Efficacy of Clostridium butyricum Supplementation Combined with Phototherapy for Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Interventions
2.2.4. Types of Comparisons
2.2.5. Types of Outcome Measurements
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.4.1. Study Selection
2.4.2. Data Extraction
2.5. Assessment of RoB
2.6. Statistical Analysis
2.6.1. Assessment of Heterogeneity
2.6.2. Assessment of Reporting Bias
2.6.3. Subgroup and Sensitivity Analysis
2.7. Quality of Evidence
3. Results
3.1. Results of Literature Search
3.2. Characteristics of the Study
3.3. Interventions
3.4. Outcome Measures
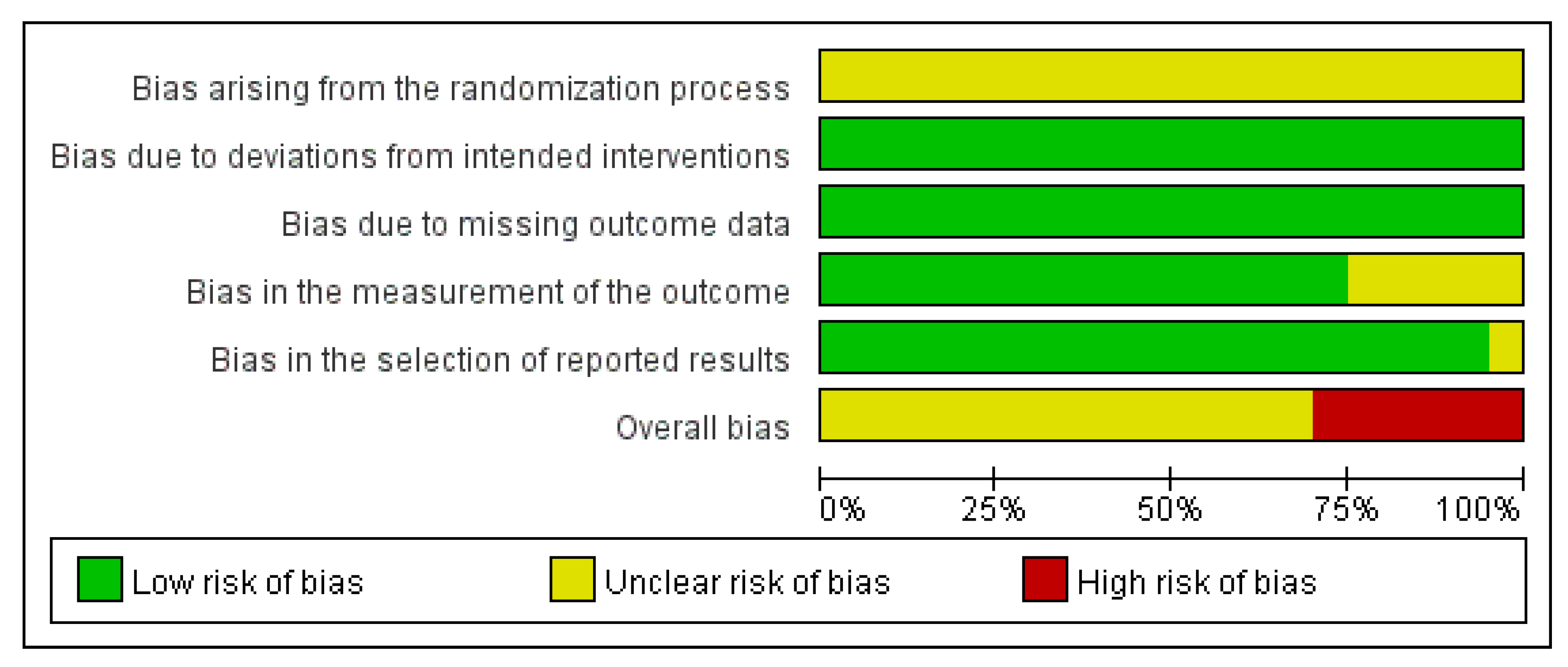
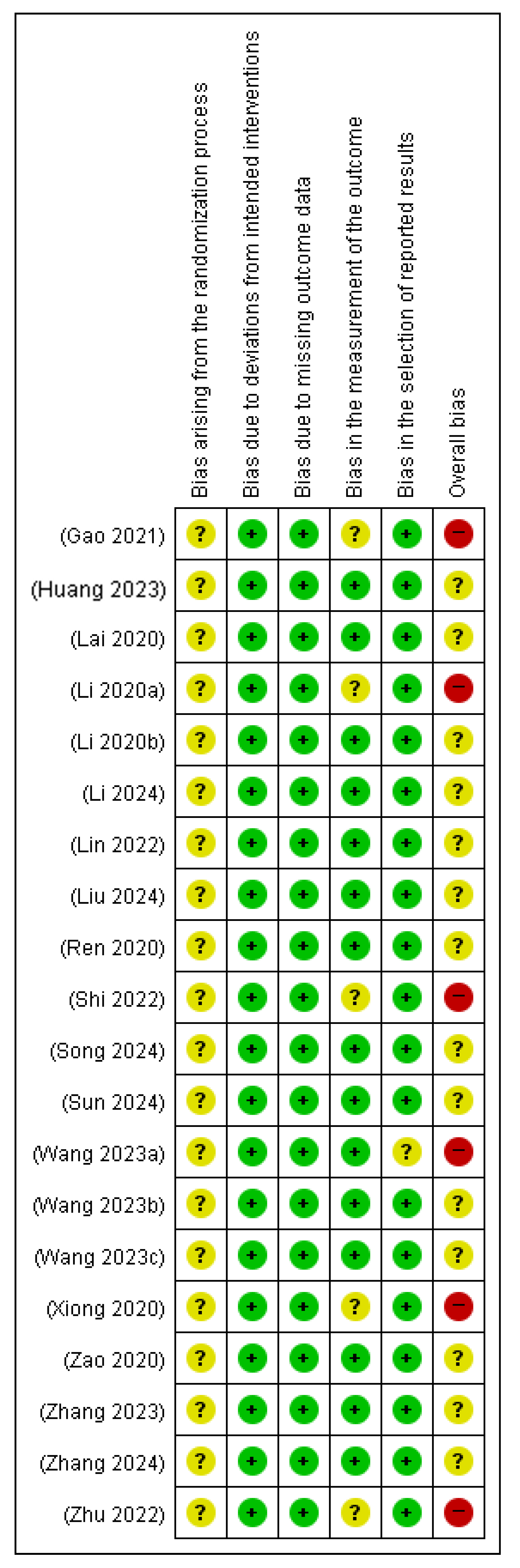
3.5. Quality Assessment
3.6. Meta-Analysis Results
3.6.1. Serum Bilirubin Level
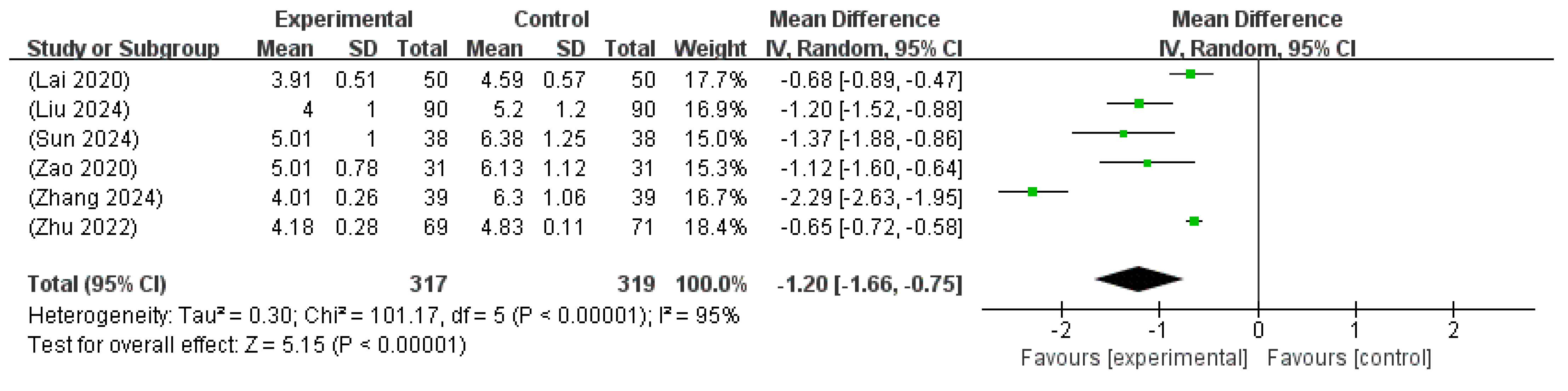
3.6.2. Duration Until Jaundice Resolution
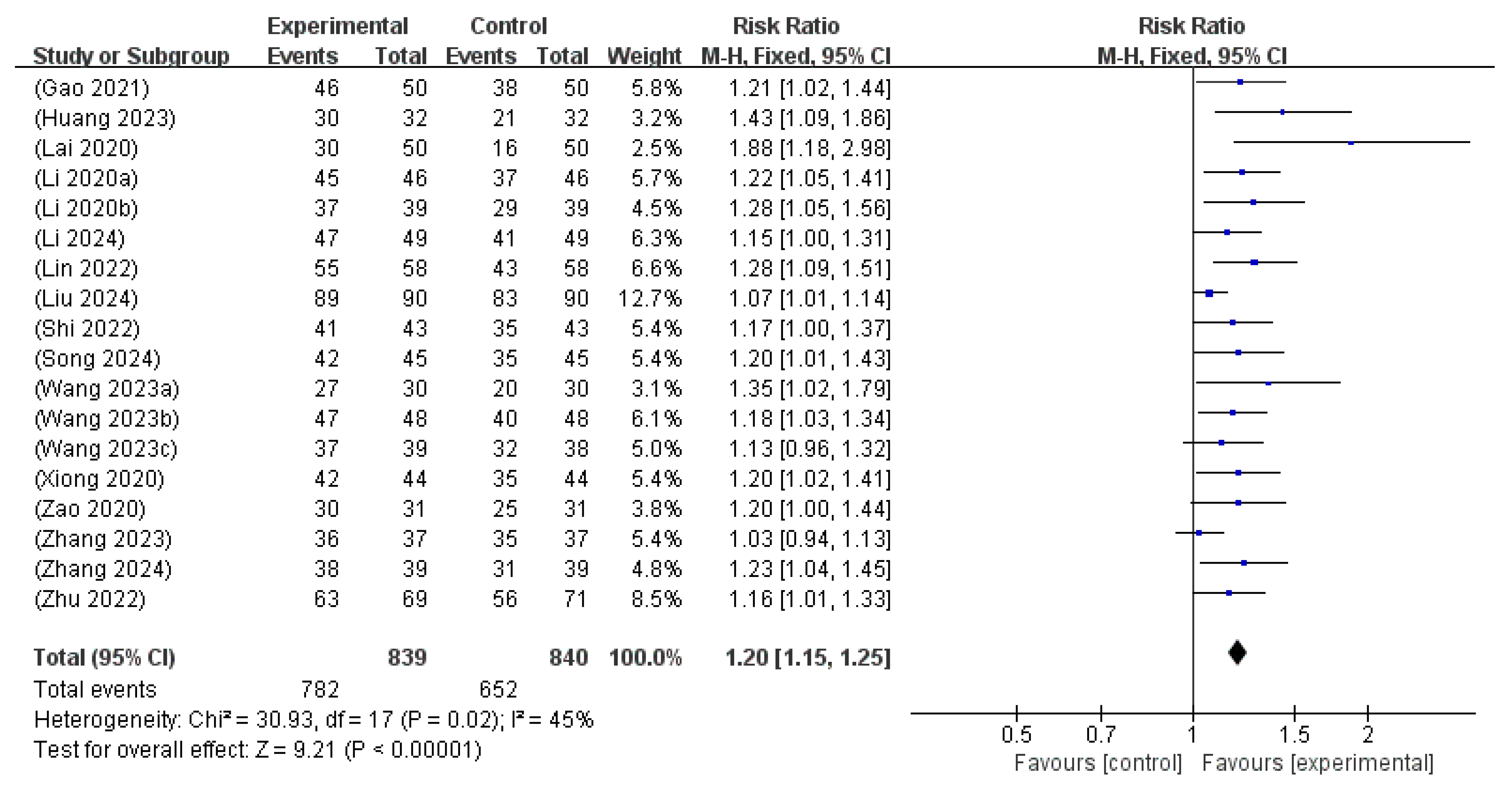
3.6.3. TER
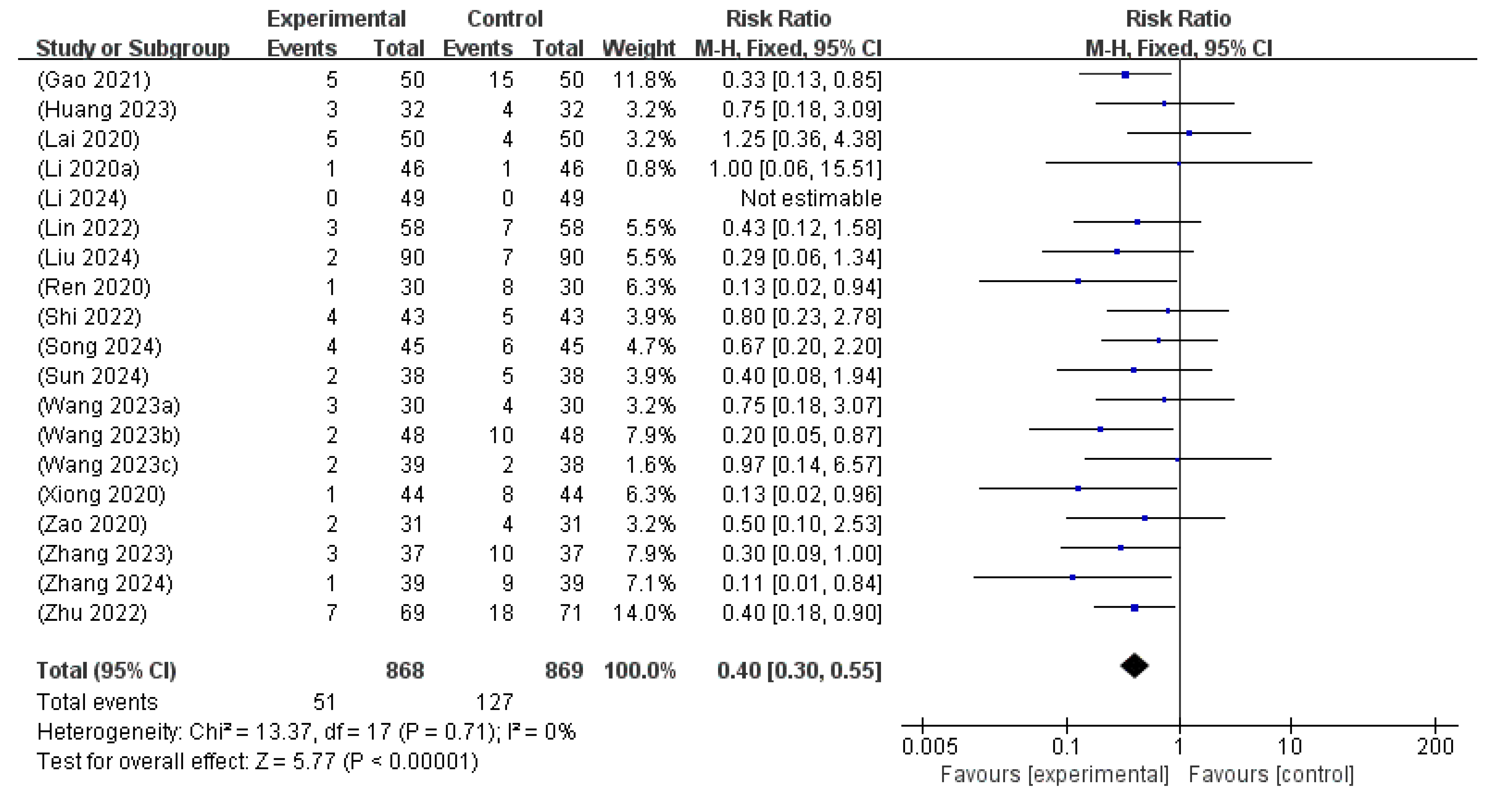
3.6.4. Adverse Events
3.6.5. Transcutaneous Bilirubin Level
3.6.6. Length of Hospital Stay
3.7. Publication Bias
3.8. Sensitivity Analyses and Subgroup Analyses
3.9. GRADE Certainty of Evidence
4. Discussion
4.1. Summary of This Review
4.2. Clinical Implications, Limitations, and Suggestions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NH | Neonatal hyperbilirubinemia |
| C. butyricum | Clostridium butyricum |
| RCTs | Randomized clinical trials |
| TER | Total effective rate |
| RoB | Risk of Bias |
| RR | Risk ratio |
| MD | Mean difference |
| SMD | Standardized mean difference |
| CI | Confidence interval |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
References
- Santosa, I.; Shoji, H.; Itoh, S.; Shimizu, T. Roles of probiotics in reduction of neonatal jaundice in term newborns. Juntendo Iji Zasshi 2022, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Lin, W.-Y.; Chen, Y.-T.; Lin, H.-Y.; Ho, H.-H.; Kuo, Y.-W.; Lin, J.H.; Huang, Y.Y.; Wang, H.S.; Chiu, H.Y.; et al. Adjuvant probiotic Bifidobacterium animalis subsp. lactis CP-9 improves phototherapeutic treatment outcomes in neonatal jaundice among full-term newborns: A randomized double-blind clinical study. Medicine 2022, 101, e31030. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yuan, T. The role of microbiota in neonatal hyperbilirubinemia. Am. J. Transl. Res. 2020, 12, 7459–7474. [Google Scholar]
- Navarro-Tapia, E.; Almeida-Toledano, L.; Sebastiani, G.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Effects of microbiota imbalance in anxiety and eating disorders: Probiotics as novel therapeutic approaches. Int. J. Mol. Sci. 2021, 22, 2351. [Google Scholar] [CrossRef]
- Duan, M.; Han, Z.H.; Huang, T.; Yang, Y.; Huang, B. Characterization of gut microbiota and short-chain fatty acids in breastfed infants with or without breast milk jaundice. Lett. Appl. Microbiol. 2021, 72, 60–67. [Google Scholar] [CrossRef]
- Mutlu, M.; Aslan, Y.; Kader, Ş.; Aktürk Acar, F. Preventive effects of probiotic supplementation on neonatal hyperbilirubinemia caused by isoimmunization. Am. J. Perinatol. 2020, 37, 1173–1176. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Zeng, L.; Yang, X.; Jiang, L.; Gui, G.; Zhang, Z. Probiotics supplementation therapy for pathological neonatal jaundice: A systematic review and meta-analysis. Front. Pharmacol. 2017, 8, 432. [Google Scholar] [CrossRef]
- Deshmukh, J.; Deshmukh, M.; Patole, S. Probiotics for management of neonatal hyperbilirubinemia: A systematic review of randomised controlled trials. J. Matern. Fetal Neonatal Med. 2017, 32, 154–163. [Google Scholar] [CrossRef]
- Sada, R.; Matsuo, H.; Motooka, D.; Kutsuna, S.; Hamaguchi, S.; Yamamoto, G.; Ueda, A. Clostridium butyricum bacteremia associated with probiotic use, Japan. Emerg. Infect. Dis. 2024, 30, 665–671. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Zhang, Y.; Wang, Y.; Wang, P.; Zhang, L. Meta-analysis of Clostridium butyricum-based dual live probiotic capsules for the treatment of neonatal hyperbilirubinemia. Chin. J. Clin. Ration. Drug Use 2022, 15, 119–121. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 January 2025).
- Guyatt, G.; Rennie, D.; Meade, M.; Cook, D. Users’ Guides to the Medical Literature: Essentials of Evidence-Based Clinical Practice, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Gao, K.; Abbas, S.; Faheem, A.; Zeeshan, M.; Masud, R.; Akram, M.; Awais, Z.; Muhammad, S.A.; Raza, A.; Laique, T. Therapeutic effect of micro-ecologics combined with blue light irradiation on neonatal jaundice: Randomized control trial. Pak. J. Med. Health Sci. 2021, 15, 2294–2296. [Google Scholar] [CrossRef]
- Huang, S. Analysis of the effect of Clostridium butyricum double-live powder combined with LED cold light source blue light irradiation in the treatment of neonatal hyperbilirubinemia. Med. Theory Pract. 2023, 36, 2788–2790. [Google Scholar] [CrossRef]
- Lai, Z.; Zhong, H.; Fang, Z. Clinical observation of as an adjunctive treatment for neonatal jaundice. Strait Pharm. J. 2020, 32, 170–171. [Google Scholar]
- Li, J.; Lin, F.; Chen, X. Observation on the therapeutic effect of Clostridium butyricum live powder combined with blue light therapy for neonatal hyperbilirubinemia. Mod. Diagn. Treat. 2020, 31, 405–407. [Google Scholar]
- Li, Y. Clinical analysis of the efficacy of probiotics combined with phototherapy in the treatment of neonatal hyperbilirubinemia. Electron. J. Clin. Med. Lit. 2020, 7, 134–135. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liang, G.; Zhang, H.; Kang, J. Effect of Clostridium butyricum dual live powder combined with intermittent blue light therapy on neonatal hyperbilirubinemia and its impact on bilirubin and neurotrophic factor levels. Chin. Med. Eng. 2024, 32, 89–92. [Google Scholar] [CrossRef]
- Lin, K. Effect of probiotics combined with intermittent blue light irradiation on neonatal hyperbilirubinemia. Guide China Med. 2022, 20, 104–106. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhao, X. efficacy of blue light combined with Clostridium butyricum dual active bacterial powder in 180 cases with neonatal pathological jaundice. Shanxi Med. J. 2024, 53, 1620–1623. [Google Scholar] [CrossRef]
- Ren, C. Effects of combined Clostridium butyricum and Bifidobacterium powder, Live, with blue light therapy on TBIL and IBIL levels in children with neonatal jaundice. Acta Med. Sin. 2020, 33, 76–79. [Google Scholar] [CrossRef]
- Shi, S.; Chen, X.; Li, J. Analysis of the efficacy of Clostridium butyricum dual live powder combined with intermittent blue light irradiation in the treatment of neonatal hyperbilirubinemia. J. Math. Med. 2022, 35, 409–411. [Google Scholar] [CrossRef]
- Song, X.; Yao, J. The effect of Clostridium butyricum dual live powder combined with blue light irradiation treatment on serum bilirubin and inflammatory index levels in neonates with hyperbilirubinemia. Reflexol. Rehabil. Med. 2024, 5, 33–39. [Google Scholar]
- Sun, L.; Lin, L.; Wei, E. Comparison of the effects of different probiotics combined with phototherapy in the treatment of neonatal pathological jaundice. Chin. J. Clin. Ration. Drug Use 2024, 17, 130–133. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J. The Clinical effect of blue light irradiation combined with probiotic intervention on neonatal hyperbilirubinemia. Friends Diabetes 2023, 254, 36–37. [Google Scholar]
- Wang, J.; Liang, K. Effect of combined Clostridium butyricum and Bifidobacterium powders, live, combined with double-sided blue light in the treatment of neonatal hyperbilirubinemia and its influence on β2-mg level. Clin. Med. Res. Pract. 2023, 3, 58–61. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J. The Effect of probiotics in adjuvant treatment of neonatal pathological jaundice on the intestinal flora and immune function of children. Res. Women’s Health China Abroad 2023, 24, 5–7. [Google Scholar]
- Xiong, X.; Hu, F. Effect of intermittent blue light irradiation combined with Clostridium butyricum on serum bilirubin levels and prognosis in neonatal jaundice. Prim. Med. Forum 2020, 24, 186–187. [Google Scholar] [CrossRef]
- Zhao, Q. Clinical effect of short-term multiple blue light irradiation combined with Clostridium butyricum in the treatment of neonatal hyperbilirubinemia. Clin. Med. Res. Pract. 2020, 13, 104–106. [Google Scholar] [CrossRef]
- Zhang, Y. Effect of Clostridium butyricum dual live powder combined with blue light irradiation on immune function in the treatment of neonatal hyperbilirubinemia. Chin. J. Clin. Ration. Drug Use 2023, 16, 105–111. [Google Scholar] [CrossRef]
- Zhang, X. Clinical observation of adjuvant treatment of neonatal hyperbilirubinemia with Clostridium butyricum live powder. Matern. Child. Nurs. 2024, 4, 3079–3081. [Google Scholar]
- Zhu, S. Observation on the effect of intermittent blue light irradiation combined with Clostridium butyricum powder in the treatment of neonatal hyperbilirubinemia. Tibet Med. 2022, 43, 47–49. [Google Scholar]
- Neonatal Jaundice. Available online: https://emedicine.medscape.com/article/974786-overview?form=fpf (accessed on 1 April 2025).
- Pearson, H.A. Life-Span of the Fetal Red Blood Cell. J. Pediatr. 1967, 70, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Bravdo, Y.; Habib, C.; Maor, I.; Mousa, J.; Shahbarat, S.; Shahak, E.; Shalata, A. The Genetics of Glucose-6-Phosphate-Dehydrogenase (G6PD) and Uridine Diphosphate Glucuronosyl Transferase 1A1 (UGT1A1) Promoter Gene Polymorphism in Relation to Quantitative Biochemical G6PD Activity Measurement and Neonatal Hyperbilirubinemia. Children 2023, 10, 1172. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Kanai, T.; Mikami, Y.; Hayashi, A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 928–939. [Google Scholar] [CrossRef]
- Lu, J.; Yao, J.; Xu, Q.; Zheng, Y.; Dong, X. Clostridium butyricum relieves diarrhea by enhancing digestive function, maintaining intestinal barrier integrity, and relieving intestinal inflammation in weaned piglets. Livest. Sci. 2020, 239, 104112. [Google Scholar] [CrossRef]
- Aslamzai, M.; Hamidi, M.F.; Halimi, A. The effects of dual-strain probiotics on the weight gain in premature neonates of Kabul City: A randomized clinical trial. Glob. Pediatr. 2023, 5, 100062. [Google Scholar] [CrossRef]
- Tao, R.; Zong, G.; Pan, Y.; Li, H.; Cheng, P.; Deng, R.; Chen, W.; Wang, A.; Xia, S.; Tang, W.; et al. Clostridium butyricum and Clostridium tyrobutyricum: Angel or devil for necrotizing enterocolitis? mSystems 2023, 8, e00732-23. [Google Scholar] [CrossRef]
- Xu, J.; Lawley, B.; Wong, G.; Otal, A.; Chen, L.; Ying, T.J.; Lin, X.; Pang, W.W.; Yap, F.; Chong, Y.S.; et al. Ethnic diversity in infant gut microbiota is apparent before the introduction of complementary diets. Gut Microbes 2020, 11, 1362–1373. [Google Scholar] [CrossRef]



| First Author (Year) | Sample Size (E/C) | Age Distribution (Mean ± SD) | Body Weight [Birth Weight] (Mean ± SD) | Gestational Age (mean ± SD) | Duration of Illness (Mean ± SD) | Type of Jaundice | Experimental Intervention (E) | Total Treatment Periods | Outcome Measurement | Adverse Events Incidence |
|---|---|---|---|---|---|---|---|---|---|---|
|
Control Intervention (C) | ||||||||||
| Gao (2021) [14] | 100 (50/50) | E: 3.58 ± 1.291 d | [E: 3.39 ± 0.475 kg] | NR | NR | NR | Probiotics + (C) | 3 d | (1)(2)(3) | E: 5/50 |
| C: 3.25 ± 1.401 d | [C: 3.28 ± 0.451 kg] | NR | NR | NR | phototherapy | C: 14/50 | ||||
| Huang (2023) [15] | 64 (32/32) | E: 16.57 ± 3.11 d | NR | NR | NR | NR | Probiotics + (C) | 5 d | (1)(2)(4)(5)(6)(7)(8) | E: 3/32 |
| C: 17.26 ± 3.52 d | NR | NR | NR | NR | phototherapy | C: 4/32 | ||||
| Lai (2020) [16] | 100 (50/50) | E: 2~6 d | normal range | full-term infant | NR | NR | Probiotics + (C) | 5 d | (1)(2)(3)(9) | E: 5/50 |
| C: 2~6 d | normal range | full-term infant | NR | NR | phototherapy | <5 d | C: 4/50 | |||
| Li (2020a) [17] | 92 (46/46) | E: 7.12 ± 2.31 d | E: 3.25 ± 0.21 kg | E: 38.75 ± 1.23 w | 4.23 ± 1.16 d | NR | Probiotics + (C) | 5 d | (1)(2)(4) | E: 1/46 |
| C: 7.15 ± 2.28 d | C: 3.24 ± 0.23 kg | C: 38.65 ± 1.05 w | 4.28 ± 1.18 d | NR | phototherapy | C: 1/46 | ||||
| Li (2020b) [18] | 78 (39/39) | E: 18.94 ± 1.06 d | NR | NR | NR | NR | Probiotics + (C) | 5 d | (1)(4) | NR |
| C: 18.22 ± 1.37 d | NR | NR | NR | NR | phototherapy | NR | ||||
| Li (2024) [19] | 98 (49/49) | E: 10.43 ± 2.02 d | E: 3.27 ± 0.33 kg | NR | NR | infectious (n = 25), hemolytic (n = 13), others (n = 11) | Probiotics + (C) | 2 w | (1)(2)(4)(7)(10)(11) | E: 0/49 |
| C: 10.85 ± 2.07 d | C: 3.18 ± 0.35 kg | NR | NR | infectious (n = 24), hemolytic (n = 15), others (n = 10) | phototherapy | C: 0/49 | ||||
| Lin (2022) [20] | 116 (58/58) | NR | NR | NR | NR | NR | Probiotics + (C) | NR | (1)(2)(4)(8)(12)(13)(14)(15) | E: 3/58 |
| NR | NR | NR | NR | NR | phototherapy | C: 7/58 | ||||
| Liu (2024) [21] | 180 (90/90) | E: 5.2 ± 0.4 d | NR | NR | NR | pathological jaundice | Probiotics + (C) | 7 d | (1)(2)(3)(4)(9)(15)(16)(17) | E: 2/90 |
| C: 5.2 ± 0.5 d | NR | NR | NR | phototherapy | C: 7/90 | |||||
| Ren (2020) [22] | 60 (30/30) | E: 15.02 ± 6.54 d | E: 4.25 ± 1.20 kg | NR | NR | NR | Probiotics + (C) | 5 d | (2)(4) | E: 1/30 |
| C: 15.01 ± 6.99 d | C: 4.30 ± 1.29 kg | NR | NR | NR | phototherapy | C: 8/30 | ||||
| Shi (2022) [23] | 86 (43/43) | E: 7.05 ± 0.75 d | NR | E: 39.63 ± 0.52 w | NR | NR | Probiotics + (C) | 5 d | (1)(2)(4)(18) | E: 4/30 |
| C: 5.52 ± 0.63 d | NR | C: 39.63 ± 0.52 w | NR | NR | phototherapy | C: 5/30 | ||||
| Song (2024) [24] | 90 (45/45) | E: 5.24 ± 1.05 d | NR | E: 38.58 ± 0.72 w | NR | NR | Probiotics + (C) | 7 d | (1)(2)(4)(8)(19)(20) | E: 4/45 |
| C: 5.29 ± 1.08 d | NR | C: 38.75 ± 0.79 w | NR | NR | phototherapy | C: 6/45 | ||||
| Sun (2024) [25] | 76 (38/38) | E: 12.56 ± 3.18 d | NR | E: 39.39 ± 1.23 w | NR | pathological jaundice | Probiotics + (C) | 5 d | (2)(4)(9)(15)(16)(17) | E: 2/45 |
| C: 12.56 ± 2.93 d | NR | C: 39.59 ± 1.26 w | NR | phototherapy | C: 5/45 | |||||
| Wang (2023a) [26] | 60 (30/30) | E: 5.37 ± 0.22 d | NR | NR | NR | NR | Probiotics + (C) | 8 d | (1)(2)(3)(4) | E: 3/30 |
| C: 5.41 ± 0.23 d | NR | NR | NR | NR | phototherapy | C: 4/30 | ||||
| Wang (2023b) [27] | 96 (48/48) | E: 12.56 ± 3.63 d | E: 3.24 ± 0.68 kg | E: 40.51 ± 0.49 w | NR | infectious (n = 28), hemolytic (n = 13), perinatal factors (n = 4), others (n = 3) | Probiotics + (C) | 2 w | (1)(2)(18)(21)(22) (23)(24)(25)(26) | E: 2/48 |
| C: 12.94 ± 3.81 d | C: 3.28 ± 0.73 kg | C: 40.38 ± 0.69 w | NR | infectious (n = 29), hemolytic (n = 11), perinatal factors (n = 5), others (n = 3) | phototherapy | C: 9/48 | ||||
| Wang (2023c) [28] | 77 (39/38) | E: 8.04 ± 1.99 d | E: 3.26 ± 0.68 kg | E: 39.35 ± 1.87 w | 6.41 ± 3.02 d | Pathological jaundice | Probiotics + (C) | 3 d | (1)(2)(27)(28) | E: 2/39 |
| C: 7.97 ± 2.36 d | C: 3.24 ± 0.61 kg | C: 39.42 ± 1.63 w | 6.29 ± 2.87 d | phototherapy | C: 2/38 | |||||
| Xiong (2020) [29] | 88 (44/44) | E: 6.25 ± 1.46 d | NR | NR | 4.23 ± 0.24 d | NR | Probiotics + (C) | 4 d | (1)(2)(4) | E: 1/44 |
| C: 6.29 ± 1.51 d | NR | NR | 4.20 ± 0.23 d | NR | phototherapy | C: 8/44 | ||||
| Zhao (2020) [30] | 62 (31/31) | E: 13.86 ± 7.67 d | E: 3.45 ± 0.88 kg | E: 40.42 ± 1.06 w | NR | infectious (n = 19), hemolytic (n = 7), perinatal factors (n = 3), others (n = 2) | Probiotics + (C) | 5 d | (1)(2)(3)(4)(9)(18)(21) | E: 2/31 |
| C: 13.68 ± 7.37 d | C: 3.52 ± 0.91 kg | C: 40.52 ± 1.09 w | NR | infectious (n = 18), hemolytic (n = 6), perinatal factors (n = 4), others (n = 3) | phototherapy | C: 4/31 | ||||
| Zhang (2023) [31] | 74 (37/37) | E: 7.01 ± 2.02 d | NR | NR | NR | infectious (n = 15), hemolytic (n = 15), breast milk jaundice (n = 7) | Probiotics + (C) | 3–10 d | (1)(2)(4)(8)(15)(17)(19)(20)(27) | E: 3/37 |
| C: 6.97 ± 2.13 d | NR | NR | NR | infectious (n = 13), hemolytic (n = 16), breast milk jaundice (n = 8) | phototherapy | C: 10/37 | ||||
| Zhang (2024) [32] | 78 (39/39) | E: 8.03 ± 1.19 d | E: 3020.23 ± 1049.90 g | E: 39.86 ± 1.75 w | NR | NR | Probiotics + (C) | 5 d | (1)(2)(4)(9) | E: 1/39 |
| C: 7.99 ± 1.21 d | C: 3020.15 ± 1049.85 g | C: 39.84 ± 1.76 w | NR | NR | phototherapy | C: 9/39 | ||||
| Zhu (2022) [33] | 140 (69/71) | E: 2.35 ± 3.16 d | E: 3.14 ± 0.15 kg | E: 39.17 ± 1.06 w | NR | hemolytic (n = 36), hepatocellular (n = 20), cholestatic jaundice (n = 11), others (n = 2) | Probiotics + (C) | 5 d | (1)(2)(4)(9) | E: 7/69 |
| C: 2.36 ± 2.26 d | C: 3.15 ± 0.13 kg | C: 40.12 ± 1.01 w | NR | hemolytic (n = 32), hepatocellular (n = 23), cholestatic jaundice (n = 10), others (n = 6) | phototherapy | C: 18/71 |
| First Author (Year) | Probiotics Information | Dosage (Time) | Frequency (Day) | Phototherapy Information | Frequency (Day) |
|---|---|---|---|---|---|
| Gao (2021) [14] | C. butyricum double living capsule | 420 mg | 2 times | NR | NR |
| Huang (2023) [15] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Wavelength: 427 to 475 nm | NR |
| Lai (2020) [16] | C. butyricum–based triple probiotic supplementation: consisting of C. butyricum, Enterococcus faecalis, and Bacillus mesentericus | 100 mg | 3 times | Wavelength: 427 to 475 nm Treatment duration: 18 h | 1 time |
| Li (2020a) [17] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Treatment duration: 2 to 6 h Pause interval: 2 to 4 h Total daily exposure: less than 10 h | 2 times |
| Li (2020b) [18] | C. butyricum probiotic supplementation | 500 mg | 3 times | Wavelength: 427 to 475 nm Treatment duration: 6 to 8 h | 1 time |
| Li (2024) [19] | C. butyricum–Bifidobacterium probiotic supplementation | NR | NR | Wavelength: 420 nm Treatment duration: 12 h Pause interval: 12 h | 1 time |
| Lin (2022) [20] | C. butyricum–based triple probiotic supplementation: consisting of C. butyricum, Enterococcus faecalis, and Bacillus mesentericus | 200 mg | 3 times | Total daily exposure: less than 18 h Pause interval: 6 h | NR |
| Liu (2024) [21] | C. butyricum–Bifidobacterium probiotic supplementation | NR | NR | Wavelength: 420 to 480 nm Treatment duration: 8 h Pause interval: 8 h | NR |
| Ren (2020) [22] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Wavelength: 420 to 470 nm Treatment duration: 12 h | NR |
| Shi (2022) [23] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 3 times | Wavelength: 420 to 470 nm Treatment duration: 14 h Pause interval: 10 h | NR |
| Song (2024) [24] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Wavelength: 425 to 475 nm Treatment duration: 3 h | 2 times |
| Sun (2024) [25] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 1 time | Treatment duration: 4 h Pause interval: 5 h Total daily exposure: Less than 16 h | NR |
| Wang (2023a) [26] | C. butyricum–Bifidobacterium probiotic supplementation | 400 mg | 3 times | Treatment duration: 8 h Pause interval: 16 h | NR |
| Wang (2023b) [27] | C. butyricum–Bifidobacterium probiotic supplementation | 400 mg | 2 times | Wavelength: 420 to 470 nm Treatment duration: 12 h Pause interval: 12 h | 1 time |
| Wang (2023c) [28] | C. butyricum probiotic supplementation | 500 mg | 2 times | Treatment duration: 8 to 12 h | NR |
| Xiong (2020) [29] | C. butyricum probiotic supplementation | 500 mg | 2 times | Treatment duration: 4 times/h | 4 times |
| Zhao (2020) [30] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Treatment duration: 8 h Pause interval: 4 h | 2 times |
| Zhang (2023) [31] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Wavelength: 425 to 475 nm Total daily exposure: less than 8 to 12 h | NR |
| Zhang (2024) [32] | C. butyricum probiotic supplementation | 500 mg | 2 times | Treatment duration: 5 to 6 h Pause interval: 2 to 4 h Total daily exposure: less than 8 to 12 h | 2 times |
| Zhu (2022) [33] | C. butyricum–Bifidobacterium probiotic supplementation | 500 mg | 2 times | Treatment duration: 8 h | 2 times |
| Outcomes | No. Participants (Studies) | Anticipated Absolute Effects (95% CI) | Relative Effect (95% CI) | Heterogeneity (I2) | Quality of Evidence (GRADE) | |
|---|---|---|---|---|---|---|
| Risk with Control Group | Risk with Intervention Group | |||||
| Total bilirubin levels | 870 (10 RCTs) | - | SMD 1.54 lower (2.21 lower to 0.86 lower) | - | 95 | ⨁⨁⨁◯ Moderate a,b |
| Direct bilirubin levels | 410 (4 RCTs) | - | SMD 0.91 lower (1.32 lower to 0.50 lower) | - | 73 | ⨁⨁⨁◯ Moderate a,b |
| Indirect bilirubin levels | 448 (6 RCTs) | - | SMD 2.03 lower (2.98 lower to 1.07 lower) | - | 94 | ⨁⨁⨁◯ Moderate a,b |
| The time of resolution of jaundice | 636 (6 RCTs) | - | MD 1.2 lower (1.66 lower to 0.75 lower) | - | 95 | ⨁⨁⨁◯ Moderate a,b |
| Total effective rate | 1679 (18 RCTs) | 776 per 1000 | 931 per 1000 (893 to 970) | RR 1.20 (1.15 to 1.25) | 45 | ⨁⨁⨁◯ Moderate a |
| Adverse events | 1737 (19 RCTs) | 147 per 1000 | 57 per 1000 (43 to 78) | RR 0.39 (0.29 to 0.53) | 0 | ⨁⨁⨁◯ Moderate a |
| Transcutaneous bilirubin levels | 502 (5 RCTs) | - | SMD 1.11 lower (1.62 lower to 0.59 lower) | - | 85 | ⨁⨁◯◯ Low a,b,c |
| Length of hospital stay | 446 (4 RCTs) | - | MD 1.66 lower (2.44 lower to 0.88 lower) | - | 94 | ⨁⨁◯◯ Low a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-J.; Go, H.-Y.; Sung, H.-K. Efficacy of Clostridium butyricum Supplementation Combined with Phototherapy for Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 1441. https://doi.org/10.3390/microorganisms13071441
Kim E-J, Go H-Y, Sung H-K. Efficacy of Clostridium butyricum Supplementation Combined with Phototherapy for Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(7):1441. https://doi.org/10.3390/microorganisms13071441
Chicago/Turabian StyleKim, Eun-Jin, Ho-Yeon Go, and Hyun-Kyung Sung. 2025. "Efficacy of Clostridium butyricum Supplementation Combined with Phototherapy for Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 7: 1441. https://doi.org/10.3390/microorganisms13071441
APA StyleKim, E.-J., Go, H.-Y., & Sung, H.-K. (2025). Efficacy of Clostridium butyricum Supplementation Combined with Phototherapy for Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis. Microorganisms, 13(7), 1441. https://doi.org/10.3390/microorganisms13071441