Differential Strain-Specific Responses of Trichoderma spp. in Mycoparasitism, Chitinase Activity, and Volatiles Production Against Moniliophthora spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Dual Culture Antagonism Assay
2.3. Chitinolytic Activity Analysis
2.3.1. Chitin Preparation from Mycelium Cell Walls
2.3.2. Commercial Chitin Preparation from Shrimp Shells
2.3.3. Semi-Quantitative Determination of Trichoderma spp. Chitinolytic Activity
2.3.4. Quantification of Total Trichoderma spp. Chitinase Activity
2.4. Volatile Organic Compounds Analysis
2.4.1. Volatiles Extraction
2.4.2. VOCs Analysis
2.5. Statistical Analysis
3. Results
3.1. Antagonistic Activity of Trichoderma Strains
3.2. Chitinolytic Activity of Trichoderma spp.
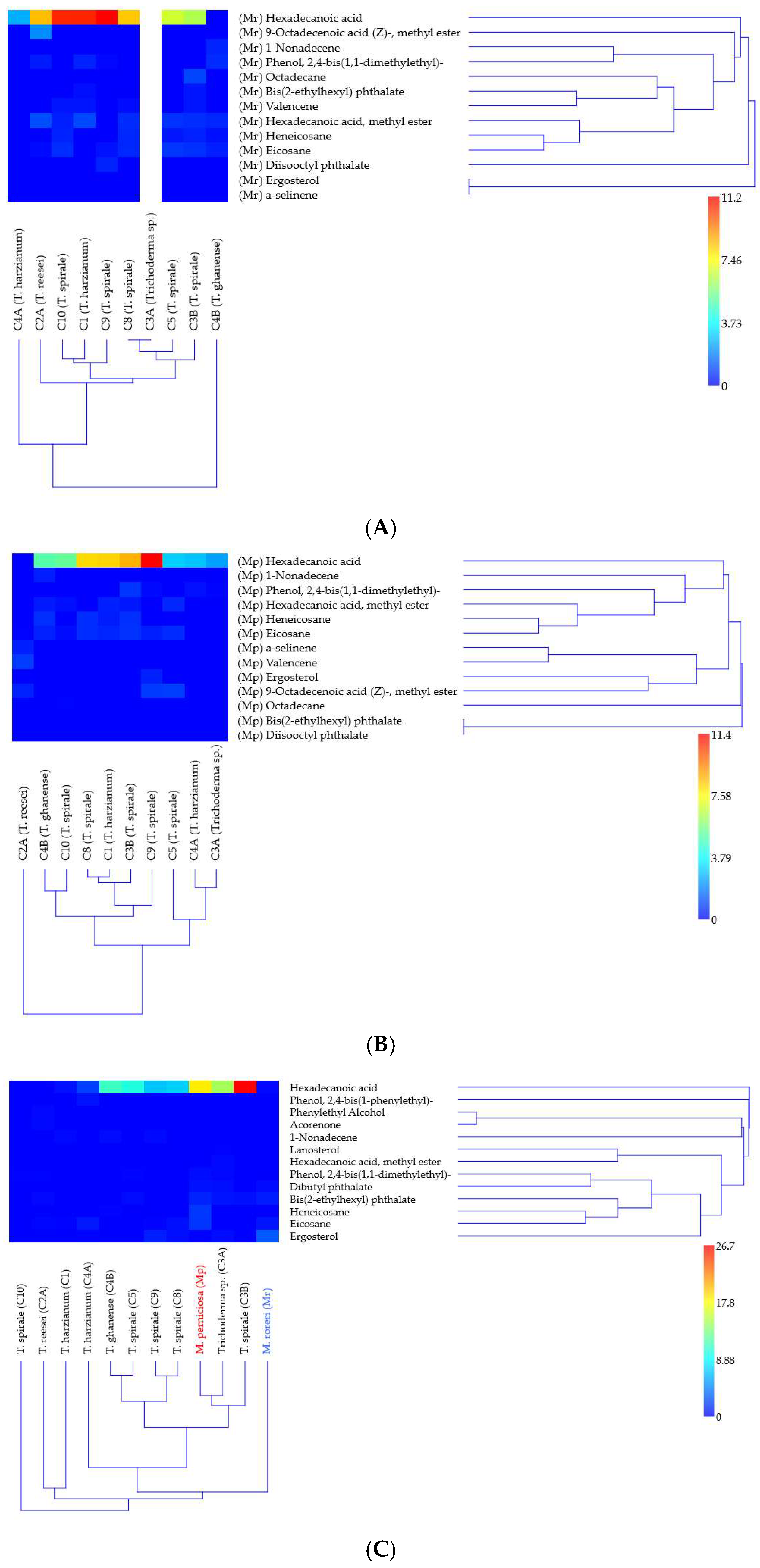
3.3. Volatile Organic Compounds and Their Limited Association with Antagonism
3.4. Integrated Analysis for Biocontrol Potential Assessment
4. Discussion
4.1. Complementary Antagonistic Profiles Against Moniliophthora spp.
4.2. Volatile Organic Compounds as Secondary Contributors to Antagonism
4.3. Strain Specialization Suggests Potential for Tailored Consortia
4.4. Methodological Considerations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MR | Moniliophthora roreri |
| MP | Moniliophthora perniciosa |
| PPI | pathogen growth inhibition |
| NAGA | N-acetyl-β-D-glucosamine |
| VOC | volatile organic compound |
| PCA | principal component analysis |
| CWDE | cell wall-degrading enzyme |
References
- Evans, H.C. Witches’ Broom Disease (Moniliophthora perniciosa): History and Biology. In Cacao Diseases: A History of Old Enemies and New Encounters; Springer: Cham, Switzerland, 2016; pp. 137–177. ISBN 978-3-319-24789-2. [Google Scholar]
- Evans, H.C. Frosty Pod Rot (Moniliophthora roreri). In Cacao Diseases: A History of Old Enemies and New Encounters; Bailey, B.A., Meinhardt, L.W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–96. ISBN 978-3-319-24789-2. [Google Scholar]
- ten Hoopen, G.M.; Krauss, U. Biological Control of Cocoa Disease. In Cacao Diseases A History of Old Enemies and New Encounters; Bailey, B.A., Meinhardt, L.W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 511–566. ISBN 978-3-319-24787-8. [Google Scholar]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Synergistic Effect of Trichoderma-Derived Antifungal Metabolites and Cell Wall Degrading Enzymes on Enhanced Biocontrol of Fusarium Oxysporum f. sp. Cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Silar, P. Hyphal Interference: Self Versus Non-Self Fungal Recognition and Hyphal Death. In Biocommunication of Fungi; Witzany, G., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–170. ISBN 978-94-007-4263-5. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Sayed, S.R.M.; Rady, A.M. Antagonistic Activity of Trichoderma harzianum and Trichoderma viride Strains against Some Fusarial Pathogens Causing Stalk Rot Disease of Maize, In Vitro. J. King Saud Univ.-Sci. 2021, 33, 101363. [Google Scholar] [CrossRef]
- Dutta, P.; Mahanta, M.; Singh, S.B.; Thakuria, D.; Deb, L.; Kumari, A.; Upamanya, G.K.; Boruah, S.; Dey, U.; Mishra, A.K.; et al. Molecular Interaction between Plants and Trichoderma Species against Soil-Borne Plant Pathogens. Front. Plant Sci. 2023, 14, 1145715. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The Structure and Synthesis of the Fungal Cell Wall. BioEssays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Thu Ha, T.T. Characterisation and Antifungal Activity of Extracellular Chitinase from a Biocontrol Fungus, Trichoderma asperellum PQ34. Mycology 2019, 11, 38–48. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Degradation of Fungal Cell Walls by Lytic Enzymes of Trichoderma harzianum. Microbiology 1989, 135, 675–682. [Google Scholar] [CrossRef]
- De La Cruz, J.; Hidalgo-Gallego, A.; Lora, J.M.; Benitez, T.; Pintor-Toro, J.A.; Llobell, A. Isolation and Characterization of Three Chitinases from Trichoderma harzianum. Eur. J. Biochem. 1992, 206, 859–867. [Google Scholar] [CrossRef]
- Chérif, M. Cytochemical Aspects of Chitin Breakdown During the Parasitic Action of a Trichoderma sp. on Fusarium Oxysporum f. sp. radicis-lycopersici. Phytopathology 1990, 80, 1406. [Google Scholar] [CrossRef]
- Agrawal, T.; Kotasthane, A.S. Chitinolytic Assay of Indigenous Trichoderma Isolates Collected from Different Geographical Locations of Chhattisgarh in Central India. Springerplus 2012, 1, 73. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Ghirardo, A.; Antritter, F.; Benz, J.P.; Schnitzler, J.; Rosenkranz, M. Sniffing Fungi—Phenotyping of Volatile Chemical Diversity in Trichoderma Species. New Phytol. 2020, 227, 244–259. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-López, N.; Cruz-López, L.; Holguín-Meléndez, F.; Guillén-Navarro, G.K.; Huerta-Palacios, G. Volatile Organic Compounds Produced by Cacao Endophytic Bacteria and Their Inhibitory Activity on Moniliophthora roreri. Curr. Microbiol. 2022, 79, 35. [Google Scholar] [CrossRef]
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma Species Isolated in Ecuador and Their Antagonistic Activities against Phytopathogenic Fungi from Ecuador and Japan. J. Gen. Plant Pathol. 2015, 81, 201–210. [Google Scholar] [CrossRef]
- Garcés-Moncayo, M.F. Evaluación De La Actividad Antagónica Y Quitinolítica De Trichoderma Spp., Para Inhibir El Crecimiento De Moniliophthora roreri Y Moniliophthora perniciosa. Master’s Thesis, Escuela Superior Politécnica Del Litoral, Guayaquil, Ecuador, 2018. [Google Scholar]
- Chóez-Guaranda, I.; Espinoza-Lozano, F.; Reyes-Araujo, D.; Romero, C.; Manzano, P.; Galarza, L.; Sosa, D. Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens. Molecules 2023, 28, 3208. [Google Scholar] [CrossRef] [PubMed]
- Avilés, D.; Espinoza, F.; Villao, L.; Alvarez, J.; Sosa, D.; Santos-Ordóñez, E.; Galarza, L. Application of Microencapsulated Trichoderma spp. against Moniliophthora roreri during the Vegetative Development of Cocoa. Sci. Agropecu. 2023, 14, 539–547. [Google Scholar] [CrossRef]
- Villavicencio-Vásquez, M.; Espinoza-Lozano, R.F.; Pérez-Martínez, S.; Castillo, D.S.D.; Villavicencio-Vásquez, M.; Espinoza-Lozano, R.F.; Pérez-Martínez, S.; Castillo, D.S.D. Foliar Endophyte Fungi as Candidate For Biocontrol Against Moniliophthora spp. of Theobroma Cacao (Malvaceae) In Ecuador. Acta Biol. Colomb. 2018, 23, 235–241. [Google Scholar] [CrossRef]
- Mallikharjuna Rao, K.L.N.; Siva Raju, K.; Ravisankar, H. Cultural Conditions on the Production of Extracellular Enzymes by Trichoderma Isolates from Tobacco Rhizosphere. Braz. J. Microbiol. 2015, 47, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.K.; Selitrennikoff, C.P. Plant and Bacterial Chitinases Differ in Antifungal Activity. Microbiology 1988, 134, 169–176. [Google Scholar] [CrossRef]
- Meena, L.K.; Jambhulkar, P.P. Determination of Chitinase Activity of Trichoderma Isolates on Colloidal Chitin Supplemented Medium. Int. J. Adv. Biochem. Res. 2024, 8, 406–410. [Google Scholar] [CrossRef]
- Liu, M.; Wei, F.; Lv, X.; Dong, X.Y.; Chen, H. Rapid and Sensitive Detection of Free Fatty Acids in Edible Oils Based on Chemical Derivatization Coupled with Electrospray Ionization Tandem Mass Spectrometry. Food Chem. 2018, 242, 338–344. [Google Scholar] [CrossRef]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for Isolation of Marine-Derived Endophytic Fungi and Their Bioactive Secondary Products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, V.; Arukha, A.P.; Rabbee, M.F.; Ameen, F.; Koul, B. Screening of the Biocontrol Efficacy of Potent Trichoderma Strains against Fusarium oxysporum f.sp. ciceri and Scelrotium rolfsii Causing Wilt and Collar Rot in Chickpea. Microorganisms 2024, 12, 1280. [Google Scholar] [CrossRef]
- Yu, N.; Gao, Y.; Chang, F.; Liu, W.; Guo, C.; Cai, H. Screening of Antagonistic Trichoderma Strains to Enhance Soybean Growth. J. Fungi 2025, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.M.D.; Fonseca, P.L.C.; Heming, N.M.; de Amorim Conceição, L.B.; de Souza Nascimento, K.T.; Gramacho, K.P.; Arevalo-Gardini, E.; Pirovani, C.P.; Guimarães Rocha, E.R. Characterization of the Microbiota Dynamics Associated with Moniliophthora roreri, Causal Agent of Cocoa Frosty Pod Rot Disease, Reveals New Viral Species. Front. Microbiol. 2022, 13, 1053562. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shahid, M.; Srivastava, M.; Sharma, A.; Singh, A.; Kumar, V.; Gupta, S.J. Chitinolytic Assay for Trichoderma Species Isolated from Different Geographical Locations of Uttar Pradesh. Afr. J. Biotechnol. 2014, 13, 4246–4250. [Google Scholar] [CrossRef]
- Khatri, D.K.; Tiwari, D.N.; Bariya, H.S. Chitinolytic Efficacy and Secretion of Cell Wall Degrading Enzymes from Trichoderma spp. in Response to Phyto-Pathological Fungi. J. App. Biol. Biotech. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- dos Santos, I.B.; Lopes, M.d.S.; Bini, A.P.; Tschoeke, B.A.P.; Verssani, B.A.W.; Figueredo, E.F.; Cataldi, T.R.; Marques, J.P.R.; Silva, L.D.; Labate, C.A.; et al. The Eucalyptus Cuticular Waxes Contribute in Preformed Defense Against Austropuccinia psidii. Front. Plant Sci. 2019, 9, 1978. [Google Scholar] [CrossRef]
- Dauda, W.P.; Singh Rana, V.; Solanke, A.U.; Krishnan, G.; Bashya, B.M.; Aggarwal, R.; Shanmugam, V. Metabolomic Analysis of Sheath Blight Disease of Rice (Oryza sativa L.) Induced by Rhizoctonia Solani Phytotoxin. J. Appl. Microbiol. 2022, 133, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Sánchez-Fernández, R.E.; Roque-Flores, G.; Lappe-Oliveras, P.; Medina-Romero, Y.M. Volatile Organic Compounds from Hypoxylon anthochroum Endophytic Strains as Postharvest Mycofumigation Alternative for Cherry Tomatoes. Food Microbiol. 2018, 76, 363–373. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Antifungal Activity of Volatile Organic Compounds from Trichoderma virens. AIP Conf. Proc. 2019, 2120, 080012. [Google Scholar]
- Siddiquee, S.; Cheong, B.E.; Taslima, K.; Kausar, H.; Hasan, M.M. Separation and Identification of Volatile Compounds from Liquid Cultures of Trichoderma harzianum by GC-MS Using Three Different Capillary Columns. J. Chromatogr. Sci. 2012, 50, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and Identification of Bioactive Compounds (Eicosane and Dibutyl Phthalate) Produced by Streptomyces Strain KX852460 for the Biological Control of Rhizoctonia solani AG-3 Strain KX852461 to Control Target Spot Disease in Tobacco Leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Jegan, P.; Sethurathinam, S.; Iyyamperumal, M.; Jacob, R.; Kathithachalam, A.; Mannu, J.; Padmanabhan, S.; Gajendiran, M. Antifungal and Plant-Growth Promoting Potency of Streptomyces rochei against Biotic Stress Caused by Race 4 Fusarium Wilt on Banana. Plant Stress 2025, 15, 100779. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Krauss, U.; Soberanis, W. Biocontrol of Cocoa Pod Diseases with Mycoparasite Mixtures. Biol. Control 2001, 22, 149–158. [Google Scholar] [CrossRef]



| Strain (Species) 1 | vs. M. roreri | vs. M. perniciosa | Solo-Culture | vs. M. roreri | vs. M. perniciosa | ||
|---|---|---|---|---|---|---|---|
| % Pathogen Inhibition 2 | Chitinase Activity 4 (mg/mL NAGA) | % Pathogen Inhibition | Chitinase Activity (NAGA) | Hexadecanoic Acid (% of Peak Area) | |||
| MR (M. roreri) | -- 3 | -- | -- | -- | 0.23 | -- | -- |
| MP (M. perniciosa) | -- | -- | -- | -- | 18.41 | -- | -- |
| C3B (“T. spirale”) | 94.68 | 39.2 | 87.5 | 31.7 | 26.65 | 6.3 | 8.73 |
| C3A (Trichoderma sp.) | 89.32 | 24.2 | 87.56 | 26.7 | 14.62 | -- | 2.39 |
| C4B (“T. gahnense”) | 95.43 | 66.7 | 93.53 | 56.7 | 11.02 | 0 | 5.07 |
| C5 (“T. spirale”) | 92.6 | 54.2 | 88.13 | 49.2 | 9.98 | 6.75 | 3.06 |
| C8 (“T. spirale”) | 96.69 | 24.2 | 91 | 21.7 | 7.17 | 8.27 | 8.14 |
| C9 (“T. spirale”) | 91.8 | 34.2 | 89.79 | 54.2 | 6.81 | 11.19 | 11.37 |
| C4A (“T. harzianum”) | 92.99 | 71.7 | 89.09 | 34.2 | 2.22 | 2.55 | 2.9 |
| C1 (“T. harzianum”) | 95.87 | 39.2 | 88 | 94.2 | 0.55 | 10.67 | 8.23 |
| C10 (“T. spirale”) | 92.59 | 24.2 | 89.39 | 46.7 | 0 | 10.65 | 5.3 |
| C2A (“T. reesei”) | 93.04 | 29.2 | 93.5 | 61.7 | 0 | 8.42 | 0 |
| Average | 93.50 | 40.7 | 89.75 | 47.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés-Moncayo, M.F.; Romero, C.A.; Pérez-Martínez, S.; Noceda, C.; Galarza, L.L.; del Castillo, D.S. Differential Strain-Specific Responses of Trichoderma spp. in Mycoparasitism, Chitinase Activity, and Volatiles Production Against Moniliophthora spp. Microorganisms 2025, 13, 1499. https://doi.org/10.3390/microorganisms13071499
Garcés-Moncayo MF, Romero CA, Pérez-Martínez S, Noceda C, Galarza LL, del Castillo DS. Differential Strain-Specific Responses of Trichoderma spp. in Mycoparasitism, Chitinase Activity, and Volatiles Production Against Moniliophthora spp. Microorganisms. 2025; 13(7):1499. https://doi.org/10.3390/microorganisms13071499
Chicago/Turabian StyleGarcés-Moncayo, María F., Christian A. Romero, Simón Pérez-Martínez, Carlos Noceda, Luís L. Galarza, and Daynet Sosa del Castillo. 2025. "Differential Strain-Specific Responses of Trichoderma spp. in Mycoparasitism, Chitinase Activity, and Volatiles Production Against Moniliophthora spp." Microorganisms 13, no. 7: 1499. https://doi.org/10.3390/microorganisms13071499
APA StyleGarcés-Moncayo, M. F., Romero, C. A., Pérez-Martínez, S., Noceda, C., Galarza, L. L., & del Castillo, D. S. (2025). Differential Strain-Specific Responses of Trichoderma spp. in Mycoparasitism, Chitinase Activity, and Volatiles Production Against Moniliophthora spp. Microorganisms, 13(7), 1499. https://doi.org/10.3390/microorganisms13071499






