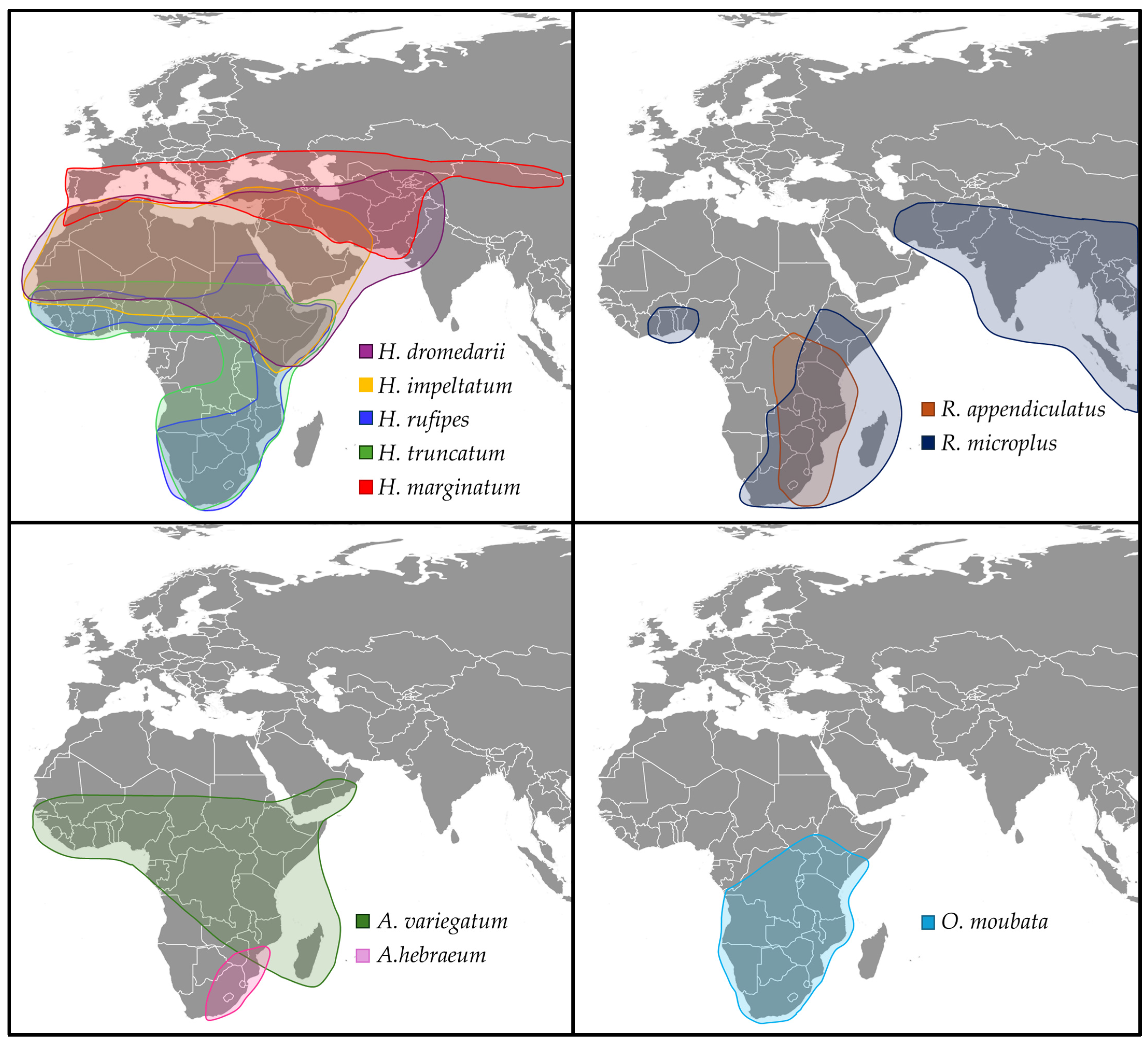
3.1. Relevant Tick Species from the African Continent
3.2. Shifts in Tick Habitats, Behavior, and Seasonal Disease Risks
3.3. Environmental Conditions Favoring Tick Expansion
3.4. Future Research and Disease Control Strategies
| Tick Species | Genus | Climate Impact | Region(s) Affected | Notes | References |
|---|---|---|---|---|---|
| R. appendiculatus | Rhipicephalus | Habitat suitability reduced with +2 °C warming. Expansion into new areas with shifting rainfall patterns. | South Africa, Northern Ethiopia | Vulnerable to rising temperatures; expanded range in some regions. | [56] |
| R. microplus | Rhipicephalus | Expanding range due to livestock movement and warming; replacing native R. decoloratus. | Tanzania, Cameroon, sub-Saharan Africa | Invasive; thrives in warmer lowlands. | [59,60,76] |
| R. decoloratus | Rhipicephalus | Retreating to highland areas with cooler temperatures. | Tanzania | Losing ground to R. microplus. | [60] |
| A. hebraeum | Amblyomma | Predicted 13% decrease in habitat by 2050 due to temperature and rainfall shifts. | Zimbabwe (Mashonaland Central), South Africa | Sensitive to climate variables. | [57] |
| A. variegatum | Amblyomma | Expanding due to animal movement and land use changes. | Multiple African countries | Climate-tolerant; generalist feeder. | [59] |
| H. truncatum | Hyalomma | Habitat suitability is decreasing in warming regions. | South Africa | Two larval peaks; impacted by temperature. | [62] |
| H. rufipes | Hyalomma | Seasonal activity altered; more generations per year possible. | Eastern Cape Province, South Africa | Adults peak Sept–March; larvae July–Nov. | [62] |
| H. marginatum | Hyalomma | Expanding into Europe due to warmer, drier summers and moderate autumn rains. | Spain, France (Var, Ardèche, Pyrénées-Orientales) | CCHFV risk in the Mediterranean Basin. | [65,77] |
| O. moubata | Ornithodoros | Expected to expand under warming; altered feeding rates and reproduction. | Eastern and Southern Africa | Soft tick; vector of ASFV and relapsing fever. | [55] |
4. Impact of Climate Change on Tick-Borne Viral Transmission in Africa and Beyond
4.1. The Role of Climate in Tick-Borne Virus Transmission
4.2. The Growing Threat of CCHFV
4.3. The Expanding Risk of CCHFV: From Africa to Emerging Hotspots in Europe
4.4. Climate Change and the Role of Migratory Birds in CCHFV Spread
4.5. Assessing the Risk of CCHFV Introduction in CCHF-Free Countries
- Infected tick vectors;
- Wildlife reservoirs;
- Livestock movement.
4.6. Challenges and Future Predictions for CCHFV Expansion
4.7. NSDV, ASFV, and the Future of Tick-Borne Viral Infections
| Tick Species | Genus | Transmitted Virus(es) | Countries/Regions Detected | Notes | References |
|---|---|---|---|---|---|
| Hyalomma spp. | Hyalomma | CCHFV | Nigeria, Senegal, Ethiopia, Kenya, South Africa, Uganda | Primary vector of CCHFV in Africa. | [49,77] |
| H. marginatum | Hyalomma | CCHFV | Spain, France, Mediterranean Basin | Expanding into Europe due to warming climate. | [50] |
| H. rufipes | Hyalomma | CCHFV | South Africa, other Sahel/northern African regions | Seasonally active; competent vector. | [103] |
| H. truncatum | Hyalomma | CCHFV | Zimbabwe, South Africa | Two larval peaks per year. | [61,74] |
| H. impeltatum | Hyalomma | CCHFV | Ethiopia | Experimental transmission confirmed. | [54] |
| H. dromedarii | Hyalomma | CCHFV | Northern Africa, Sahel | Common on camels. | [74] |
| R. appendiculatus | Rhipicephalus | NSDV, Theileria parva, CCHFV | Kenya, East Africa, Uganda | Major vector in East Africa. | [31,104] |
| R. microplus | Rhipicephalus | Babesia spp., possibly CCHFV | Tanzania, Cameroon, sub-Saharan Africa, Mexico, parts of Asia, South and Central America | Invasive; spreading via livestock trade. | [44,76] |
| R. decoloratus | Rhipicephalus | Possibly CCHFV | Kenya, Tanzania | Replaced by R. microplus in warmer lowlands. | [60,105] |
| A.variegatum | Amblyomma | Ehrlichia ruminantium, NSDV | Sub-Saharan Africa | Affects cattle and small ruminants. | [104,106] |
| A. hebraeum | Amblyomma | Ehrlichia ruminantium, NSDV | South Africa (e.g., Eastern Cape, Limpopo, KwaZulu-Natal) | Peak in summer; key heartwater vector. | [46,47] |
| O. moubata | Ornithodoros | ASFV, Borrelia duttonii | Eastern and Southern Africa, Malawi | Found in traditional dwellings. Soft tick vector. | [36,55] |
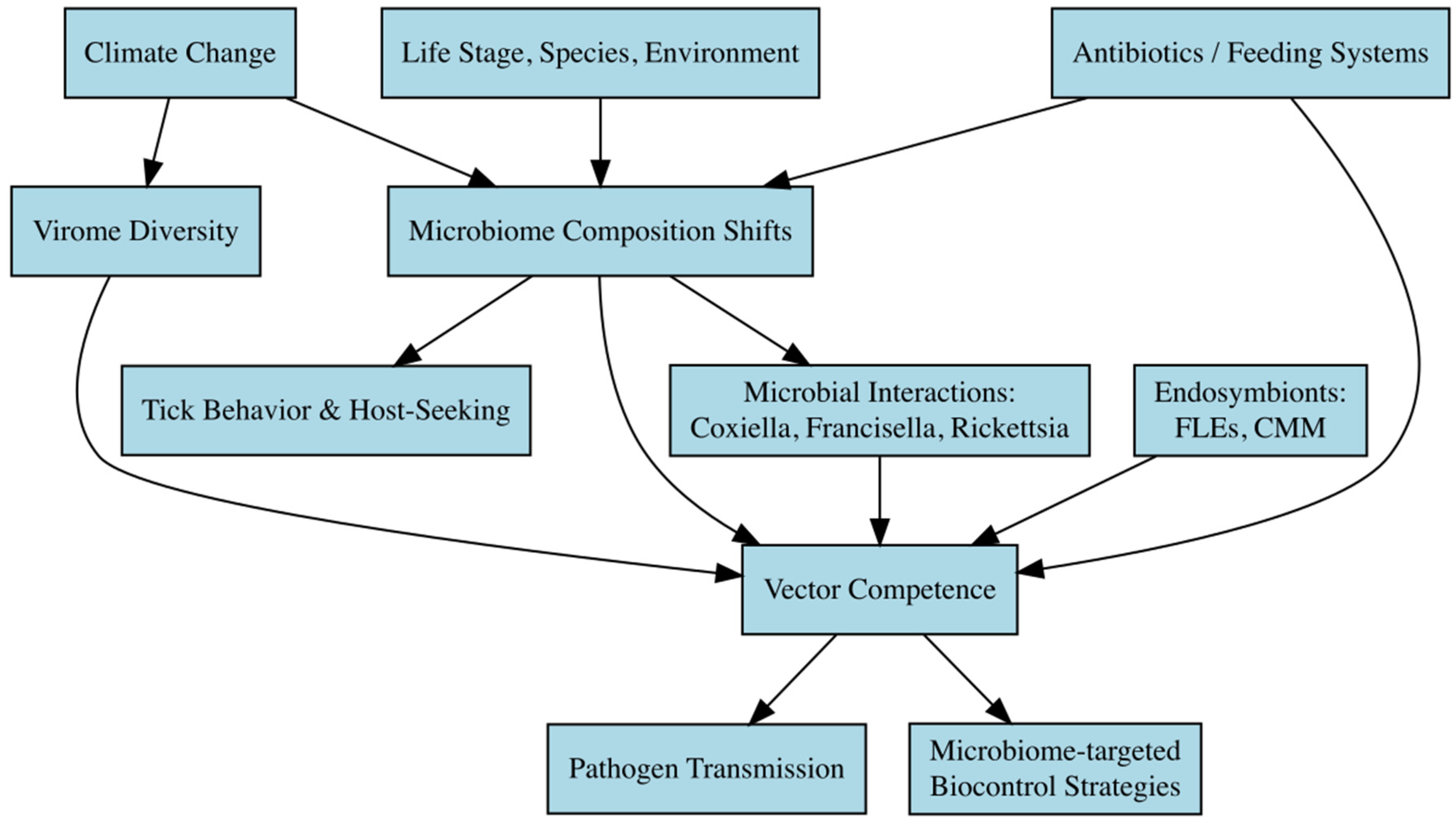
5. Microbiome Shifts and Vector Competence
5.1. Climate Change, Microbiome Composition, and Tick Behavior
5.2. Key Microbial Players and Their Interactions in Ticks
5.3. Microbiome Diversity and Vector Competence
5.4. The Impact of Climate on Tick Virome Diversity
5.5. The Role of Antibiotics and Microbiome Perturbation in Vector Competence
6. Immune Mechanisms Driving Vector Competence in Ticks
6.1. Innate Immune Pathways in Ticks and Their Role in Vector Competence
6.2. Tick Immune Interactions with Viruses in a Changing Climate: Insights from ASFV
7. Strategies for Monitoring and Mitigating Emerging Tick-Borne Viral Threats in Africa
7.1. Strengthening Surveillance and One Health Approaches
7.2. Developing Alternative Tick Control Strategies
7.3. Antiviral Control Strategies
7.4. Enhancing Healthcare Infrastructure and International Cooperation
8. Conclusions
- Strengthening surveillance by scaling up molecular tick identification and TBV monitoring across diverse ecological zones;
- Adopting a One Health approach to integrating human, animal, and environmental health responses in TBV control strategies;
- Investing in vaccine development and prioritizing scalable and broadly effective candidates for TBVs, particularly for high-risk livestock populations;
- Promoting innovative control strategies, such as anti-tick vaccines and microbiome-targeted interventions, as sustainable alternatives to chemical acaricides;
- Enhancing healthcare infrastructure and diagnostics, especially in high-burden, low-resource settings, to improve outbreak response and clinical outcomes;
- Facilitating international collaboration, data sharing, and standardized protocols to track and manage the transboundary spread of TBVs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A. | Amblyomma |
| AMP | antimicrobial peptide |
| ASFV | African swine fever virus |
| CCHF | Crimean–Congo hemorrhagic fever |
| CCHFV | Orthonairovirus haemorrhagiae (Crimean–Congo hemorrhagic fever virus) |
| CMM | Candidatus Midichloria mitochondrii |
| H. | Hyalomma |
| I. | Ixodes |
| IMD | immune deficiency |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| MDPI | Multidisciplinary Digital Publishing Institute |
| NSDV | Orthonairovirus nairobiense (Nairobi sheep disease orthonairovirus) |
| O. | Ornithodoros |
| R. | Rhipicephalus |
| RNAi | RNA interference |
| ROS | reactive oxygen species |
| sfRNAs | subgenomic flavivirus RNAs |
| siRNAs | small interfering RNAs |
| TBV | Tick-borne virus |
References
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.; Schmidt, K.; Ozkul, A.; Groschup, M.H. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antivir. Res. 2013, 98, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Tsergouli, K.; Tsioka, K.; Mirazimi, A. Crimean-Congo Hemorrhagic Fever: Tick-Host-Virus Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 213. [Google Scholar] [CrossRef]
- Lizinfeld, I.; Pshenichnaya, N.; Parolina, L. A comprehensive analysis of climate change impact on crimean-congo hemorrhagic fever spread (Russia, Kazakhstan, Turkey and Iran from 1999 to 2022). Int. J. Infect. Dis. 2025, 152, 107465. [Google Scholar] [CrossRef]
- Chanda, M.M.; Kharkwal, P.; Dhuria, M.; Prajapathi, A.; Yogisharadhya, R.; Shome, B.R.; Shivachandra, S.B. Quantifying the influence of climate, host and change in land-use patterns on occurrence of Crimean Congo Hemorrhagic Fever (CCHF) and development of spatial risk map for India. One Health 2023, 17, 100609. [Google Scholar] [CrossRef]
- Léger, E.; Vourc’h, G.; Vial, L.; Chevillon, C.; McCoy, K.D. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 2013, 59, 219–244. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; Venzal, J.M. Climate niches of tick species in the Mediterranean region: Modeling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2007, 44, 1130–1138. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef]
- Olwoch, J.M.; Van Jaarsveld, A.S.; Scholtz, C.H.; Horak, I.G. Climate change and the genus Rhipicephalus (Acari: Ixodidae) in Africa. Onderstepoort J. Vet. Res. 2007, 74, 45–72. [Google Scholar] [CrossRef]
- Nuttall, P.A. Climate change impacts on ticks and tick-borne infections. Biologia 2022, 77, 1503–1512. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef] [PubMed]
- Lontsi-Demano, M.; Ngnindji-Youdje, Y.; Laroche, M.; Bamou, R.; Talom, A.D.; Abah, S.; Fopa, F.; Mamoudou, A.; Tchuinkam, T. Cattle trading favors the introduction and establishment of the invasive tick Rhipicephalus (Boophilus) microplus in Menoua Division, West Region of Cameroon. J. Entomol. Zool. Stud. 2020, 8, 207–214. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R. Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Pollet, T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol. 2021, 43, e12813. [Google Scholar] [CrossRef]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Wong, G.; Babuadze, G.; Camp, J.V.; Ergonul, O.; Kobinger, G.P.; Chinikar, S.; Nowotny, N. Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe. Microorganisms 2021, 9, 1907. [Google Scholar] [CrossRef]
- Eslava, M.; Carlos, S.; Reina, G. Crimean-Congo Hemorrhagic Fever Virus: An Emerging Threat in Europe with a Focus on Epidemiology in Spain. Pathogens 2024, 13, 770. [Google Scholar] [CrossRef]
- de la Fuente, J. Controlling ticks and tick-borne diseases… looking forward. Ticks Tick-Borne Dis. 2018, 9, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.J.; Johnson, N.; Mansfield, K.L. Vectorial dynamics underpinning current and future tick-borne virus emergence in Europe. J. Gen. Virol. 2024, 105, 002041. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Celina, S.S.; Italiya, J.; Tekkara, A.O.; Černý, J. Crimean-Congo haemorrhagic fever virus in ticks, domestic, and wild animals. Front. Vet. Sci. 2024, 11, 1513123. [Google Scholar] [CrossRef]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Spengler, J.R.; Estrada-Peña, A.; Garrison, A.R.; Schmaljohn, C.; Spiropoulou, C.F.; Bergeron, É.; Bente, D.A. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antivir. Res. 2016, 135, 31–47. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papa, A. Crimean-Congo hemorrhagic fever: Risk for emergence of new endemic foci in Europe? Travel. Med. Infect. Dis. 2010, 8, 139–143. [Google Scholar] [CrossRef]
- Mild, M.; Simon, M.; Albert, J.; Mirazimi, A. Towards an understanding of the migration of Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2010, 91, 199–207. [Google Scholar] [CrossRef]
- Baron, M.; Holzer, B. Nairobi sheep disease virus/Ganjam virus. Rev. Sci. Tech. Off. Int. Des. Épizoot. 2015, 34, 411–417. [Google Scholar] [CrossRef]
- Krasteva, S.; Jara, M.; Frias-De-Diego, A.; Machado, G. Nairobi sheep disease virus: A historical and epidemiological perspective. Front. Vet. Sci. 2020, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Wang, H.; Cha, J.; Wang, X. The Geographical Coexist of the Migratory Birds, Ticks, and Nairobi Sheep Disease Virus May Potentially Contribute to the Passive Spreading of Nairobi Sheep Disease. Transbound. Emerg. Dis. 2023, 2023, 5598142. [Google Scholar] [CrossRef]
- Tulman, E.R.; Delhon, G.A.; Ku, B.K.; Rock, D.L. African Swine Fever Virus. In Lesser Known Large dsDNA Viruses; Van Etten, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 43–87. [Google Scholar]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Anholt, H.; Hillman, V.; Vaughan, J.; Smyth, N. The soft tick ornithodoros moubata and its role in the epidemiology of African swine fever in Central Malawi. J. Wildl. Dis. 2023, 59, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; de la Torre, A.; Sánchez-Vizcaíno, J.; Bellini, S. 10. Biosecurity measures against African swine fever in domestic pigs. In Understanding and Combatting African Swine Fever; Wageningen Academic: Wageningen, The Netherlands, 2021; pp. 263–281. [Google Scholar]
- Brookes, V.J.; Barrett, T.E.; Ward, M.P.; Roby, J.A.; Hernandez-Jover, M.; Cross, E.M.; Donnelly, C.M.; Barnes, T.S.; Wilson, C.S.; Khalfan, S. A scoping review of African swine fever virus spread between domestic and free-living pigs. Transbound. Emerg. Dis. 2021, 68, 2643–2656. [Google Scholar] [CrossRef]
- Li, M.; Zheng, H. Insights and progress on epidemic characteristics, pathogenesis, and preventive measures of African swine fever virus: A review. Virulence 2025, 16, 2457949. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Brites-Neto, J.; Duarte, K.M.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301–315. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Ixodid and Argasid Ticks. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 1049–1063. [Google Scholar]
- Onyiche, T.E.; MacLeod, E.T. Hard ticks (Acari: Ixodidae) and tick-borne diseases of sheep and goats in Africa: A review. Ticks Tick-Borne Dis. 2023, 14, 102232. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Olds, C.L.; Mason, K.L.; Scoles, G.A. Rhipicephalus appendiculatus ticks transmit Theileria parva from persistently infected cattle in the absence of detectable parasitemia: Implications for East Coast fever epidemiology. Parasites Vectors 2018, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Mnisi, S.S.; Mphuthi, M.B.; Ramatla, T.; Mofokeng, L.S.; Thekisoe, O.; Syakalima, M. Molecular detection and genetic characterization of Ehrlichia ruminantium harbored by Amblyomma hebraeum ticks of domestic ruminants in North West Province, South Africa. Animals 2022, 12, 2511. [Google Scholar] [CrossRef]
- Meyer, D.F.; Moumène, A.; Rodrigues, V. Microbe Profile: Ehrlichia ruminantium–stealthy as it goes. Microbiology 2023, 169, 001415. [Google Scholar] [CrossRef]
- Sajid, M.; Kausar, A.; Iqbal, A.; Abbas, H.; Iqbal, Z.; Jones, M. An insight into the ecobiology, vector significance and control of Hyalomma ticks (Acari: Ixodidae): A review. Acta Trop. 2018, 187, 229–239. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Gargili, A.; Thangamani, S.; Bente, D. Influence of laboratory animal hosts on the life cycle of Hyalomma marginatum and implications for an in vivo transmission model for Crimean-Congo hemorrhagic fever virus. Front. Cell. Infect. Microbiol. 2013, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.M.; Linthicum, K.J.; Bailey, C.L.; Watts, D.M.; Dohm, D.J.; Moulton, J.R. Replication of Crimean-Congo Hemorrhagic Fever Virus in Four Species of Ixodid Ticks (Acari) Infected Experimentally. J. Med. Entomol. 1990, 27, 537–542. [Google Scholar] [CrossRef]
- Gordon, S.W.; Linthicum, K.J.; Moulton, J.R. Transmission of Crimean-Congo hemorrhagic fever virus in two species of Hyalomma ticks from infected adults to cofeeding immature forms. Am. J. Trop. Med. Hyg. 1993, 48, 576–580. [Google Scholar] [CrossRef]
- Zeller, H.G.; Cornet, J.P.; Camicas, J.L. Experimental transmission of Crimean-Congo hemorrhagic fever virus by west African wild ground-feeding birds to Hyalomma marginatum rufipes ticks. Am. J. Trop. Med. Hyg. 1994, 50, 676–681. [Google Scholar] [CrossRef]
- Dohm, D.J.; Logan, T.M.; Linthicum, K.J.; Rossi, C.A.; Turell, M.J. Transmission of Crimean-Congo Hemorrhagic Fever Virus by Hyalomma impeltatum (Acari: Ixodidae) after Experimental Infection. J. Med. Entomol. 2014, 33, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Abdissa, A.; Trape, J.-F. New concepts for the old challenge of African relapsing fever borreliosis. Clin. Microbiol. Infect. 2009, 15, 400–406. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Climate change decreases habitat suitability for some tick species (Acari: Ixodidae) in South Africa. Onderstepoort J. Vet. Res. 2003, 70, 79–93. [Google Scholar] [PubMed]
- Tagwireyi, P.; Ndebele, M.; Chikurunhe, W. Climate change diminishes the potential habitat of the bont tick (Amblyomma hebraeum): Evidence from Mashonaland Central Province, Zimbabwe. Parasit. Vectors 2022, 15, 237. [Google Scholar] [CrossRef]
- Hadgu, M.; Menghistu, H.T.; Girma, A.; Abrha, H.; Hagos, H. Modeling the potential climate change-induced impacts on future genus Rhipicephalus (Acari: Ixodidae) tick distribution in semi-arid areas of Raya Azebo district, Northern Ethiopia. J. Ecol. Environ. 2019, 43, 43. [Google Scholar] [CrossRef][Green Version]
- Nyangiwe, N.; Yawa, M.; Muchenje, V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: A review. S. Afr. J. Anim. Sci. 2018, 48, 829–841. [Google Scholar] [CrossRef]
- Lynen, G.; Zeman, P.; Bakuname, C.; Di Giulio, G.; Mtui, P.; Sanka, P.; Jongejan, F. Shifts in the distributional ranges of Boophilus ticks in Tanzania: Evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients. Exp. Appl. Acarol. 2008, 44, 147–164. [Google Scholar] [CrossRef]
- Yawa, M.; Nyangiwe, N.; Muchenje, V.; Kadzere, C.T.; Mpendulo, T.C.; Marufu, M.C. Ecological preferences and seasonal dynamics of ticks (Acari: Ixodidae) on and off bovine hosts in the Eastern Cape Province, South Africa. Exp. Appl. Acarol. 2018, 74, 317–328. [Google Scholar] [CrossRef]
- Rechav, Y. Seasonal activity and hosts of the vectors of Crimean-Congo haemorrhagic fever in South Africa. S. Afr. Med. J. 1986, 69, 364–368. [Google Scholar]
- Ronai, I. The Impact of Climate Change on Tick Host-Seeking Behaviour; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Cumming, G. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology 2002, 83, 255–268. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Lima-e-Silva, A.A.d. Anthropogenic Changes in Land Use Impact the Emergence and Transmission of Infectious Diseases. J. Microbiol. Biotechnol. 2024, 9. [Google Scholar] [CrossRef]
- White, R.J.; Razgour, O. Emerging zoonotic diseases originating in mammals: A systematic review of effects of anthropogenic land-use change. Mammal. Rev. 2020, 50, 336–352. [Google Scholar] [CrossRef]
- Faust, C.L.; McCallum, H.I.; Bloomfield, L.S.; Gottdenker, N.L.; Gillespie, T.R.; Torney, C.J.; Dobson, A.P.; Plowright, R.K. Pathogen spillover during land conversion. Ecol. Lett. 2018, 21, 471–483. [Google Scholar] [CrossRef]
- Ledwaba, M.B.; Nozipho, K.; Tembe, D.; Onyiche, T.E.; Chaisi, M.E. Distribution and prevalence of ticks and tick-borne pathogens of wild animals in South Africa: A systematic review. Curr. Res. Parasitol. Vector Borne Dis. 2022, 2, 100088. [Google Scholar] [CrossRef] [PubMed]
- Makwarela, T.G.; Djikeng, A.; Masebe, T.M.; Nkululeko, N.; Nesengani, L.T.; Mapholi, N.O. Vector abundance and associated abiotic factors that influence the distribution of ticks in six provinces of South Africa. Vet. World 2024, 17, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.C.; Muñoz, Á.G.; Cousin, R.; Shumake-Guillemot, J. Climate drivers of vector-borne diseases in Africa and their relevance to control programmes. Infect. Dis. Poverty 2018, 7, 15–36. [Google Scholar] [CrossRef]
- Djiman, T.A.; Biguezoton, A.S.; Saegerman, C. Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health. Pathogens 2024, 13, 697. [Google Scholar] [CrossRef]
- ECDC. Tick Maps. Available online: https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps?etrans=uk (accessed on 12 June 2025).
- Madder, M.; Horak, I.; Stoltsz, H. Tick Importance—Disease Transmission. Available online: https://afrivip.org/sites/default/files/Ticks-importance/index/ (accessed on 12 June 2025).
- Marques, R.; Krüger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jiménez-García, D. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Vet. Res. 2020, 51, 81. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Moo-Llanes, D.; Ibarra-Cerdeña, C.; Romero-Salas, D.; Cruz-Romero, A.; López-Hernández, K.; Aguilar-Dominguez, M. Worldwide comparison between the potential distribution of Rhipicephalus microplus (Acari: Ixodidae) under climate change scenarios. Med. Vet. Entomol. 2023, 37, 745–753. [Google Scholar] [CrossRef]
- Bah, M.T.; Grosbois, V.; Stachurski, F.; Muñoz, F.; Duhayon, M.; Rakotoarivony, I.; Appelgren, A.; Calloix, C.; Noguera, L.; Mouillaud, T. The Crimean-Congo haemorrhagic fever tick vector Hyalomma marginatum in the south of France: Modelling its distribution and determination of factors influencing its establishment in a newly invaded area. Transbound. Emerg. Dis. 2022, 69, e2351–e2365. [Google Scholar] [CrossRef]
- Estrada-Pena, A. Tick-borne pathogens, transmission rates and climate change. Front. Biosci. 2009, 14, 2674–2687. [Google Scholar] [CrossRef][Green Version]
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, drivers, and challenges of vector-borne disease emergence. Vector-Borne Zoonotic Dis. 2020, 20, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Dhakal, T.; Kim, T.-S.; Lee, D.-H.; Jang, G.-S.; Oh, Y. Climate change influences the spread of African swine fever virus. Vet. Sci. 2022, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Danielová, V.; Fialová, A.; Malý, M.; Kříž, B.; Nuttall, P.A. Increased relative risk of tick-borne encephalitis in warmer weather. Front. Cell. Infect. Microbiol. 2018, 8, 90. [Google Scholar] [CrossRef]
- Gray, J.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Maqbool, M.; Sajid, M.S.; Saqib, M.; Anjum, F.R.; Tayyab, M.H.; Rizwan, H.M.; Rashid, M.I.; Rashid, I.; Iqbal, A.; Siddique, R.M.; et al. Potential Mechanisms of Transmission of Tick-Borne Viruses at the Virus-Tick Interface. Front. Microbiol. 2022, 13, 846884. [Google Scholar] [CrossRef]
- Temur, A.I.; Kuhn, J.H.; Pecor, D.B.; Apanaskevich, D.A.; Keshtkar-Jahromi, M. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa-Underestimated for Decades. Am. J. Trop. Med. Hyg. 2021, 104, 1978–1990. [Google Scholar] [CrossRef]
- Chiuya, T.; Masiga, D.K.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef]
- Ilboudo, A.K.; Oloo, S.O.; Sircely, J.; Nijhof, A.M.; Bett, B. Spatial analysis and risk mapping of Crimean-Congo hemorrhagic fever (CCHF) in Sub-saharan Africa. Sci. Rep. 2025, 15, 2292. [Google Scholar] [CrossRef]
- Spengler, J.R.; Bergeron, É.; Spiropoulou, C.F. Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr. Opin. Virol. 2019, 34, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lule, S.A.; Gibb, R.; Kizito, D.; Nakanjako, G.; Mutyaba, J.; Balinandi, S.; Owen, L.; Jones, K.E.; Abubakar, I.; Lutwama, J.J.; et al. Widespread exposure to Crimean-Congo haemorrhagic fever in Uganda might be driven by transmission from Rhipicephalus ticks: Evidence from cross-sectional and modelling studies. J. Infect. 2022, 85, 683–692. [Google Scholar] [CrossRef]
- Belobo, J.T.E.; Kenmoe, S.; Kengne-Nde, C.; Emoh, C.P.D.; Bowo-Ngandji, A.; Tchatchouang, S.; Sowe Wobessi, J.N.; Mbongue Mikangue, C.A.; Tazokong, H.R.; Kingue Bebey, S.R.; et al. Worldwide epidemiology of Crimean-Congo Hemorrhagic Fever Virus in humans, ticks and other animal species, a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009299. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Messina, J.P.; Wint, G.R.W. The Spatial Distribution of Crimean-Congo Haemorrhagic Fever and Its Potential Vectors in Europe and Beyond. Insects 2023, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Celina, S.S.; Černý, J.; Samy, A.M. Mapping the potential distribution of the principal vector of Crimean-Congo haemorrhagic fever virus Hyalomma marginatum in the Old World. PLoS Negl. Trop. Dis. 2023, 17, e0010855. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.; Stephenson, B.; Brouwer, A.; Martinez, M.; de la Torre, A.; Bosch, J.; Foley-Fisher, M.; Bonilauri, P.; Lindström, A.; Ulrich, R.G.; et al. Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J. Appl. Microbiol. 2012, 112, 246–257. [Google Scholar] [CrossRef]
- Fanelli, A.; Buonavoglia, D. Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF-free countries in southern and Western Europe: A semi-quantitative risk assessment. One Health 2021, 13, 100290. [Google Scholar] [CrossRef]
- Nasirian, H. Ticks infected with Crimean-Congo hemorrhagic fever virus (CCHFV): A decision approach systematic review and meta-analysis regarding their role as vectors. Travel. Med. Infect. Dis. 2022, 47, 102309. [Google Scholar] [CrossRef]
- Shaibu, J.O.; Daodu, O.B.; Akinyemi, K.O.; Audu, R.A.; Bola Oyefolu, A.O. A systematic review of Crimean-Congo Haemorrhagic fever virus in Sub-Sahara Africa, 1969–2022. medRxiv 2022. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, S.; Balaraman, A.K.; Mehta, R.; Sah, S. The silent spread: Why we need increased awareness of Crimean-Congo haemorrhagic fever. Infect. Dis. 2025, 57, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; Mulei, S.; Whitmer, S.; Nyakarahuka, L.; Cossaboom, C.M.; Shedroff, E.; Morales-Betoulle, M.; Krapiunaya, I.; Tumusiime, A.; Kyondo, J. Crimean-Congo hemorrhagic fever cases diagnosed during an outbreak of Sudan virus disease in Uganda, 2022–2023. PLOS Neglected Trop. Dis. 2024, 18, e0012595. [Google Scholar] [CrossRef] [PubMed]
- Hartlaub, J.; Gutjahr, B.; Fast, C.; Mirazimi, A.; Keller, M.; Groschup, M.H. Diagnosis and Pathogenesis of Nairobi Sheep Disease Orthonairovirus Infections in Sheep and Cattle. Viruses 2021, 13, 1250. [Google Scholar] [CrossRef]
- Li, Y.; Salman, M.; Shen, C.; Yang, H.; Wang, Y.; Jiang, Z.; Edwards, J.; Huang, B. African Swine Fever in a commercial pig farm: Outbreak investigation and an approach for identifying the source of infection. Transbound. Emerg. Dis. 2020, 67, 2564–2578. [Google Scholar] [CrossRef]
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016, 58, 82. [Google Scholar] [CrossRef]
- Zhou, R.; Sprong, H.; Liu, Q.; Krafft, T.; Estrada-Peña, A. Mapping the potential suitable habitats for Hyalomma rufipes (Acari: Ixodidae) in Africa and Western Eurasia. PLOS Neglected Trop. Dis. 2025, 19, e0012923. [Google Scholar] [CrossRef]
- Davies, F.G. Nairobi Sheep Disease; Taylor & Francis Group: Abingdon-on-Thames, UK, 2019; pp. 191–204. [Google Scholar]
- Nyangiwe, N.; Harrison, A.; Horak, I.G. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp. Appl. Acarol. 2013, 61, 371–382. [Google Scholar] [CrossRef]
- Esemu, S.N.; Besong, W.O.; Ndip, R.N.; Ndip, L.M. Prevalence of Ehrlichia ruminantium in adult Amblyomma variegatum collected from cattle in Cameroon. Exp. Appl. Acarol. 2013, 59, 377–387. [Google Scholar] [CrossRef]
- de Angeli Dutra, D.; Salloum, P.M.; Poulin, R. Vector microbiome: Will global climate change affect vector competence and pathogen transmission? Parasitol. Res. 2023, 122, 11–17. [Google Scholar] [CrossRef]
- Narasimhan, S.; Fikrig, E. Tick microbiome: The force within. Trends Parasitol. 2015, 31, 315–323. [Google Scholar] [CrossRef]
- Mucheka, V.T.; Pillay, A.; Mukaratirwa, S. Prevalence of tick-borne pathogens in Rhipicephalus species infesting domestic animals in Africa: A systematic review and meta-analysis. Acta Trop. 2023, 246, 106994. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Esser, H.J.; Weiss, S.J.; Jansen, P.A.; Foley, J.E. Tick microbiomes in neotropical forest fragments are best explained by tick-associated and environmental factors rather than host blood source. Appl. Environ. Microbiol. 2021, 87, e02668-20. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, E.; Kariuki, E.; Floriano, A.M.; Castelli, M.; Tafesse, Y.M.; Magoga, G.; Kumsa, B.; Montagna, M.; Sassera, D. Multi-country investigation of the diversity and associated microorganisms isolated from tick species from domestic animals, wildlife and vegetation in selected african countries. Exp. Appl. Acarol. 2021, 83, 427–448. [Google Scholar] [CrossRef]
- Khogali, R.; Bastos, A.; Getange, D.; Bargul, J.L.; Kalayou, S.; Ongeso, N.; Verhoeven, J.T.P.; Kabii, J.; Ngiela, J.; Masiga, D.; et al. Exploring the microbiomes of camel ticks to infer vector competence: Insights from tissue-level symbiont-pathogen relationships. Sci. Rep. 2025, 15, 5574. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, A.; Kumar, D.; Bobo, C.; Rashid, M.I.; Durrani, A.Z.; Sajid, M.S.; Karim, S. Tick-Borne Pathogens Shape the Native Microbiome Within Tick Vectors. Microorganisms 2020, 8, 1299. [Google Scholar] [CrossRef] [PubMed]
- Mota, T.F.; Fukutani, E.R.; Martins, K.A.; Salgado, V.R.; Andrade, B.B.; Fraga, D.B.; Queiroz, A.T. Another tick bites the dust: Exploring the association of microbial composition with a broad transmission competence of tick vector species. Microbiol. Spectr. 2023, 11, e02156-23. [Google Scholar] [CrossRef]
- Hajdušek, O.; Síma, R.; Ayllón, N.; Jalovecká, M.; Perner, J.; de la Fuente, J.; Kopáček, P. Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 26. [Google Scholar] [CrossRef]
- Sesmero-García, C.; Cabanero-Navalon, M.D.; Garcia-Bustos, V. The Importance and Impact of Francisella-like Endosymbionts in Hyalomma Ticks in the Era of Climate Change. Diversity 2023, 15, 562. [Google Scholar] [CrossRef]
- Gall, C.A.; Reif, K.E.; Scoles, G.A.; Mason, K.L.; Mousel, M.; Noh, S.M.; Brayton, K.A. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016, 10, 1846–1855. [Google Scholar] [CrossRef]
- Liebig, K.; Boelke, M.; Grund, D.; Schicht, S.; Springer, A.; Strube, C.; Chitimia-Dobler, L.; Dobler, G.; Jung, K.; Becker, S. Tick populations from endemic and non-endemic areas in Germany show differential susceptibility to TBEV. Sci. Rep. 2020, 10, 15478. [Google Scholar] [CrossRef]
- Budachetri, K.; Kumar, D.; Crispell, G.; Beck, C.; Dasch, G.; Karim, S. The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector. Microbiome 2018, 6, 141. [Google Scholar] [CrossRef]
- Ni, X.B.; Pei, Y.; Ye, Y.T.; Shum, M.H.; Cui, X.M.; Wu, Y.Q.; Pierce, M.P.; Zhao, L.; Wang, G.P.; Wei, J.T.; et al. Ecoclimate drivers shape virome diversity in a globally invasive tick species. ISME J. 2024, 18, wrae087. [Google Scholar] [CrossRef]
- Caragata, E.P.; Short, S.M. Vector microbiota and immunity: Modulating arthropod susceptibility to vertebrate pathogens. Curr. Opin. Insect Sci. 2022, 50, 100875. [Google Scholar] [CrossRef] [PubMed]
- Militzer, N.; Pinecki Socias, S.; Nijhof, A.M. Changes in the Ixodes ricinus microbiome associated with artificial tick feeding. Front. Microbiol. 2023, 13, 1050063. [Google Scholar] [CrossRef]
- Talactac, M.R.; Hernandez, E.P.; Hatta, T.; Yoshii, K.; Kusakisako, K.; Tsuji, N.; Tanaka, T. The antiviral immunity of ticks against transmitted viral pathogens. Dev. Comp. Immunol. 2021, 119, 104012. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Molecular characterization of tick-virus interactions. Front. Biosci. 2009, 14, 2466–2483. [Google Scholar] [CrossRef]
- Fogaça, A.C.; Sousa, G.; Pavanelo, D.B.; Esteves, E.; Martins, L.A.; Urbanová, V.; Kopáček, P.; Daffre, S. Tick immune system: What is known, the interconnections, the gaps, and the challenges. Front. Immunol. 2021, 12, 628054. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Macaluso, K.R. Microbial invasion vs. tick immune regulation. Front. Cell. Infect. Microbiol. 2017, 7, 390. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Nuss, A.B.; Meyer, J.M.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; De La Fuente, J.; Ribeiro, J.M.; Megy, K. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.-F.; Wang, Q.; Zhang, B.; Ji, P. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340.E13. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Pal, U. Immunity-related genes in Ixodes scapularis—Perspectives from genome information. Front. Cell. Infect. Microbiol. 2014, 4, 116. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Tykalová, H.; Watson, M.; Sharma, M.; Sterken, M.G.; Obbard, D.J.; Lewis, S.H.; McFarlane, M.; Bell-Sakyi, L.; Barry, G. Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res. 2014, 42, 9436–9446. [Google Scholar] [CrossRef]
- Hart, C.E.; Thangamani, S. Tick-virus interactions: Current understanding and future perspectives. Parasite Immunol. 2021, 43, e12815. [Google Scholar] [CrossRef]
- Inoue, N.; Hanada, K.; Tsuji, N.; Igarashi, I.; Nagasawa, H.; Mikami, T.; Fujisaki, K. Characterization of phagocytic hemocytes in Ornithodoros moubata (Acari: Ixodidae). J. Med. Entomol. 2001, 38, 514–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borovičková, B.; Hypša, V. Ontogeny of tick hemocytes: A comparative analysis of Ixodes ricinus and Ornithodoros moubata. Exp. Appl. Acarol. 2005, 35, 317–333. [Google Scholar] [CrossRef]
- Leite, T.H.; Ferreira, Á.G.; Imler, J.-L.; Marques, J.T. Distinct roles of hemocytes at different stages of infection by dengue and zika viruses in Aedes aegypti mosquitoes. Front. Immunol. 2021, 12, 660873. [Google Scholar] [CrossRef]
- Tassetto, M.; Kunitomi, M.; Andino, R. Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in Drosophila. Cell 2017, 169, 314–325.E13. [Google Scholar] [CrossRef]
- Pereira, L.S.; Oliveira, P.L.; Barja-Fidalgo, C.; Daffre, S. Production of reactive oxygen species by hemocytes from the cattle tick Boophilus microplus. Exp. Parasitol. 2001, 99, 66–72. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Slonchak, A.; Hugo, L.E.; Freney, M.E.; Hall-Mendelin, S.; Amarilla, A.A.; Torres, F.J.; Setoh, Y.X.; Peng, N.Y.; Sng, J.D.; Hall, R.A. Zika virus noncoding RNA suppresses apoptosis and is required for virus transmission by mosquitoes. Nat. Commun. 2020, 11, 2205. [Google Scholar] [CrossRef]
- Palmer, W.J.; Jiggins, F.M. Comparative Genomics Reveals the Origins and Diversity of Arthropod Immune Systems. Mol. Biol. Evol. 2015, 32, 2111–2129. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.D.; Capelli-Peixoto, J.; Mesquita, R.D.; Kalil, S.P.; Pohl, P.C.; Braz, G.R.; Fogaca, A.C.; Daffre, S. Exploring the immune signalling pathway-related genes of the cattle tick Rhipicephalus microplus: From molecular characterization to transcriptional profile upon microbial challenge. Dev. Comp. Immunol. 2016, 59, 1–14. [Google Scholar] [CrossRef]
- Shaw, D.K.; Wang, X.; Brown, L.J.; Chávez, A.S.O.; Reif, K.E.; Smith, A.A.; Scott, A.J.; McClure, E.E.; Boradia, V.M.; Hammond, H.L. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 2017, 8, 14401. [Google Scholar] [CrossRef] [PubMed]
- Romeo, Y.; Lemaitre, B. Drosophila immunity: Methods for monitoring the activity of Toll and Imd signaling pathways. Innate Immun. 2008, 379–394. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Pereira De Oliveira, R.; Hutet, E.; Lancelot, R.; Paboeuf, F.; Duhayon, M.; Boinas, F.; Pérez de León, A.A.; Filatov, S.; Le Potier, M.F.; Vial, L. Differential vector competence of Ornithodoros soft ticks for African swine fever virus: What if it involves more than just crossing organic barriers in ticks? Parasit. Vectors 2020, 13, 618. [Google Scholar] [CrossRef]
- de Oliveira, V.L.; Almeida, S.C.; Soares, H.R.; Crespo, A.; Marshall-Clarke, S.; Parkhouse, R.M. A novel TLR3 inhibitor encoded by African swine fever virus (ASFV). Arch. Virol. 2011, 156, 597–609. [Google Scholar] [CrossRef]
- AFAYIBO, D.J.A.; Yang, J.; Hao, R.; Zhang, Z.; Sun, H.; Luo, J.; Ren, Q.; Tadele, B.A.; Guan, G.; Niu, Q. Protein profile and protein interaction network analysis of Ornithodoros moubata during African swine fever virus infection. Parasitol. Res. 2025; preprint. [Google Scholar] [CrossRef]
- Taylor, D.; Ogihara, M. Climate influences on reproduction and immunity in the soft tick, Ornithodoros moubata. In Climate, Ticks and Disease; CABI: Wallingford, UK, 2021; pp. 74–83. [Google Scholar]
- Nielebeck, C.; Kim, S.H.; Pepe, A.; Himes, L.; Miller, Z.; Zummo, S.; Tang, M.; Monzón, J.D. Climatic stress decreases tick survival but increases rate of host-seeking behavior. Ecosphere 2023, 14, e4369. [Google Scholar] [CrossRef]
- Ajayi, O.M.; Oyen, K.J.; Davies, B.; Finch, G.; Piller, B.D.; Harmeyer, A.A.; Wendeln, K.; Perretta, C.; Rosendale, A.J.; Benoit, J.B. Egg hatching success is influenced by the time of thermal stress in four hard tick species. J. Med. Entomol. 2023, 61, 110–120. [Google Scholar] [CrossRef]
- Busby, A.T.; Ayllón, N.; Kocan, K.M.; Blouin, E.F.; De La Fuente, G.; Galindo, R.; Villar, M.; De La Fuente, J. Expression of heat shock proteins and subolesin affects stress responses, Anaplasma phagocytophilum infection and questing behaviour in the tick, Ixodes scapularis. Med. Vet. Entomol. 2012, 26, 92–102. [Google Scholar] [CrossRef]
- Damian, D. Navigating the Landscape of Tick Diversity: Integrating Molecular Approaches for Enhanced Control Measures. Vector-Borne Zoonotic Dis. 2025, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sorvillo, T.E.; Rodriguez, S.E.; Hudson, P.; Carey, M.; Rodriguez, L.L.; Spiropoulou, C.F.; Bird, B.H.; Spengler, J.R.; Bente, D.A. Towards a sustainable one health approach to crimean–congo hemorrhagic fever prevention: Focus areas and gaps in knowledge. Trop. Med. Infect. Dis. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Hewson, R. Understanding Viral Haemorrhagic Fevers: Virus Diversity, Vector Ecology, and Public Health Strategies. Pathogens 2024, 13, 909. [Google Scholar] [CrossRef]
- Kasaija, P.D.; Contreras, M.; Kirunda, H.; Nanteza, A.; Kabi, F.; Mugerwa, S.; de la Fuente, J. Inspiring Anti-Tick Vaccine Research, Development and Deployment in Tropical Africa for the Control of Cattle Ticks: Review and Insights. Vaccines 2022, 11, 99. [Google Scholar] [CrossRef]
- Aguilar-Díaz, H.; Quiroz-Castañeda, R.E.; Salazar-Morales, K.; Cossío-Bayúgar, R.; Miranda-Miranda, E. Tick Immunobiology and Extracellular Traps: An Integrative Vision to Control of Vectors. Pathogens 2021, 10, 1511. [Google Scholar] [CrossRef]
- Mateos-Hernández, L.; Obregón, D.; Wu-Chuang, A.; Maye, J.; Bornères, J.; Versillé, N.; de la Fuente, J.; Díaz-Sánchez, S.; Bermúdez-Humarán, L.G.; Torres-Maravilla, E.; et al. Anti-Microbiota Vaccines Modulate the Tick Microbiome in a Taxon-Specific Manner. Front. Immunol. 2021, 12, 704621. [Google Scholar] [CrossRef]
- Wu-Chuang, A.; Obregon, D.; Mateos-Hernández, L.; Cabezas-Cruz, A. Anti-tick microbiota vaccines: How can this actually work? Biologia 2022, 77, 1555–1562. [Google Scholar] [CrossRef]
- Zens, K.D. Tick-borne encephalitis-viral transmission and considerations for vaccination. Ther. Umschau. Rev. Ther. 2022, 79, 471–481. [Google Scholar] [CrossRef]
- Ozdarendeli, A. Crimean–Congo Hemorrhagic Fever Virus: Progress in Vaccine Development. Diagnostics 2023, 13, 2708. [Google Scholar] [CrossRef] [PubMed]
- WHO. Prioritizing Diseases for Research and Development in Emergency Contexts. Available online: https://www.emro.who.int/pandemic-epidemic-diseases/news/list-of-blueprint-priority-diseases.html (accessed on 16 April 2025).
- Arias, M.; De la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F. Approaches and perspectives for development of African swine fever virus vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef]
- Yadav, P.D.; Vincent, M.J.; Khristova, M.; Kale, C.; Nichol, S.T.; Mishra, A.C.; Mourya, D.T. Genomic analysis reveals Nairobi sheep disease virus to be highly diverse and present in both Africa, and in India in the form of the Ganjam virus variant. Infect. Genet. Evol. 2011, 11, 1111–1120. [Google Scholar] [CrossRef]
- Wasonga, C.; Kiraithe, M.M.; Nyamache, A. Comparative Studies of Nairobi-Sheep-Disease Virus Strains Infectivity, Immunogenicty and Cross-Protectivity in BALB/C Mice Model. Int. J. Innov. Sci. Res. Technol. 2024, 9. [Google Scholar] [CrossRef]
- Muriuki, N.P.; Nyamache, A.; Ateya, L.O.; Binepal, Y.S.; Wasonga, C.; Kiraithe, M.M.; Ithinji, G. Evaluation of Infectivity and Immunogenicity of Sugar Stabilized Nairobi Sheep Disease Vaccine. Int. J. Innov. Sci. Res. Technol. 2024, 9. [Google Scholar] [CrossRef]
- Nkengasong, J.N.; Tessema, S.K. Africa Needs a New Public Health Order to Tackle Infectious Disease Threats. Cell 2020, 183, 296–300. [Google Scholar] [CrossRef]
- Okesanya, O.J.; Olatunji, G.D.; Kokori, E.; Olaleke, N.O.; Adigun, O.A.; Manirambona, E.; Lucero-Prisno, D.E., 3rd. Looking Beyond the Lens of Crimean-Congo Hemorrhagic Fever in Africa. Emerg. Infect. Dis. 2024, 30, 1319–1325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, C.L.; Becker, S.C. Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond. Microorganisms 2025, 13, 1509. https://doi.org/10.3390/microorganisms13071509
Ebert CL, Becker SC. Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond. Microorganisms. 2025; 13(7):1509. https://doi.org/10.3390/microorganisms13071509
Chicago/Turabian StyleEbert, Cara Leonie, and Stefanie C. Becker. 2025. "Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond" Microorganisms 13, no. 7: 1509. https://doi.org/10.3390/microorganisms13071509
APA StyleEbert, C. L., & Becker, S. C. (2025). Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond. Microorganisms, 13(7), 1509. https://doi.org/10.3390/microorganisms13071509





