Identification of Parasitic Infections by Analyzing Honeybees, Honey, and Pollen Using Droplet Digital RT-PCR
Abstract
1. Introduction
2. Materials and Methods
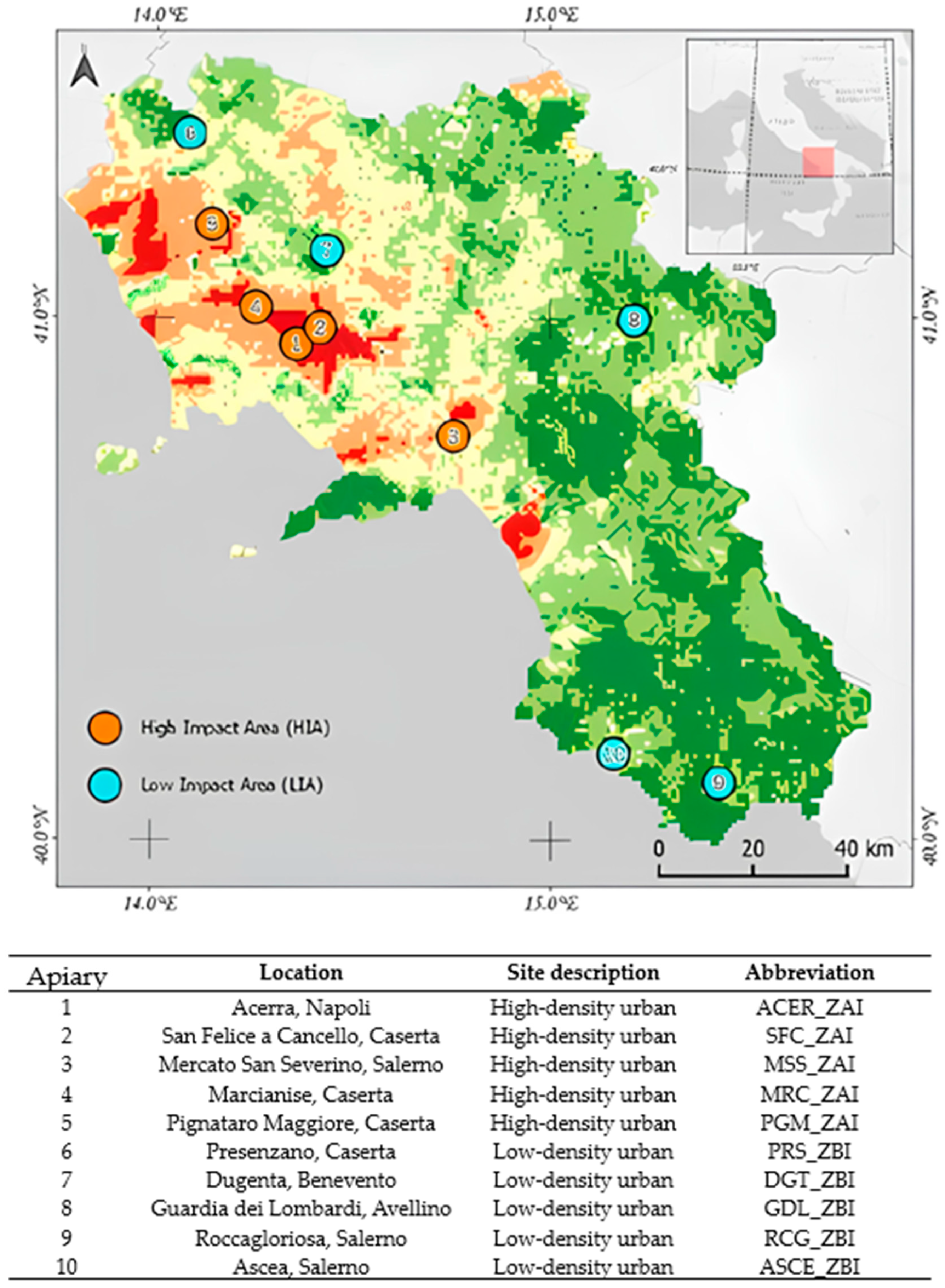
2.1. Study Area and Sampling
2.2. DNA Extraction
2.3. Droplet Digital RT-PCR (dd RT-PCR) for Parasitic Load Detection
2.4. Test Performance Assessment
2.5. Statistical Analyses
3. Results
3.1. Performance Evaluation of dd-PCR Assay
3.2. Honeybees, Honey, and Pollen Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO, Geneva. Soil-Transmitted Helminth Infections (Updated 2023 January 18). 2023. Available online: https://www.who.int/en/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 23 December 2024).
- Rossi, F.; Santonicola, S.; Amadoro, C.; Marino, L.; Colavita, G. Food and Drinking Water as Sources of Pathogenic Protozoans: An Update. Appl. Sci. 2024, 14, 5339. [Google Scholar] [CrossRef]
- Grellet, A.; Mila, H. Endoparasitic Diseases in Breeding Kennels: A Frequent and Complex Problem Requiring a Holistic Approach. Animals 2024, 14, 2357. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Badri, M.; Olfatifar, M.; Karim, R.; Modirian, E.; Houshmand, E.; Abdoli, A.; Nikoonejad, A.; Sotoodeh, S.; Zargar, A.; Samimi, R.; et al. Global prevalence of intestinal protozoan contamination in vegetables and fruits: A systematic review and meta-analysis. Food Control 2022, 133, 108656. [Google Scholar] [CrossRef]
- López Ureña, N.M.; Chaudhry, U.; Calero Bernal, R.; Cano Alsua, S.; Messina, D.; Evangelista, F.; Betson, M.; Lalle, M.; Jokelainen, P.; Ortega Mora, L.M.; et al. Contamination of Soil, Water, Fresh Produce, and Bivalve Mollusks with Toxoplasma gondii Oocysts: A Systematic Review. Microorganisms 2022, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.A.; Wu, F.; Anwar, Z.; Saif, I.; ul Akbar, N.; Gul, N.; Ali, I.; Feng, H.; Wang, W. The Presence of Toxoplasma gondii in Soil, Their Transmission, and Their Influence on the Small Ruminants and Human Population: A Review. Microb. Pathog. 2021, 158, 104850. [Google Scholar] [CrossRef]
- González-Alcaraz, M.N.; Malheiro, C.; Cardoso, D.N.; Prodana, M.; Morgado, R.G.; van Gestel, C.A.M.; Loureiro, S. Bioaccumulation and Toxicity of Organic Chemicals in Terrestrial Invertebrates; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2020; pp. 149–189. [Google Scholar] [CrossRef]
- Hristovski, M.; Cvetkovik, A.; Cvetkovik, I.; Dukoska, V. Concept of one health-a new professional imperative. Maced. J. Med. Sci. 2010, 3, 229–232. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Ortega Polo, R.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Marta Guarna, M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Girotti, S.; Ghini, S.; Ferri, E.; Bolelli, L.; Colombo, R.; Serra, G.; Porrini, C.; Sangiorgi, S. Bioindicators and biomonitoring: Honeybees and hive products as pollution impact assessment tools for the Mediterranean area. Euro Mediterr. J. Environ. Integr. 2020, 5, 62. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology and Foodborne Pathogens in Honey. Crit. Rev. Food Sci. Nutr. 2015, 57, 1852–1862. [Google Scholar] [CrossRef]
- Bellucci, V.; Lucci, S.; Bianco, P.; Ubaldi, A.; Felicioli, A.; Porrini, C.; Mutinelli, F.; Battisti, S.; Spallucci, V.; Cersini, A.; et al. Monitoring honey bee health in five natural protected areas in Italy. Vet. Ital. 2019, 55, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Trudel-Ferland, M.; Collard, M.È.; Goulet-Beaulieu, V.; Jubinville, E.; Hamon, F.; Jean, J. Evaluation of a new automated viral RNA extraction platform for hepatitis A virus and human norovirus in testing of berries, lettuce, and oysters. Int. J. Food Microbiol. 2024, 416, 110664. [Google Scholar] [CrossRef] [PubMed]
- Mancusi, A.; Fulgione, A.; Girardi, S.; Di Maro, O.; Capuano, F.; Proroga, Y.T.R.; Cristiano, D. Droplet Digital PCR (ddPCR) Analysis for Detecting Shiga-Toxin-Producing Escherichia coli (STEC). Appl. Sci. 2022, 12, 3654. [Google Scholar] [CrossRef]
- Haque, R.; Roy, S.; Siddique, A.; Mondal, U.; Rahman, S.M.; Mondal, D.; Houpt, E.; Petri, W.A., Jr. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 2007, 76, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, M.; Savini, J.; Delobel, J.; Broll, H.; Damant, A.; Paoletti, J.; Van den Eede, G. Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing Part 2 European Network of GMO Laboratories European Union Reference Laboratory for Genetically Modified Food and Feed—Part 2; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Cazeaux, C.; Lalle, M.; Durand, L.; Aubert, D.; Favennec, L.; Dubey, J.P.; Geffard, A.; Villena, I.; La Carbona, S. Evaluation of Real-Time QPCR-Based Methods to Detect the DNA of the Three Protozoan Parasites Cryptosporidium Parvum, Giardia Duodenalis and Toxoplasma gondii in the Tissue and Hemolymph of Blue Mussels (M. edulis). Food Microbiol. 2022, 102, 103870. [Google Scholar] [CrossRef]
- Srisutham, S.; Saralamba, N.; Malleret, B.; Rénia, L.; Dondorp, A.M.; Imwong, M. Four human Plasmodium species quantification using droplet digital PCR. PLoS ONE 2017, 12, e0175771. [Google Scholar] [CrossRef]
- Baltrušis, P.; Höglund, J. Digital PCR: Modern solution to parasite diagnostics and population trait genetics. Parasit. Vectors 2023, 16, 143. [Google Scholar] [CrossRef]
- ISO 17468; Microbiology of the Food Chain—Technical Requirements and Guidance on Establishment or Revision of a Standardized Reference Method. International Organization for Standardization, Beuth: Berlin, Germany, 2016.
- ISO 16140-1; Microbiology of the Food Chain—Method Validation—Part 1—Vocabulary. International Organization for Standardization, Beuth: Berlin, Germany, 2016.
- ISO 16140-2; Microbiology of the Food Chain—Method Validation—Part 2—Protocol for the Validation of Alternative (Proprietary) Methods Against a Reference Method. International Organization for Standardization, Beuth: Berlin, Germany, 2016.
- Chalmers, R.M.; Katzer, F.; La Carbona, S.; Lalle, M.; Razakandrainibe, R.; Robertson, L.J.; Robinson, G.; Šoba, B.; Temesgen, T.; Mayer-Scholl, A. A Guide to Standardise Artificial Contamination Procedures with Protozoan Parasite Oocysts or Cysts during Method Evaluation, Using Cryptosporidium and Leafy Greens as Models. Food Control 2022, 134, 108678. [Google Scholar] [CrossRef]
- Signorelli, D.; D’Auria, L.J.; Di Stasio, A.; Gallo, A.; Siciliano, A.; Esposito, M.; De Felice, A.; Rofrano, G. Application of a Quality-Specific Environmental Risk Index for the Location of Hives in Areas with Different Pollution Impacts. Agriculture 2023, 13, 998. [Google Scholar] [CrossRef]
- Berrouch, S.; Escotte-Binet, S.; Amraouza, Y.; Flori, P.; Aubert, D.; Villena, I.; Hafid, J. Cryptosporidium spp., Giardia duodenalis and Toxoplasma gondii Detection in Fresh Vegetables Consumed in Marrakech, Morocco. Afr. Health Sci. 2020, 20, 1669–1678. [Google Scholar] [CrossRef]
- Flamminii, F.; Consalvo, A.; Cichelli, A.; Chiaudani, A. Assessing Mineral Content and Heavy Metal Exposure in Abruzzo Honey and Bee Pollen from Different Anthropic Areas. Foods 2024, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Izah, S.C. Honey as a Natural Antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Salkova, D.; Panayotova-Pencheva, M. Honey Bees and Their Products as Indicators of Environmental Pollution: A Review. Agric. Sci. Technol. 2016, 8, 175–182. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, L.C.; Djabayan-Djibeyan, P.; Prato, J.G.; Garcia Rios, C.A.; Carrero, J.C.; Trelis, M.; Fuentes, M.V. Field study of parasitic contamination of fruits, vegetables and leafy greens in the Ecuadorian Andes. F1000Research 2023, 12, 532. [Google Scholar] [CrossRef]
| Sample | Sampling Period | Sampling Site | |
|---|---|---|---|
| Honey bees | S1 | June | Nocera (SA) |
| S2 | June | Guardia Lombardi | |
| S3 | June | Palma Campania (NA) | |
| S4 | July | Presenzano (CE) | |
| S5 | July | Acerra (NA) | |
| S6 | July | Pignataro (CE) | |
| S7 | July | Marcianise (CE) | |
| S8 | July | Mercato S.S. (SA) | |
| S9 | July | Nocera (SA) | |
| S10 | July | Palma Campania (NA) | |
| S11 | July | Mercato S.S. (SA) | |
| S12 | August | San Felice Cancello (CE) | |
| S13 | August | San Felice Cancello (CE) | |
| S14 | August | Dugenta (BN) | |
| S15 | September | Roccagloriosa (SA) | |
| S16 | September | Ascea (SA) | |
| Honey | S17 | June | Guardia Lombardi (AV) |
| S18 | June | Guardia Lombardi (AV) | |
| S19 | July | Acerra (NA) | |
| S20 | July | Pignataro (CE) | |
| S21 | July | Marcianise (CE) | |
| S22 | July | Presenzano (CE) | |
| S23 | July | Mercato S.S. (SA) | |
| S24 | August | Dugenta (BN) | |
| S25 | August | Dugenta (BN) | |
| S26 | August | San Felice Cancello (CE) | |
| S27 | September | Presenzano (CE) | |
| S28 | September | Ascea (SA) | |
| Pollen | S29 | June | Guardia Lombardi (AV) |
| S30 | June | Guardia Lombardi (AV) | |
| S31 | July | Acerra (NA) | |
| S32 | July | Acerra (NA) | |
| S33 | July | Mercato S.S. (SA) | |
| S34 | August | Dugenta (BN) | |
| S35 | August | Dugenta (BN) | |
| S36 | August | San Felice Cancello (CE) |
| Primer Name | Sequence 5′–3′ | Concentrations | Reference |
|---|---|---|---|
| AF1 | CACAGAAGGGACAGAAGT | 500 nM | [15] |
| AF2 | TCGCCTTCATCTACAGTC | 500 nM | |
| AF probe | FAM—CTCTCCTCCAAGACGGCTGG—BHQ | 250 nM | |
| GD 80 For | GACGGCTCAGGACAACGGTT | 360 nM | [16] |
| GD 127 Rev | TTGCCAGCGGTGTCCG | 360 nM | |
| GD 105 probe | FAM—CCCGCGGCGGTCCCTGCTAG—BHQ1 | 90 nM | |
| COWP For | CAAATTGATACCGTTTGTCCTTCTG | 600 nM | [17] |
| COWP Rev | GGCATGTCGATTCTAATTCAGCT | 600 nM | |
| Crypto probe | FAM—TGCCATACATTGTTGTCCTGACAAATTGAAT—BHQ1 | 240 nM |
| 100 gc/μL | 10 gc/μL | 1 gc/μL | ||
|---|---|---|---|---|
| Honeybee | Toxoplasma gondii | 0.62 | 2.93 | 37.89 |
| Giardia intestinalis | 0.52 | 4.92 | 51.22 | |
| Cryptosporidium spp. | 0.41 | 5.72 | 51.07 | |
| Honey | Toxoplasma gondii | 0.64 | 7.87 | 49.33 |
| Giardia intestinalis | 0.64 | 7.86 | 65 | |
| Cryptosporidium spp. | 0.83 | 11.67 | 50 | |
| Pollen | Toxoplasma gondii | 3.75 | 29.88 | NOT DETECTED |
| Giardia intestinalis | 6.74 | 43.09 | NOT DETECTED | |
| Cryptosporidium spp. | 3.84 | 48.23 | NOT DETECTED |
| Sampling Period | Sample | Sampling Site | Toxoplasma gondii | Giardia intestinalis | Cryptosporidium spp. | |||
|---|---|---|---|---|---|---|---|---|
| DDPCR | copies/μL | DDPCR | copies/μL | DDPCR | copies/μL | |||
| June | S1 | Nocera (SA) | − | − | + | 4.6 | − | − |
| June | S2 | Guardia Lombardi (AV) | + | 14.1 | + | 13.8 | + | 12.4 |
| June | S3 | Palma Campania (NA) | + | 21.5 | + | 18.8 | + | 6.8 |
| July | S4 | Presenzano (CE) | + | 2.5 | + | 1.1 | + | 1 |
| July | S5 | Acerra (NA) | + | 3.2 | + | 5.6 | + | 4.6 |
| July | S6 | Pignataro (CE) | + | 2.1 | + | 4.3 | − | − |
| July | S7 | Marcianise (CE) | − | − | − | - | − | − |
| July | S8 | Mercato S.S. (SA) | − | − | + | 1.6 | − | − |
| July | S9 | Nocera (SA) | − | − | + | 9.6 | + | 19.2 |
| July | S10 | Palma Campania (NA) | − | − | + | 6 | + | 5.9 |
| July | S11 | Mercato S.S. (SA) | − | − | − | - | − | − |
| August | S12 | San Felice Cancello (CE) | + | 1 | + | 1.6 | + | 0.7 |
| August | S13 | San Felice Cancello (CE) | − | − | − | - | − | − |
| August | S14 | Dugenta (BN) | + | 3.7 | + | 6.5 | + | 4.3 |
| September | S15 | Roccagloriosa (SA) | − | − | − | - | − | − |
| September | S16 | Ascea (SA) | − | − | + | 2.3 | − | − |
| Prevalence (%) | 43.75 | 75 | 50 | |||||
| IC 95% | 23.1–66.8 | 50.5–89.8 | 28–72 | |||||
| Sampling Period | Sample | Sampling Site | Toxoplasma gondii | Giardia intestinalis | Cryptosporidium spp. | |||
|---|---|---|---|---|---|---|---|---|
| DDPCR | copies/μL | DDPCR | copies/μL | DDPCR | copies/μL | |||
| June | S17 | Guardia Lombardi (AV) | − | − | − | − | − | − |
| June | S18 | Guardia Lombardi (AV) | − | − | − | − | − | − |
| July | S19 | Acerra (NA) | − | − | − | − | − | − |
| July | S20 | Pignataro (CE) | + | 1.5 | − | − | − | − |
| July | S21 | Marcianise (CE) | − | − | + | 0.13 | − | − |
| July | S22 | Presenzano (CE) | − | − | − | − | − | − |
| July | S26 | Mercato S.S. (SA) | + | 1.2 | − | − | − | − |
| August | S24 | Dugenta (BN) | − | − | − | − | − | − |
| August | S23 | Dugenta (BN) | − | − | − | − | + | 0.13 |
| August | S25 | San Felice Cancello (CE) | − | − | − | − | − | − |
| September | S27 | Presenzano (CE) | − | − | − | − | − | − |
| September | S28 | Ascea (SA) | + | 0.8 | − | − | − | − |
| Prevalence (%) | 25 | 8.3 | 8.3 | |||||
| IC 95% | 8.9–53.2 | 1.5–35.4 | 1.5–35.4 | |||||
| Sampling Period | Sample | Sampling Site | Toxoplasma gondii | Giardia intestinalis | Cryptosporidium spp. | |||
|---|---|---|---|---|---|---|---|---|
| DDPCR | copies/μL | DDPCR | copies/μL | DDPCR | copies/μL | |||
| June | S29 | Guardia Lombardi (AV) | + | 7.8 | + | 7.8 | + | 7.9 |
| June | S30 | Guardia Lombardi (AV) | − | − | − | − | − | − |
| July | S31 | Acerra (NA) | + | 1.1 | − | − | − | − |
| July | S32 | Acerra (NA) | − | − | − | − | − | − |
| July | S33 | Mercato S.S. (SA) | + | 24.3 | + | 14.9 | + | 30.6 |
| August | S34 | Dugenta (BN) | + | 0.7 | + | 0.4 | + | 1.2 |
| August | S35 | Dugenta (BN) | − | − | − | − | − | − |
| August | S36 | San Felice Cancello (CE) | + | 2.2 | − | − | + | 4.4 |
| Prevalence (%) | 62.5 | 37.5 | 50 | |||||
| IC 95% | 30.6–86.3 | 13.7–69.4 | 21.5–78.5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Auria, L.J.; Mancusi, A.; Proroga, Y.T.R.; Dini, I.; Cardellicchio, T.; Di Maro, O.; De Vita, S.; Egidio, M.; Marrone, R.; Rofrano, G. Identification of Parasitic Infections by Analyzing Honeybees, Honey, and Pollen Using Droplet Digital RT-PCR. Microorganisms 2025, 13, 1487. https://doi.org/10.3390/microorganisms13071487
D’Auria LJ, Mancusi A, Proroga YTR, Dini I, Cardellicchio T, Di Maro O, De Vita S, Egidio M, Marrone R, Rofrano G. Identification of Parasitic Infections by Analyzing Honeybees, Honey, and Pollen Using Droplet Digital RT-PCR. Microorganisms. 2025; 13(7):1487. https://doi.org/10.3390/microorganisms13071487
Chicago/Turabian StyleD’Auria, Luigi Jacopo, Andrea Mancusi, Yolande Thérèse Rose Proroga, Irene Dini, Tiziana Cardellicchio, Orlandina Di Maro, Sabato De Vita, Marica Egidio, Raffaele Marrone, and Giuseppe Rofrano. 2025. "Identification of Parasitic Infections by Analyzing Honeybees, Honey, and Pollen Using Droplet Digital RT-PCR" Microorganisms 13, no. 7: 1487. https://doi.org/10.3390/microorganisms13071487
APA StyleD’Auria, L. J., Mancusi, A., Proroga, Y. T. R., Dini, I., Cardellicchio, T., Di Maro, O., De Vita, S., Egidio, M., Marrone, R., & Rofrano, G. (2025). Identification of Parasitic Infections by Analyzing Honeybees, Honey, and Pollen Using Droplet Digital RT-PCR. Microorganisms, 13(7), 1487. https://doi.org/10.3390/microorganisms13071487








