Abstract
The sterol demethylation inhibitors (DMIs) are among the most widely used fungicides for controlling black Sigatoka (Mycosphaerella fijiensis) and yellow Sigatoka (Mycosphaerella musicola) in banana plantations in Brazil. Black Sigatoka is considered more important due to causing yield losses of up to 100% in commercial banana crops under predisposing conditions. In contrast, yellow Sigatoka is important due to its widespread occurrence in the country. This study aimed to determine the current sensitivity levels of Mf and Mm populations to DMI fungicides belonging to the chemical group of triazoles. Populations of both species were sampled from commercial banana plantations in Registro, Vale do Ribeira, São Paulo (SP), Ilha Solteira, Northwestern SP, and Janaúba, Northern Minas Gerais, and were further characterized phenotypically. Additionally, allelic variation in the CYP51 gene was analyzed in populations of these pathogens to identify and characterize major mutations and/or mechanisms potentially associated with resistance. Sensitivity to the triazoles propiconazole and tebuconazole was determined by calculating the 50% inhibitory concentration of mycelial growth (EC50) based on dose–response curves ranging from 0 to 5 µg mL−1. Variation in sensitivity to fungicides was evident with all nine Mf isolates showing moderate resistance levels to both propiconazole or tebuconazole, while 11 out of 42 Mm strains tested showed low to moderate levels of resistance to these triazoles. Mutations leading to CYP51 substitutions Y136F, Y461N/H, and Y463D in Mm and Y461D, G462D, and Y463D in Mf were associated with low or moderate levels of resistance to the triazoles. Interestingly, Y461H have not been reported before in Mm or Mf populations, and this alteration was found in combination with V106D and A446S. More complex CYP51 variants and CYP51 promoter inserts associated with upregulation of the target protein were not detected and can explain the absence of highly DMI-resistant strains in Brazil. Disease management programs that minimize reliance on fungicide sprays containing triazoles will be needed to slow down the further evolution and spread of novel CYP51 variants in Mf and Mm populations in Brazil.
1. Introduction
The banana Sigatoka disease complex (BSDC) encompasses black Sigatoka (caused by Mycosphaerella fijiensis, Mf) and yellow Sigatoka (caused by M. musicola, Mm). Black Sigatoka was initially reported in Brazil in 1998 in the Amazon region and has since been detected in 19 states, including São Paulo and Bahia, the country’s primary banana-producing states [1,2,3,4,5]. This disease is a significant constraint to banana production, capable of reducing yields by up to 100%. However, there are reports suggesting that black Sigatoka may have been misidentified as the less devastating yellow Sigatoka, which was first identified in the Amazon region in 1944 and is widespread across all banana-growing regions of Brazil, causing yield losses of up to 50% [6,7,8,9].
The BSDC diseases are polycyclic, with pathogens Mf and Mm exhibiting a mixed reproductive system enabling dispersal via asexual conidia over short distances by rain splash and sexually produced ascospores over long distances by air [10,11]. Populations of Mf and Mm in Brazil, Mexico, and the Philippines show high genotypic variation due to sexual reproduction and gene flow from distant pathogen migration [6,8,12,13]. Genetic resistance to BSDC is generally absent or partial in most commercial banana cultivars, necessitating disease control strategies primarily based on calendar-based systemic or protectant fungicide sprays [6].
In regions with high disease pressure, extensive fungicide applications, up to 52 sprays of protectant or 26 sprays of systemic fungicides per year, are applied, leading to increased production costs and environmental impact [5,6,14]. Conversely, in the Ribeira Valley of Brazil, black Sigatoka control involves weekly disease monitoring and frequent fungicide applications, resulting in 15–20 sprays per year [5]. Control heavily relies on systemic site-specific quinone outside inhibitor (QoI), demethylation inhibitor (DMI), and the more recently introduced succinate dehydrogenase inhibitors (SDHIs) fungicides. However, this intensive use of fungicides exerts selective pressure on pathogen populations, leading to the emergence, selection, and spread of fungicide-resistant strains [15,16,17,18,19,20].
Several studies on fungicide resistance in populations of Mf and Mm from Brazil have been carried out. Concerning QoIs, the first surveys in Brazil initially showed no resistance in Mf populations from the Amazon and Ribeira Valley in São Paulo, as well as in Mm populations from the Federal District and São Paulo sampled from 2008 to 2018 [6,21,22]. However, recent data indicate varying levels of QoI resistance, with 10.0% and 9.4% of Mf and Mm isolates, respectively, carrying the G143A substitution in cytochrome b, particularly in Southeastern Brazil (São Paulo and Minas Gerais), a region with an intensive fungicide use [14,19].
Similarly, resistance to SDHI fungicides has also been detected in Mf and Mm populations in Southeastern Brazil sampled during 2020–2021, a few years after its labeling [20]. Globally, SDHI fungicides approved for BSDC management in banana plantations include boscalid, fluopyram, fluxapyroxad, and isopyrazam [23]. In Brazil, only one SDHI fungicide co-formulation (Collis™ from BASF) with the SDHI boscalid and the QoI kresoxim-methyl is labeled for BSDC [20]. Some fungal isolates with resistance to boscalid and fluxapyroxad showed Sdh target site alterations (SdhC N55D, SdhB E196Q, and SdhD K66N) [20]. None of these substitutions have been associated with SDHI fungicide resistance in BSDC pathogens, based on the latest survey conducted by the SDHI Working Group in 2022 [23]. Continued monitoring for other mutations and resistance mechanisms such as multiple Sdh paralogs and overexpression of efflux pumps is critical for effective disease control strategies [20].
Regarding resistance to DMI fungicides, there is evidence of reduced sensitivity in Mf populations since 2008–2009 [21,22]. The DMI fungicides, introduced in the 1980s, are among the most widely used for controlling Sigatoka diseases [24,25]. DMIs inhibit the biosynthesis of ergosterol, an essential component of the fungal cell membrane [26]. Their target is 14α-demethylase encoded by the CYP51 gene, a member of the cytochrome P450 family [27]. Studies on plant pathogens have shown that target site mutations appear to be the least predictable for CYP51 [28]. Given the frequent spray of DMI fungicides in banana plantations, the resulting selection pressure may lead to increased frequency of new Mf and Mm genotypes with reduced sensitivity to these fungicides. Continuous monitoring and detailed investigation into resistance evolution and spread in favorable agroecosystems are crucial for effective disease management and sustainable control strategies [3,10,29,30,31].
Mechanisms of resistance to DMI involving CYP51 mutations have been reported in Mf and Mm both elsewhere and in Brazil [15,24], with CYP51 overexpression being linked to tandem repeats in Mf [17]. In Brazil, CYP51 mutations resulting in G462D and Y463H in Mf [14] and A381G, Y461N, and Y463H in Mm [1,14,24] have been associated with DMI resistance in insensitive strains of the pathogens from the Federal District (Central-western Brazil) and Ribeira Valley region in São Paulo (Southeastern Brazil). A comprehensive study on 266 Mf strains originating from the Americas, Africa, and Asia showed that CYP51 substitutions Y136F, A313G, H380N, D460E/V, ΔY461, Y461D/N/S, G462A/D, and Y463D/H/N/S were only present in strains with reduced sensitivity to DMIs [15,17,25]. CYP51 substitutions T18I, D106V, and A446S were frequently found in both DMI-sensitive and -resistant strains. Accumulation of mutations resulting in combinations of CYP51 substitutions were identified. CYP51 variants [V106D, A313G, and D460V], [T18I, V106D, Y136F, and Y463D] and [T18I, V106D, A313G, and Y463D/H/N] in combination with promoter inserts containing tandem copies or palindromic motifs leading to overexpression of the fungicide target protein were most frequently found in insensitive strains [25].
Our study aims to provide updated insights into DMI resistance prevalence and mechanisms in Mycosphaerella species associated with BSDC in Brazil, contributing to more sustainable approaches for disease control.
Therefore, based on these premises, the main objectives of our study were: (i) to determine the current sensitivity levels of populations of Mf and Mm from banana plantations in the Ribeira Valley, in Northwestern São Paulo, and in Northern Minas Gerais (Southeastern Brazil) to DMI fungicides; and (ii) to determine the variation in the CYP51 gene to identify key mutations and to characterize the mechanisms associated with resistance to DMI fungicides locally.
2. Materials and Methods
2.1. Sampling of Diseased Plants and Isolation of the Fungal Pathogens
The Brazilian Ministry of Environment/National System for the Management of Genetic Heritage and Associated Traditional Knowledge; SisGen issued Certificates #A64D0EA and A100786 authorizing the scientific activities associated with the collection of botanical and fungal material from the banana agroecosystems in the Cerrado’s and Atlantic Forest’s biomes and access to the genetic diversity of Mycosphaerella species.
During 2020, diseased plants were obtained from commercial banana plantations from Northwest São Paulo (populations designated SPNW-C and SPNW-O), from Ribeira Valley in Southern São Paulo (SPVR-CI), and from Northern Minas Gerais (MGN-C), Brazil. These geographic populations were associated with distinct disease management systems: (i) intensive management (population SPVR-CI, sampled from Jacupiranga, Registro, and Sete Barras counties, in the Ribeira Valley); (ii) reduced management (population SPNW-C, obtained from Ilha Solteira county, in Northwest São Paulo, and MGN-C, sampled from Janaúba, Northern Minas Gerais); and (iii) organic management, with no fungicide applications (population SPNW-O, also obtained from Ilha Solteira).
In Ribeira Valley (SPVR-CI), the management strategy involved intensive fungicide spraying, with 8 to 14 preventive applications annually for chemical control of black Sigatoka [14,19]. This region is renowned as a major center for banana production in São Paulo state and Brazil. The predominant banana cultivars there are the BSDC-susceptible Prata (Musa spp. AAB, commonly known as “Lady Finger” banana) and Nanica (Musa spp. AAA, Cavendish subgroup) [9,19,31,32]. In the second geographic population, located in Northwestern São Paulo (SPNW-C), and the third site, in Northern Minas Gerais (MGN-C), fungicide use is reduced to four to five preventive sprays targeting yellow Sigatoka [19]. The SPNW-C area encompasses 40 hectares dedicated to the susceptible Maçã variety (triploid AAB) [19]. Conversely, in MGN-C, the banana plantation comprises Prata and Nanica varieties [19]. At the final sampled site (SPNW-O), no fungicides were used. This area includes several small family plantations with banana plants of various varieties and ages in Ilha Solteira county [19].
Fragments (approximately 20 × 20 cm) of banana leaves showing symptoms of either yellow Sigatoka or black Sigatoka diseases from different ages and cultivars were collected. At each location, sampling points consisted of 50 m2 areas, where approximately five plants were sampled. The leaf fragments were placed in paper bags and transported to the Molecular Plant Pathology Lab at UNESP, Ilha Solteira Campus. Samples were refrigerated until pathogen isolation.
Pathogen isolation followed the methodology of [24] with modifications. Leaves with apparent disease symptoms were rinsed with running water. Samples taken from the leaves, including the lesion area and its marginal parts; disinfected with a 75% ethanol solution; and then rinsed with distilled water to remove excess alcohol, approximately for a minute each. Subsequently, they were dried on sterilized filter paper and incubated in Petri dishes containing water agar medium (15 g L−1 agar supplemented with 50 μg mL−1 chloramphenicol and streptomycin). Fungal mycelial growth was analyzed under a stereoscopic microscope to distinguish characteristic sporodochia of Mf and Mm [19,20]. Direct isolation was performed by transferring conidia from the sporodochia formed in water agar medium to plates containing potato dextrose agar (PDA: 20.8 g L−1 potato dextrose and 15 g L−1 agar) using the streaking technique, followed by incubation for five days at 25 °C under a 12 h photoperiod [7]. Colonies were purified by transferring pure microcolonies of Mf or Mm isolates to new PDA plates and incubating for five days under the same conditions.
For long-term preservation of isolates, sterilized 0.5 cm2 filter paper fragments were subsequently transferred onto the surfaces of growing fungal colonies, maintained under incubation at 25 °C under a 12 h photoperiod until complete colonization of the fragments. Filter paper fragments colonized by fungal mycelial growth were transferred to Petri dishes and dried under sterile conditions in a laminar flow hood for 24 h. Subsequently, these colonized and dried paper fragments were transferred to cryotubes with silica gel and cryopreserved in a −20 °C freezer. These isolates constitute a subset of the population examined for QoI resistance by [19] and for SDHI resistance by [20].
2.2. Molecular Identification of Species
For the molecular identification of the two target species, the isolates were reactivated on PDA medium and grown for 10 days at 25 °C under a 12 h photoperiod. Approximately 1 cm2 of mycelial culture per isolate was then lyophilized for 48 h. The lyophilized mycelium was used for DNA extraction using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA), following the manufacturer’s instructions. The quantification of extracted DNA was performed using a NanoDrop® 2000c spectrophotometer (Thermo Fisher Scientific, USA) to achieve final dilutions at a concentration of 100 ng μL−1.
Specific PCR primers were used for the molecular identification of Mf (CYP51_Pfijien_F1 and R1) and Mm (CYP51A_Mm_F523 and R2457) based on the amplification of the CYP51 gene (Table 1). Polymerase chain reaction (PCR) reactions were performed using a ProFlex thermal cycler (Applied Biosystems, Carlsbad, CA, USA). Reactions were prepared in a total volume of 25 μL, consisting of 1 × PCR buffer (Applied Biosystems, Foster City, CA, USA), 1.5 mM MgCl2, 60 μM dNTPs, 0.2 μM of each primer, 1.5 U Taq DNA polymerase (Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA), and 100 ng of genomic DNA. The PCR conditions for Mf were as follows: initial denaturation at 95 °C for 5 min, followed by 36 amplification cycles with temperatures of 94 °C for 30 s, 58 °C for 60 s, and 72 °C for 60 s, with a final extension at 72 °C for 7 min. For Mm, the conditions were similar, with the only difference being in the annealing temperature, which was 59 °C. The resulting amplicons were analyzed by agarose gel electrophoresis, and the species were identified based on the size of the fragments obtained. Positive identification of Mf was confirmed by amplification of a 1700 bp fragment, while for Mm, a 1900 bp fragment was amplified. Positive controls for both species and negative controls using DNA extracted from the basidiomycete fungus Rhizoctonia solani AG-1 IA and the ascomycete fungus Pyricularia oryzae Triticum lineage, available in our laboratory, were included.

Table 1.
Specific primers used for PCR amplification aimed at species identification and for Sanger sequencing to determine allelic variation in the CYP51 gene in Mycospharella fijiensis and M. musicola *.
2.3. Identification of Allelic Variation of the CYP51 Gene
To assess allelic variation of the CYP51 gene in the Mf and Mm isolates, specific primers targeting the CYP51 gene were used for PCR amplification and sequencing. We conducted PCR amplification and sequencing of a total of nine isolates of Mf and forty-two isolates of Mm from the four BSDC populations sampled. The primers used in this study (Table 1) were either the ones used in other studies [33] or were designed specifically for this study. These particular primers were designed using Primer3 software within Geneious Prime version R 9.0.5 (Biomatters, Auckland, New Zealand). These primers also cover the promoter region of the CYP51 gene, allowing the presence of repetitive elements within the region to be verified, indicated as another mechanism associated with resistance to DMI through upregulation of gene expression [25]. Reference CYP51 sequences used for primer design were obtained from NCBI/GenBank® or from a specific publication [33], which included: genomic scaffold sequence NW_006921538 and EF581093.1 for Mf and MF521833 and MF521834 for Mm. These sequences were derived from Mf strains CIRAD86 (Cameroon, 2006), Bo_1 (Philippines, 2013), and CaM10-6 (Costa Rica, 2014) and from Mm strains 2SJA and 9SJA (Brazil, 2014) [1,10,24,33].
The PCR reactions for amplifying and sequencing the CYP51 gene were carried out in a final volume of 30 µL, containing water, 50 ng of total DNA, 0.3 µM of each primer, 0.2 mM of each dNTP, 2.5 mM MgCl2, 3 µL of 10× buffer, and 0.5 U of Taq DNA polymerase (Sigma-Aldrich, St. Louis, MO, USA). Amplifications were performed using a ProFlex thermal cycler (Applied Biosystems, USA) with an initial denaturation at 95 °C for 5 min, followed by 36 cycles of 94 °C for 30 s, 1 min at the specific annealing temperature for each primer pair (see Table 1), and 72 °C for 1 min, with a final extension at 72 °C for 7 min.
PCR products were purified and Sanger-sequenced at the sequencing facilities of Macrogen Inc. in Seoul, South Korea. To ensure accuracy, four or six complementary sequences were generated for each CYP51 gene fragment amplified for Mf or Mm, respectively, which included internal primers (Table 1). DNA sequences were analyzed and aligned using Geneious R 9.0.5 software (Biomatters, New Zealand) to identify alleles, haplotypes, and distinguish non-synonymous mutations leading to amino acid changes in inferred protein sequences. The same reference sequences described previously were used for annotation and derivation of CYP51 protein sequences from the experimental sequence data obtained in our study. The amino acid substitutions in the CYP51 variants detected were depicted as lollipops graphs built using the R software version 4.2.0 libraries ggplot2, dplyr, hrbrthemes, data.table, tidyverse, readxl, glue, ggtext, and ggrepel, and the functions geom_segment, ggtitle, geom_point, and geom_text_repel [34].
2.4. Phenotypic Assessment of In Vitro Sensitivity to DMI
For the phenotyping of sensitivity to DMI fungicides, a total of 9 isolates of Mf and 42 isolates of Mm were selected: 9 isolates of Mf from SPVR-CI, 15 isolates of Mm from MGN-C, 13 isolates of Mm from SPNW-C, and 14 isolates of Mm from SPNW-O.
Fungicide sensitivity testing was carried out with the triazoles propiconazole and tebuconazole. Formulated propiconazole (Tilt™ EC, active ingredient at 250 g L−1, Syngenta S.A., Brazil) was diluted in deionized water to obtain a stock solution of 25 µg mL−1. Formulated tebuconazole (Riza™ 200 EC, active ingredient at 200 g L−1, FMC Química do Brasil Ltda, Paulínia, Brazil) was diluted to obtain a stock solution of 20 µg mL−1. The final tested doses for propiconazole and tebuconazole were 0, 0.001, 0.01, 0.05, 0.1, 1, and 5 µg mL−1. The fungicides tested at different doses were mixed with PDA medium supplemented with chloramphenicol and streptomycin, both at a concentration of 50 μg mL−1. Sensitivity tests were performed in 90 mm diameter Petri plates.
Mycelial fragment suspensions for the fungicide sensitivity testing were obtained from the fungal colonies grown for 10 days on PDA at 25 °C under dark conditions. Mycelial samples with an area of 1 cm2 were collected and macerated in crucibles using ceramic pistils under sterile conditions in a laminar flow hood. The samples were then resuspended in 5 mL of sterilized deionized water containing 0.05 mL−1 of Tween-20. Subsequently, 5 µL of the inoculum suspension was carefully pipetted onto 0.5 cm diameter sterile filter paper discs, positioned on top of PDA medium containing different fungicide concentrations. The 90 mm diameter plates were sealed with plastic film to prevent drying and external contamination and incubated for 15 days at 25 °C and a 12 h photoperiod, after which growth was measured.
2.5. Experimental Design, Statistical Analyses, and Data Depiction
The experimental design consisted of complete randomized blocks with four replicates per treatment and experiments in duplicate. Sensitivity to the two DMI fungicides was assessed by determining the effective concentration of 50% to inhibit fungal growth (EC50, in µg mL−1), estimated using a dose–response function implemented in the Excel macro ED50plus v1.0 [35]. For hypothesis testing, isolates were grouped by the CYP51 protein variants detected, by geographical populations of the pathogens, and also by species. Analysis of variance (ANOVA) and the Scott-Knott test (at 5% probability) for means comparison were performed in the R environment using the statistical packages agricolae and ScottKnott [34]. We also classified the individual isolates according to their sensitivity to the DMI fungicides propiconazole and tebuconazole using the phenotypic classes presented in Table 2. We also determined the correlation between the EC50 values for propiconazole and tebuconazole to look for evidence of cross-resistance between the two fungicides.

Table 2.
Sensitivity classes for phenotypic classification of Mycosphaerella fijiensis and M. musicola isolates to the DMI fungicides propiconazole and tebuconazole based on EC50 values.
The boxplot figures depicting the contrast among the CYP51 protein variants detected, among geographical populations of the pathogens, and between isolates grouped by species were built using the R software library tidyverse 1.3.1, which included the packages ggplot, purrr, tibble, dplyr, tidyr, stringr, readr, and forcats and the functions ggplot, geom_boxplot, stat_summary, geom_jitter, ggtitle, theme, and geom_text (35). The whole set of color palettes chosen to build figures with accessibility features included color-blind-safe and print-friendly colors, using the resources from Color Brewer 2.0 (URL: https://colorbrewer2.org/#type=sequential&scheme=BuGn&n=3, accessed on 1 November 2023).
3. Results
3.1. Identification of Allelic Variation of the CYP51 Gene
In the present study, we characterized a total of 51 isolates of the BSDC pathogens, comprising 42 from Mm and 9 from Mf. A total of eight different CYP51 protein variants were detected among these isolates (Figure 1). Mm exhibited the highest diversity of protein variants of the CYP51 gene (N = 5). CYP51 variant A ([V106D and A446S] from Mm was most frequently found in the populations tested (N = 31), while variants B [V106D, Y136F, and A446S] (N = 2), C [V106D, A446S, and Y461H] (N = 3), D [V106D, A446S, Y461N, and Y463D] (N = 1) and E [V106D, A446S, and Y463D] (N = 5) were detected at much lower numbers. As for the Mf population (N = 9) three CYP51 variants were detected: variant F [T18I, V106D, and Y463D] (N = 5), G [T18I, V106D, and Y461D] (N = 3), and H [T18I, V106D, and G462D] (N = 1) (Figure 1).
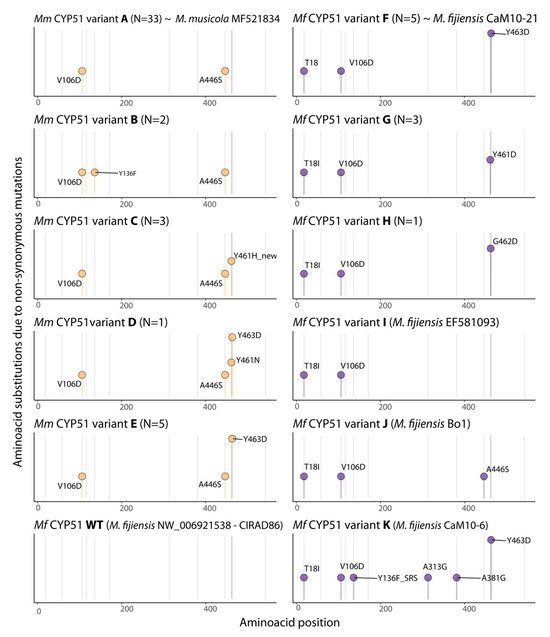
Figure 1.
Lollipop depiction of non-synonymous amino acid substitutions conferred by mutations in the CYP51 gene of isolates of Mycosphaerella musicola (at left) and Mycosphaerella fijiensis (at right) sampled from four geographical populations from distinct fungicide management systems in Minas Gerais and São Paulo, Brazil. CYP51 variants A to E were only detected in isolates of Mm, while CYP51 variants F, G, and H were found in Mf isolates. Orange lollipops indicate amino acid substitutions in the inferred CYP51 sequence for Mm while purple lollipops mark CYP51 substitutions found in Mf. When the protein variant detected has shown similarity with reference sequences from the GenBank/NCBI, the sequence code is indicated at the respective variant (e.g., CYP51 variant A was identical to Mm MF521834; variant F was identical to the sequence from Mf CaM10-21). CYP51 WT and variants I, J, and K were not detected in our study.
CYP51 substitutions V106D and A446S were detected in all Mm isolates (N = 42), while substitution T18I was absent in Mm but detected in all nine Mf isolates. CYP51 substitutions associated with azole resistance were found less frequently. CYP51 Y136F (N = 2), Y461H (N = 3), and Y461N (N = 1) were found in Mm, whereas Y461D (N = 3) and G462D (N = 1) were detected in Mf. CYP51 Y463D was found in both species, being detected in five and six isolates of Mf and Mm, respectively (Figure 2).
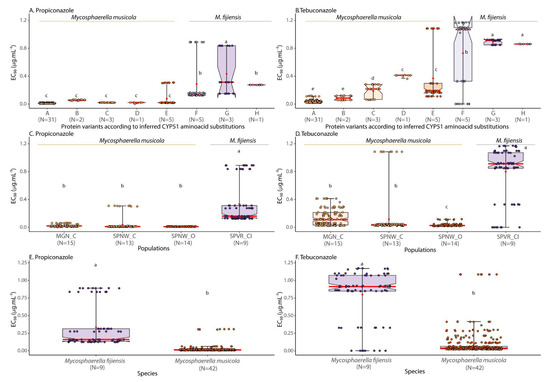
Figure 2.
Boxplot comparing the effects of CYP51 protein variants, geography, and species in the levels of sensitivity (EC50 in µg mL−1) to DMI fungicides propiconazole and tebuconazole in BSDC populations sampled from banana plantations in São Paulo and Minas Gerais, Brazil. Red dots indicate the average EC50 values for each category. Means followed by the same lowercase letter are not significantly different by the ScottKnott test at 5% probability.
No CYP51 promoter modifications linked with overexpression, based on the insertion and duplication of a 19 bp repeat element with a palindromic core TAAATCTCGTACGATAGCA), were found in the nine Mf strains tested and the sensitive reference Bo-1 [17,33]. In contrast, the highly resistant Mf reference strain Ca10-6 harbored five extra copies of this element in the promoter, as previously described [10,25]. A single copy of an almost identical 19 bp element, TAAATCTCGTACGATTGCA (with a single base difference highlighted with underline), without replications was also present in the CYP51 promoter (−118 bp upstream) of the 42 Mm strains.
3.2. Phenotypic Assessment of In Vitro Sensitivity to DMI Triazole Fungicides and Determination of EC50
Based on the average EC50 values, there were significant differences among the eight CYP51 variants detected in the population sampling of the BSDC pathogens (Table 3, Figure 2A,B, Supplementary Files S1 and S2). EC50 from variant G [T18I, V106D, and Y461D] from Mf was significantly higher for both DMI fungicides (EC50 propiconazole = 0.43 ± 0.30 µg mL−1; EC50 tebuconazole = 0.89 ± 0.03 µg mL−1), followed by variants F [T18I, V106D, and Y463D] (EC50 propiconazole = 0.29 ± 0.31 µg mL−1; EC50 tebuconazole = 0.73 ± 0.48 µg mL−1) and H [T18I, V106D, and G462D] (EC50 propiconazole = 0.27 ± 0.001 µg mL−1; EC50 tebuconazole = 0.86 ± 0.001 µg mL−1), also from Mf. Altogether, these three CYP51 variants from Mf had higher average EC50 values than all the variants detected in Mm isolates, which ranged from 0.01 to 0.07 µg mL−1 for propiconazole and from 0.03 to 0.37 µg mL−1 for tebuconazole (Table 3, Figure 2A,B).

Table 3.
Summary outlining five specific substitutions and their corresponding CYP51 protein variants and impact on resistance levels to DMI fungicides in Mycosphaerella fijiensis (Mf) and M. musicola (Mm) from the BSDC in Southeastern Brazil *.
For propiconazole, Mm strains harboring CYP51 variant A ([V106D and A446S]) showed sensitivity, whereas variants B ([V106D, Y136F, and A446S]), C ([V106D, A446S, and Y461H]), D ([V106D, A446S, Y461N, and Y463D]), and E ([V106D, A446S, and Y463D]) strains of Mm were classified as lowly resistant. Mf CYP51variants F, G, and H were all classified as moderately resistant. Regarding tebuconazole, CYP51 variants A strains B of Mm were classified as sensitive while variant B strains showed low levels of resistance. Mm CYP51 variant C, D, and E strains and Mf strains carrying CYP51 variants F, G, and H all showed moderate resistance levels to tebuconazole (Table 3). None of the BSDC isolates tested showed a highly resistant phenotype for either propiconazole and/or tebuconazole according to the resistance level categories presented in Table 2.
There were also significant differences among geographical populations of the pathogens (Table 3, Figure 2C,D, Supplementary File S2). The population SPVR_CI with Mf isolates showed significantly higher levels of resistance to both fungicides than all the other three Mm populations sampled (MGN-C, SPNW-C, SPNW-O). The comparison between species indicated that Mf (EC50 propiconazole = 0.33 ± 0.29 µg mL−1; EC50 tebuconazole = 0.80 ± 0.36 µg mL−1) was significantly more resistant than Mm (EC50 propiconazole = 0.02 ± 0.05 µg mL−1; EC50 tebuconazole = 0.10 ± 0.18 µg mL−1) for both DMI fungicides (Table 3, Figure 2E,F). Among the Mm populations, population SPNW-C contained the least DMI-sensitive isolate belonging to CYP51 variant E together with sensitive isolates carrying variant A. Population MGN-C contained the remaining CYP51 variant E isolates and isolates carrying variants C and D, all being lowly resistant or moderately resistant to propiconazole and tebuconazole, respectively. Only isolates that were sensitive to propiconazole and sensitive and/or lowly resistant to tebuconazole were detected in population SPNW-O (Figure 2).
The linear model in Figure 3 describes the correlation between the DMI fungicides propiconazole and tebuconazole and the EC50 values from 51 isolates of Mycosphaerella spp. The coefficient of determination (R2 = 0.458) indicates a moderate correlation, suggesting that the linear model explains a portion of the variability in the data. The root mean square error (RMSE = 0.330) reflects the predictive capacity of the model. Based on this linear relationship, we detected significant (p < 0.001) cross-resistance between propiconazole and tebuconazole for Mycosphaerella spp. isolates.
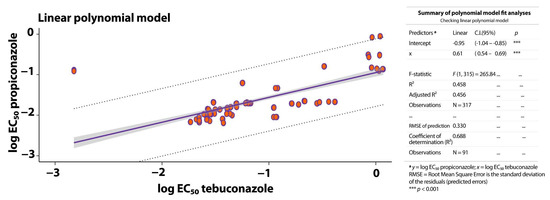
Figure 3.
Correlation between the log EC50 data for sensitivity of the BSDC pathogens Mycosphaerella spp. (N = 51) to the DMI fungicides propiconazole and tebuconazole. The scatterplot illustrates the relationship between propiconazole concentrations (y-axis) and tebuconazole concentrations (x-axis) and the corresponding EC50 values. The purple lines represent the linear regression model.
4. Discussion
Resistance to the DMI fungicides propiconazole and tebuconazole was prevalent among the populations of Mf and Mm examined in banana plantations from Southeastern Brazil, with all 9 Mf strains and 11 out of 42 Mm strains tested showing different levels of resistance. These strains carried a range of CYP51 alterations, F136Y, Y461D/H/N, G462D, and Y463D, all known to affect azole binding (Figure 2, Table 3). The evolution of DMI target site resistance in Mf and Mm indicates that the frequently sprayed DMI fungicides, within the BSDC management system, exerted a high selection pressure on pathogen populations [24,28]. This underscores the urgent need to implement integrated disease management strategies aimed at reducing exclusive reliance on DMI fungicides and incorporating less aggressive practices to mitigate and slow down resistance development [19,20,36].
It is important to highlight that all isolates in this study were also analyzed for resistance to QoI and SDHI fungicides in previous studies, and many exhibited multiple resistance [19,20,37]. For example, out of the nine Mf isolates examined, one showed also resistance to the QoI fungicides azoxystrobin and trifloxystrobin [19,37], while all demonstrated reduced sensitivity to the SDHI fungicides boscalid and fluxapyroxad [20]. Regarding Mm, among the 42 isolates evaluated, seven exhibited resistance to QoI fungicides, and all isolates showed some degree of resistance to SDHI fungicides [20].
The disease management system for the BSDC may have contributed to the significant differences among the populations sampled regarding their resistance levels to DMI fungicides. For instance, the Mf population from Vale do Ribeira (SPVR_CI) exhibited significantly higher levels of resistance to DMI fungicides compared with the three sampled Mm populations. As similar CYP51 mutations have evolved in Mm and Mf populations, additional resistance mechanisms might have evolved in Mf populations. It is noteworthy that banana plantations in Vale do Ribeira are subject to more intense fungicide spraying, with 8 to 14 annual preventive applications of fungicides for black Sigatoka control [19,20,37]. In contrast, banana plantations from the other sampled regions, such as Northwest São Paulo (SPNW-C) and Northern Minas Gerais (MGN_C), are subjected to less intensive fungicide management systems (where fungicide use is reduced to four or five preventive sprays for Sigatoka control), including even no fungicide use (SPNW_O), probably resulting in the absence of isolates with moderate resistance levels in the latter population (Figure 2). These findings underscore the significant influence of the fungicide management system on the evolution and spread of fungicide resistance within the BSDC [20] while also suggesting the presence of other contributing factors to this complex dynamic. These factors may include specific agricultural practices, genetic characteristics of local fungal populations, and multifaceted interactions among fungi, host plants, and the surrounding environment [36].
A total of 28 different amino acid alterations present in 60 different variants of the CYP51 protein have been identified in Mf strains sampled globally [25]. CYP51 alterations Y136F, A313G, H380N, A381G, D460E/V, ΔY461, Y461D/N/S, G462A/D, and Y463D/H/N/S have all been linked to DMI resistance. Other alterations, including T18I, A19E, Y59F, V106D, V117L, R416G, and A446S have been found in DMI-sensitive isolates and seem to have evolved at random as they are not located near substrate recognition sites or the haem-binding domain of the CYP51 protein [15,28]. In this study, we identified CYP51 substitutions T18I, V106D, Y461D, G462D, and Y463D in the nine Mf isolates originating from the Ribeira Valley in Southern São Paulo (SPVR-CI) (Figure 1, Table 3). CYP51 V106D seems to be present in all Mf and Mm strains that were globally sampled and tested so far [10,14,25]. The equivalent residue at position 107, D107, is also conserved in CYP51 of the closely related fungus Zymoseptoria tritici (aka Mycosphaerella graminicola), which also shares 81% amino acid sequence similarity. Therefore, it is likely that a rare random mutation, resulting in D106V, has evolved in the reference strain CIRAD86, as was also suggested in an earlier study [15]. CYP51 substitution T18I has been found at high frequencies in Mf populations sampled in Columbia, Ecuador, and the Philippines but seems to be absent in Cameroon. For Mf, CYP51 substitution A446S was exclusively found in the majority of isolates sampled in the Philippines, most of them also carrying mutations associated with resistance. A 3D model of CYP51 from Mf has shown that amino acid residues at positions 18, 106, and 446 are not near to any of the six substrate recognition sites (SRSs) or the haem-binding cavity and, therefore, are not involved in azole binding. However, an additive effect on DMI insensitivity of the A446S substitution in combination with other mutations cannot be ruled out [25]. CYP51 substitutions G462D and Y463D have been reported previously in Mf strains from Brazil, and these alterations together with Y461D are also found globally in Mf populations, often in combination with other mutations [10,14,25]. These mutations are located on a region of the protein at the haem end that is specific for fungi. Molecular modeling and azole docking studies on CYP51 from Z. tritici has shown that substitutions at positions 461–463 cause azole resistance by moving key residues V134 and or Y136, located on the access channel end, further away from the binding pocket. However, loss of the tyrosine residue at position 463 has probably the greatest impact on azole binding [38].
Mutations resulting in CYP51 substitutions V106D, Y136F, A446S, Y461D/H/N, and Y463D were detected in the 42 Mm strains. CYP51 V106D and A446S have been found before in Brazilian Mm populations and are not associated with DMI resistance [24]. Y136F, Y461D/N, and Y463D have also evolved in Mf populations, showing a parallel evolution of CYP51 in these closely related pathogens under selection of DMI fungicides. CYP51 alterations Y461N and the combination of A381G and Y463H have been reported previously for Brazilian strains [6,14,20]. CYP51 Y461H in Mm is a novel discovery, reported for the first time here and, so far, not found in any BSDC population that has been sampled and tested so far. CYP51 Y136F was detected for the time in Mm as part of this study. A substitution at Y136 (Y137 in Z. tritici), or its equivalent in other species, is the most frequently observed modification of CYP51 in pathogenic fungi [28]. V136 and Y137 are located on the access channel end of the binding pocket. Molecular modeling and azole docking studies on Z. tritici CYP51 shows that the mutant F137 residue is pushed into a position preventing the proper binding of triadimenol, which explains the observed high levels of resistance in corresponding Z. tritici field isolates [38]. However, the same study predicted a much smaller influence of the Y137F substitution on the binding of other azole agents. This also explains the low levels of resistance to propiconazole and tebuconazole in this study and support the hypothesis that this mutation was likely selected in Mm populations after exposure to triadimenol in the past.
The analysis of mutation combinations present in the Mf and Mm isolates allowed the identification of eight distinct CYP51 protein variants. These CYP51 variants containing between 2 and 4 amino acid alterations were designated as follows: (A) [V106D and A446S] (Mm, N = 33); (B) [V106D, Y136F, and A446S] (Mm, N = 2); (C) [V106D, A446S, and Y461N] (Mm, N = 3); (D) [V106D, A446S, Y461H, and Y463D] (Mm, N = 1); (E) [V106D, A446S, and Y463D] (Mm, N = 3); F) [T18I, V106D, and Y463D] (Mf, N = 5); (G) [T18I, V106D, and Y461D] (Mf, N = 3) and (H) [T18I, V106D, and G462D] (Mf, N = 1). These haplotypes highlight significant mutation diversity, revealing a complex genetic landscape within these fungal populations, but even more complex CYP51 haplotypes have evolved in Mf populations under strong selection pressure by DMI fungicides, with up to six amino acid substitutions reported for some variants (e.g., [V106D, K171R, A313G, A446S, D460E, and Y461N] and [T18I, V106D, Y136F, A313G, A381G, and Y461D]) present in populations sampled in the Philippines and Costa Rica [10,25].
Recently, another mechanism of DMI resistance in Mf was identified, involving overexpression of the CYP51 gene due to the in tandem insertion of small repetitive elements in its promoter region [17]. These repetitive sequences were also transmitted across generations of the fungal pathogen [10]. Strains of Mf sampled in Africa, Asia, and South America showing complex CYP51 variants in combination with the presence of up to six repetitive elements in their promoter, thereby enhancing the expression up to five-fold, were found to show high levels of resistance to different DMI fungicides [17,28,33]. However, CYP51 promoter inserts were not detected in the BSDC isolates examined in this study, and differences in sensitivity to propiconazole and tebuconazole between the two species cannot be explained by this mechanism. MDR (multidrug resistance) due to overexpression of efflux pumps was already detected in a selection of Mf and Mm isolates examined in this study previously [20].
In summary, the current study represents a significant contribution to an improved understanding of the mechanisms, emergence, and spread of DMI fungicide resistance in BSDC populations in Brazil. It also underscores the impact of extensive use of chemical fungicides and the associated risks of further selecting and dispersing BSDC strains with higher levels of DMI resistance [19,20,36]. To mitigate these risks, it is imperative to consider innovative disease management approaches that minimize reliance on preventive fungicide sprays with medium to high resistance risk [36].
One proposed solution to fungicide resistance involves implementing a smart spraying system that is not based on fixed spray schedules but rather on real-time information about the temporal dynamics of airborne inoculum of these phytopathogens (i.e., aerobiology data) and epidemic risks [36,39,40]. This system would enable more precise and timely decision-making on when and what to spray. For example, if the presence of fungal inoculum with resistance alleles to major fungicide groups such as DMIs is detected, the system would alternatively recommend spraying only low-resistance risk active ingredients, thereby reducing further selective pressure on populations and the likelihood of resistance fixation [36].
5. Conclusions
DMI fungicide resistance prevalence: Widespread resistance to DMI fungicides was observed among BSDC populations in banana plantations of Southeastern Brazil. All nine Mf isolates showing moderate resistance levels to propiconazole or tebuconazole, while 26% of the Mm strains (11 out of 42) tested showed low to moderate levels of resistance to these DMI fungicides.
Mutation diversity and resistance association: Mutations leading to CYP51 substitutions Y136F, lY461N/H, and Y463D in Mm and Y461D, G462D, and Y463D in Mf were associated with low or moderate levels of resistance to DMIs. More complex CYP51 variants and CYP51 promoter inserts associated with upregulation of the target protein were not detected and can explain the absence of highly DMI-resistant strains in Brazil.
Influence of fungicide management system: Variations in resistance levels were strongly influenced by the intensity of fungicide management practices. The Mf population from Vale do Ribeira exhibited notably higher resistance due to more intense fungicide spraying compared with regions with less intensive fungicide use.
Recommendations for integrated disease management: Implement integrated disease management strategies to reduce reliance on fungicides and adopt practices to mitigate resistance development. Develop and deploy smart spraying systems guided by real-time aerobiology data to optimize fungicide application and preserve efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13071439/s1. Supplementary File S1. Characterization of isolates of Mycosphaerella fijiensis and M. musicola from the BSDC, CYP51 variants, population origins, mean EC50 for propiconazole and tebuconazole, and phenotypic classes. Supplementary File S2. Analysis of variance of the effects of species, geographical populations, CYP51 variants, and isolates in the levels of sensitivity to DMI fungicides propiconazole and tebuconazole in Mycosphaerella fijiensis and M. musicola from banana plantations in São Paulo and Minas Gerais, Brazil.
Author Contributions
Conceptualization, P.C.C. and S.I.M.; methodology, A.G.d.S., T.C.S., and P.C.C.; software, P.C.C.; validation, A.G.d.S., T.C.S., T.Y.K.O., F.S.C.J., D.M.d.S., L.M.d.D.P.G., and G.V.L.; formal analysis, A.G.d.S., T.C.S., and P.C.C.; investigation, A.G.d.S., T.C.S., T.Y.K.O., F.S.C.J., D.M.d.S., L.M.d.D.P.G., G.V.L., M.C.d.G.G., and B.A.F.; resources, P.C.C.; data curation, A.G.d.S., T.C.S., and P.C.C.; writing, A.G.d.S., T.C.S., S.I.M., and P.C.C.; writing—review and editing, A.G.d.S., T.C.S., S.I.M., B.A.F., M.C.d.G.G., and P.C.C.; visualization, M.C.d.G.G., G.H.G., and P.C.C.; supervision, P.C.C., S.I.M., and G.H.G.; project administration, P.C.C.; funding acquisition, P.C.C. and B.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (São Paulo Research Foundation, Brazil), Grant/Award Numbers: 2017/50456-1, 2018/21197-0, 2019/12509-1, 2020/07611-9, and 2021/03402-9; CNPq (Brazilian National Council for Scientific and Technological Development), Grant/Award Number: Pq-1C 311895/2022-0; CAPES (Coordination for the Improvement of Higher Level Personnel), Grant/Award Numbers: CAPES/Pro-equipment Program 775202/2012, CAPES/AUXPE Program 88881.593505/2020-01—UNESP Ilha Solteira Campus, CAPES PrInt Program/UNESP, CAPES studentship Program 001); and Newton Fund/BBSRC (Biotechnology and Biological Sciences Research Council, United Kingdom), Grant/Award Number: BB/S018867/2, awarded by BBSRC under the BBSRC-FAPESP Antimicrobial Resistance and Insecticide Pest Resistance in Livestock and Agriculture Program. The article processing charges were supported in full by São Paulo State University’s Vice-Presidency for Research (PROPE) Special Grant for Publication (Public Call 6/2025). Continuing from the aforementioned support, this research was underpinned by the following grants: São Paulo Research Foundation (FAPESP) Grant Number 2021/04977-5 to G.H.G., the joint funding from Brazilian National Council for Scientific and Technological Development (CNPq), FAPESP, and Coordination for the Improvement of Higher Level Personnel (CAPES) Grant Number 405934/2022-0 (The National Institute of Science and Technology INCT Funvir), and CNPq Grant Number 301058/2019-9 to G.H.G, all from Brazil. Additionally, this study received funding from the Joint Canada–Israel Health Research Program, jointly supported by the Azrieli Foundation, Canada’s International Development Research Centre, Canadian Institutes of Health Research, and the Israel Science Foundation (G.H.G).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The CYP51 experimental sequence data from Mf and Mm populations sampled in Southeastern Brazil and that supports the findings of allelic variation in the target genes for demethylation inhibitor (DMI) triazole fungicides sensitivity will be available at the GenBank/NCBI database. Upon publication, the complete data set of phenotypic data presented in this study will be publicly available at the Mendeley Data repository.
Acknowledgments
We extend our sincere thanks to the Provost of Inclusion and Belonging at the University of São Paulo (USP) for awarding a postdoctoral fellowship to T.C.S. This support greatly contributed to the successful completion of our research project, and we appreciate the institution’s commitment to academic excellence and inclusivity.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Brito, F.S.D. Diversidade Genética de Populações de Mycosphaerella musicola e Caracterização Do Efetor LysM Em Mycosphaerella graminicola. Ph.D. Dissertation, University of Brasilia, Brasília, Brazil, 2015. [Google Scholar]
- Ferrari, J.T.; Nogueira, E.M.d.C.; Gasparotto, L.; Hanada, R.E.; Louzeiro, I.M. Ocorrência de Sigatoka negra em bananeiras do Estado de São Paulo. SciELO Arq. Do Inst. Biológico 2021, 72, 133–134. [Google Scholar] [CrossRef]
- Gasparotto, L.; Pereira, J.C.R.; Pereira, M.C.N.; Costa, M.M. Controle Quimico da Sigatoka Negra da Bananeira: Trifloxistrobin, Propiconazole e Difenoconazole; Comunicado Técnico—Embrapa Amazônia Ocidental: Manaus, Brazil, 2000; p. 1. [Google Scholar]
- Ramos, J.B.; Bragança, C.A.D.; Rocha, L.S.; Oliveira, A.d.S.; Cordeiro, Z.J.M.; Haddad, F. First Report of Black Sigatoka of Banana Caused by Mycosphaerella fijiensis in Bahia, Brazil. Plant Dis. 2018, 102, 2035. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, C.D.N.; Pozza, E.A.; Moraes, W.S.; Rocha, H.S.; Costa, F.C.L. Modelling Black Sigatoka Epidemics with Seasonal Dispersal of Mycosphaerella fijiensis Ascospores over a Banana Plantation in the Ribeira Valley, São Paulo, Brazil. Eur. J. Plant Pathol. 2021, 161, 463–474. [Google Scholar] [CrossRef]
- Brito, F.S.D.; Fraaije, B.; Miller, R.N.G. Sigatoka Disease Complex of Banana in Brazil: Management Practices and Future Directions. OutLook Pest Manag. 2015, 26, 78–81. [Google Scholar] [CrossRef]
- Gomes, L.; Douhan, G.; Bibiano, L.; Maffia, L.; Mizubuti, E. Mycosphaerella musicola Identified as the Only Pathogen of the Sigatoka Disease Complex Present in Minas Gerais State, Brazil. Plant Dis. 2013, 97, 1537–1543. [Google Scholar] [CrossRef]
- Gomes, L.I.S.; Douhan, G.W.; Lehner, M.S.; Bibiano, L.B.J.; Mizubuti, E.S.G. Yellow Sigatoka Epidemics Caused by a Panmictic Population of Mycosphaerella musicola in Brazil. Plant Pathol. 2018, 67, 295–302. [Google Scholar] [CrossRef]
- Nomura, E.S.; Moraes, W.d.S.; Kobori, R.T.; Penteado, L.A.d.C. Doenças. In Cultivo da bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 107–134. ISBN CDD.634.722. [Google Scholar]
- Chong, P. The Origin, Versatility and Distribution of Azole Fungicide Resistance in the Banana Black Sigatoka Pathogen Pseudocercospora fijiensis. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Burt, P.J.A. Windborne Dispersal of Sigatoka Leaf Spot Pathogens. Grana 1994, 33, 108–111. [Google Scholar] [CrossRef]
- Manzo-Sánchez, G.; Orozco-Santos, M.; Islas-Flores, I.; Martínez-Bolaños, L.; Guzmán-González, S.; Leopardi-Verde, C.L.; Canto-Canché, B. Genetic Variability of Pseudocercospora fijiensis, the Black Sigatoka Pathogen of Banana (Musa spp.) in Mexico. Plant Pathol. 2019, 68, 513–522. [Google Scholar] [CrossRef]
- Mendoza, M.J.; Ardales, E. Population Structure of the Banana Black Sigatoka Pathogen [Pseudocercospora fijiensis (M. Morelet) Deighton] in Luzon, Philippines. Philipp. Agric. Sci. 2019, 102, 211–219. [Google Scholar]
- Malimpensa, J.R. Caracterização Da Resistência a Fungicidas Em Populações de Fungos Associados a Lesões de Sigatokas Em Bananais Do Vale Do Ribeira (SP). Master’s Thesis, Instituto Biológico, São Paulo, Brazil, 2018. [Google Scholar]
- Cañas-Gutiérrez, G.P.; Angarita-Velásquez, M.J.; Restrepo-Flórez, J.M.; Rodríguez, P.; Moreno, C.X.; Arango, R. Analysis of the CYP51 Gene and Encoded Protein in Propiconazole-Resistant Isolates of Mycosphaerella fijiensis. Pest Manag. Sci. 2009, 65, 892–899. [Google Scholar] [CrossRef]
- Churchill, A.C.L. Mycosphaerella Fijiensis, the Black Leaf Streak Pathogen of Banana: Progress towards Understanding Pathogen Biology and Detection, Disease Development, and the Challenges of Control. Mol. Plant Pathol. 2011, 12, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Trujillo, C.; Chong, P.; Stergiopoulos, I.; Cordovez, V.; Guzman, M.; De Wit, P.J.G.M.; Meijer, H.J.G.; Scalliet, G.; Sierotzki, H.; Lilia Peralta, E.; et al. A New Mechanism for Reduced Sensitivity to Demethylation-Inhibitor Fungicides in the Fungal Banana Black Sigatoka Pathogen Pseudocercospora fijiensis. Mol. Plant Pathol. 2018, 19, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Grice, K.; Vawdrey, L.B.; Stammler, G.C.; Koch, A.; Wilson, D.; Matthews, N. Yellow Sigatoka (Mycosphaerella musicola) Populations Develop Resistance to QoI Fungicides in Australia. In Modern Fungicides and Antifungal Compounds; Dehne, H.W., Deising, H.B., Fraaije, B., Gisi, U., Hermann, D., Mehl, A., Oerke, E.C., Russell, P.E., Stammler, G., Kuck, K.H., et al., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2013; Volume 7, pp. 269–270. ISBN 978-3-941261-13-6. [Google Scholar]
- Oliveira, T.Y.K.; Silva, T.C.; Moreira, S.I.; Christiano, F.S.; Gasparoto, M.C.G.; Fraaije, B.A.; Ceresini, P.C. Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil. Agronomy 2022, 12, 2952. [Google Scholar] [CrossRef]
- Silva, T.C.; Moreira, S.I.; de Souza, D.M.; Christiano, F.S.; Gasparoto, M.C.G.; Fraaije, B.A.; Goldman, G.H.; Ceresini, P.C. Resistance to Site-Specific Succinate Dehydrogenase Inhibitor Fungicides Is Pervasive in Populations of Black and Yellow Sigatoka Pathogens in Banana Plantations from Southeastern Brazil. Agronomy 2024, 14, 666. [Google Scholar] [CrossRef]
- Gomes, L.I.S.; Bibiano, L.B.J.; da Silva, G.F.; Hanada, R.E.; Mizubuti, E.S.G. Baseline Sensitivity of Brazilian Mycosphaerella fijiensis Isolates to Protectant and Systemic Fungicides. Trop. Plant Pathol. 2014, 39, 172–177. [Google Scholar] [CrossRef]
- Hanada, R.; Gasparotto, L.; Moreira, A. Avaliação da sensibilidade de Mycosphaerella fijiensis oriundos de plátanos aos fungicidas propiconazole e azoxystrobina. Divulg. Da Rev. De Ciências Agrárias Amaz. J. Agric. Environ. Sci. 2015, 58, 21–26. [Google Scholar] [CrossRef][Green Version]
- FRAC—Fungicide Resistance Action Committee Recommendations for Bananas. Available online: https://www.frac.info/frac-teams/working-groups/banana-group/recommendations-for-bananas (accessed on 17 January 2023).
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic Diversity and Azole Fungicide Sensitivity in Pseudocercospora musae Field Populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef]
- Chong, P.; Essoh, J.N.; Arango Isaza, R.E.; Keizer, P.; Stergiopoulos, I.; Seidl, M.F.; Guzman, M.; Sandoval, J.; Verweij, P.E.; Scalliet, G.; et al. A World-Wide Analysis of Reduced Sensitivity to DMI Fungicides in the Banana Pathogen Pseudocercospora fijiensis. Pest Manag. Sci. 2021, 77, 3273–3288. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Liao, M.; Xiao, M.; Xiao, W.; Yang, S.; Wan, J. Expression and Homology Modelling of Sterol 14α-Demethylase of Magnaporthe grisea and Its Interaction with Azoles. Pest Manag. Sci. 2009, 65, 260–265. [Google Scholar] [CrossRef]
- Zhan, J.; Stefanato, F.L.; McDonald, B.A. Selection for Increased Cyproconazole Tolerance in Mycosphaerella graminicola through Local Adaptation and in Response to Host Resistance. Mol. Plant Pathol. 2006, 7, 259–268. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Fraaije, B.A. Contrasting levels of genetic predictability in the evolution of resistance to major classes of fungicides. Mol. Ecol. 2021, 30, 5318–5327. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide Resistance Management: Maximizing the Effective Life of Plant Protection Products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Martínez-Bolaños, L.; Téliz-Ortiz, D.; Rodríguez-Maciel, J.C.; Mora-Aguilera, J.A.; Nieto-Ángel, D.; Cortés-Flores, J.I.; Mejía-Sánchez, D.; Nava-Diaz, C.; Silva-Aguayo, G. Resistencia a fungicidas en poblaciones de Mycosphaerella fijiensis del sureste mexicano. Agrociencia 2012, 46, 707–717. [Google Scholar]
- Moraes, W.d.S.; Nomura, E.S. Monitoramento Da Sigatoka e Controle Químico. In Cultivo da bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 135–144. ISBN CDD.634.722. [Google Scholar]
- Rangel, A.; Penteado, L.A.C.; Tonet, R.M. Cultura Da Banana, 2nd ed.; Boletim Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2002.
- Chong, P.; Vichou, A.-E.; Schouten, H.J.; Meijer, H.J.G.; Isaza, R.E.A.; Kema, G.H.J. Pfcyp51 Exclusively Determines Reduced Sensitivity to 14α-Demethylase Inhibitor Fungicides in the Banana Black Sigatoka Pathogen Pseudocercospora fijiensis. PLoS ONE 2019, 14, e0223858. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2022. [Google Scholar]
- Vargas, M.H. ED50plus v1.0. 2000. Available online: https://archive.org/details/ed50v10_zip (accessed on 2 March 2024).
- Ceresini, P.C.; Silva, T.C.; Vicentini, S.N.C.; Júnior, R.P.L.; Moreira, S.I.; Castro-Ríos, K.; Garcés-Fiallos, F.R.; Krug, L.D.; De Moura, S.S.; Da Silva, A.G.; et al. Strategies for Managing Fungicide Resistance in the Brazilian Tropical Agroecosystem: Safeguarding Food Safety, Health, and the Environmental Quality. Trop. Plant Pathol 2024, 49, 36–70. [Google Scholar] [CrossRef]
- Oliveira, T.Y.K. de Evidence of Resistance to Strobilurin Fungicides in Contemporary Populations of Mycosphaerella fijiensis and M. musicola from Banana Plantations in Southeastern Brazil. Master’s Thesis, Universidade Estadual Paulista (UNESP), Ilha Solteira, Brazil, 2022. [Google Scholar]
- Mullins, J.G.L.; Parker, J.E.; Cools, H.J.; Martel, C.M.; Togawa, R.C.; Lucas, J.A.; Fraaije, B.A.; Kelly, D.E.; Kelly, S.L. Molecular modelling of the emergence of azoles resistance in Mycosphaerella graminicola. PLoS ONE 2012, 6, e20973. [Google Scholar] [CrossRef]
- Vicentini, S.N.C.; Hawkins, N.J.; King, K.M.; Moreira, S.I.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Portalanza, D.; Garcés-Fiallos, F.R.; Krug, L.D.; West, J.S.; et al. Aerobiology of the Wheat Blast Pathogen: Inoculum Monitoring and Detection of Fungicide Resistance Alleles. Agronomy 2023, 13, 1238. [Google Scholar] [CrossRef]
- West, J.; Burt, P. Applied Aspects of Aerobiology. Asp. Appl. Biol. 2008, 89, i–88. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).