Multi-Faceted Roles of Stress Granules in Viral Infection
Abstract
1. Introduction
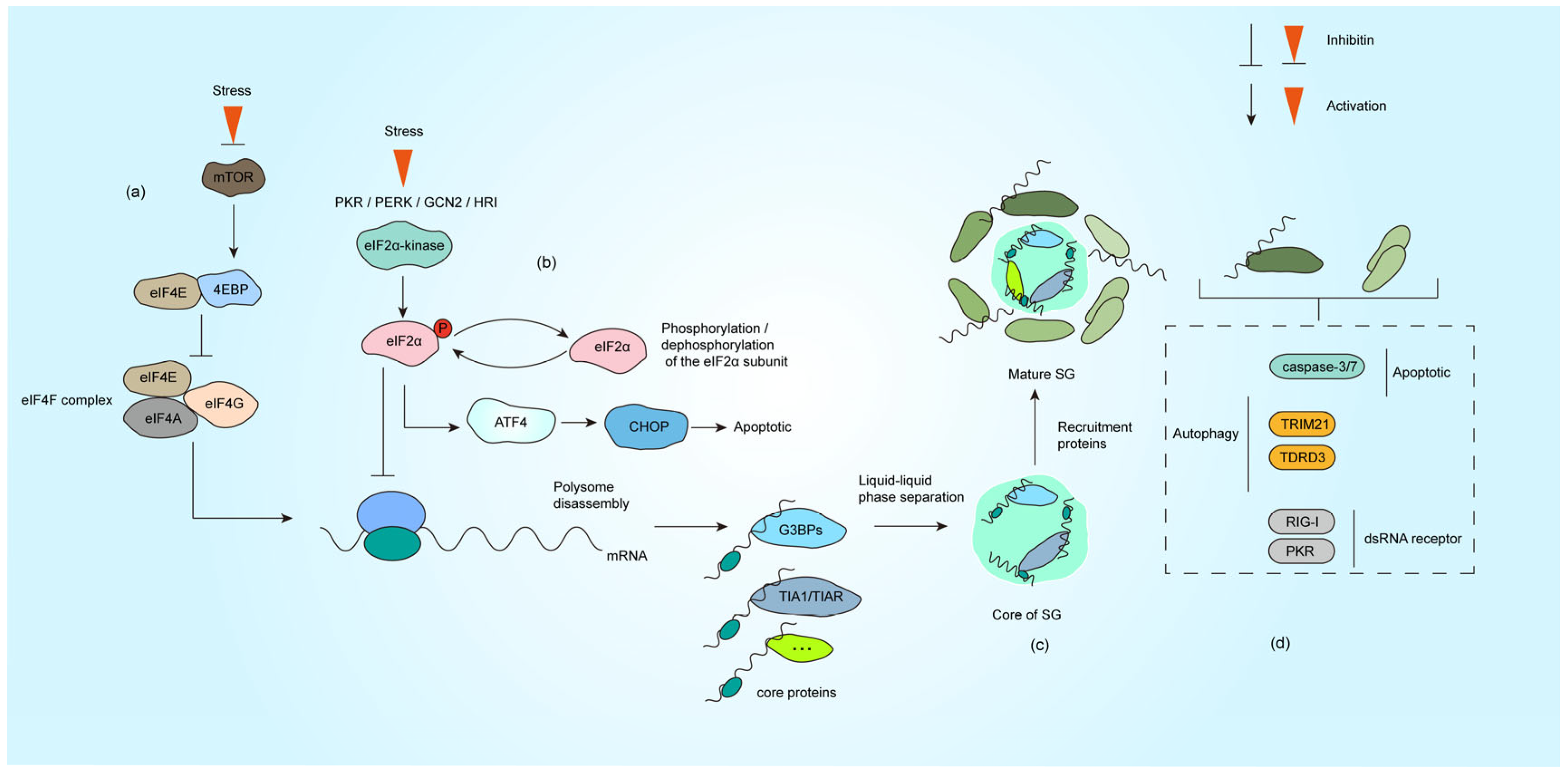
2. Stress Granules: Fundamental Concepts
2.1. Structure and Composition of SG
2.2. Mechanisms of SG Formation
3. Antiviral Functions of SG and Viral Countermeasures
3.1. SG Antiviral Defense via Viral RNA Sequestration
3.2. Stress Granule Formation and Innate Immune Signaling Pathways
3.3. Synergistic Role of SG and Autophagy in Viral Infection
3.4. SG and Apoptotic Signaling Pathways
3.5. Viral Suppression of SG Formation via Different Strategies
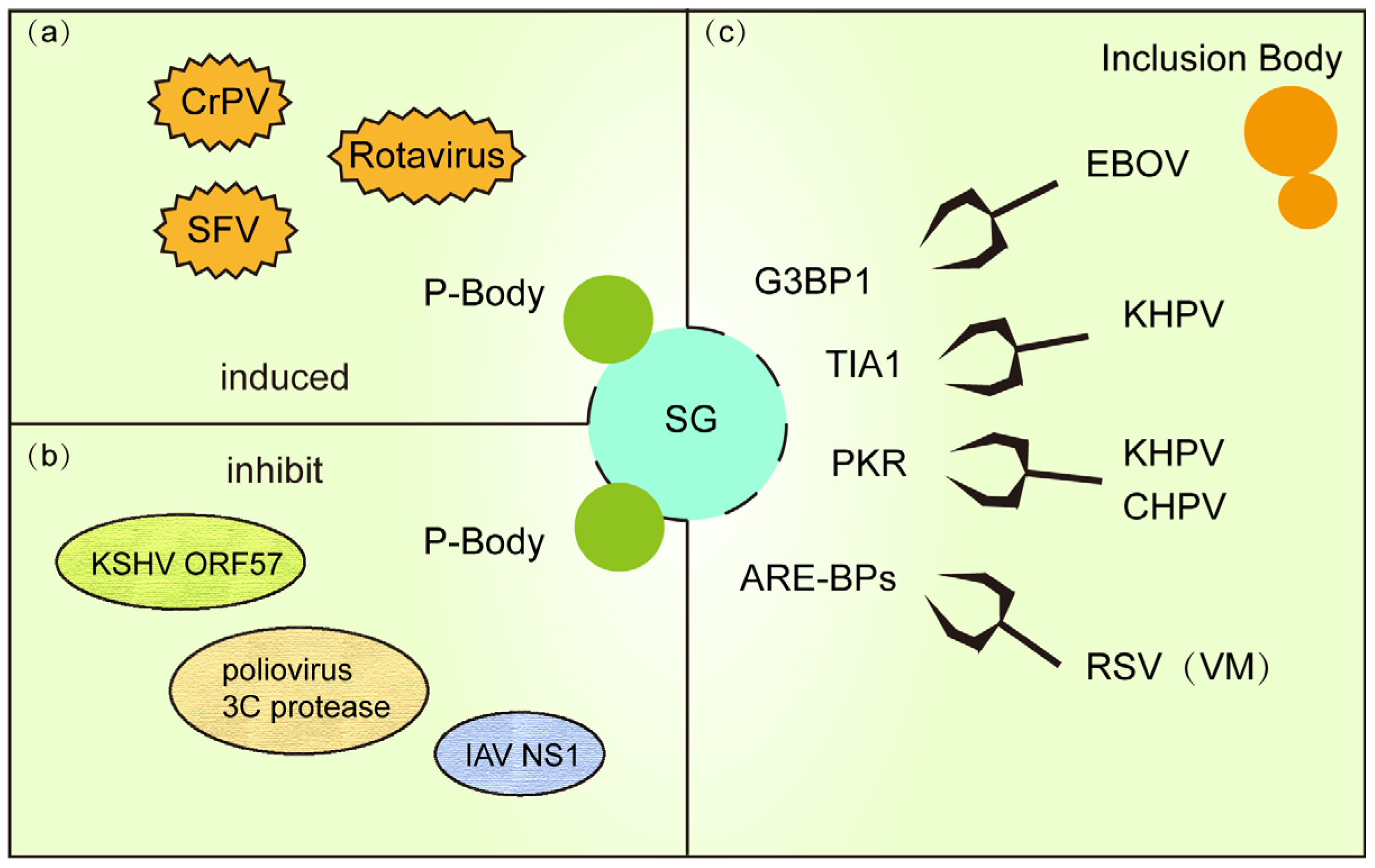
4. Stress Granule Crosstalk with Other Granules in Viral Infection
4.1. PB and SG: Cooperative Antiviral Defense
4.2. Viral Disruption of SG Formation via Inclusion Body Hijacking
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRV | Pseudorabies virus |
| SG | Stress granules |
| PB | Processing bodies |
| IB | Inclusion bodies |
| G3BP1/2 | Ras-GTPase-activating protein-binding proteins |
| TIA1 | T-cell intracellular antigen-1 |
| RBPs | RNA-binding proteins |
| LCDs | Low-complexity domains |
| PICs | Preinitiation complexes |
| ISR | Integrated stress response |
| dsRNA | double-stranded RNA |
| PABP1 | poly(A)-binding protein |
| HSV | herpes simplex virus |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| IAV | Influenza A virus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| RLR | RIG-I-like receptor |
| NDV | Newcastle disease virus |
| HDAC6 | Histone deacetylase 6 |
| HTLV-1 | Human T-cell leukemia virus type 1 |
| MAVS | Mitochondrial antiviral-signaling protein |
| WNV | West Nile virus |
| HCV | Hepatitis C virus |
| EV71 | Enterovirus 71 |
| FMDV | Foot-and-mouth disease virus |
| ASFV | African swine fever virus |
| PEDV | Porcine epidemic diarrhea virus |
| MNV | Murine norovirus |
| CJHIKV | Chikungunya virus |
| GETV | Getah virus |
| RSV | Respiratory syncytial virus |
References
- Hofmann, S.; Kedersha, N.; Anderson, P.; Ivanov, P. Molecular mechanisms of stress granule assembly and disassembly. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118876. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005, 169, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e328. [Google Scholar] [CrossRef] [PubMed]
- Rayman, J.B.; Karl, K.A.; Kandel, E.R. TIA-1 Self-Multimerization, Phase Separation, and Recruitment into Stress Granules Are Dynamically Regulated by Zn(2). Cell Rep. 2018, 22, 59–71. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Y.; Wang, H.; Chai, Y.; Weng, W.; Zhang, Y.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; et al. Viral evasion of PKR restriction by reprogramming cellular stress granules. Proc. Natl. Acad. Sci. USA 2022, 119, e2201169119. [Google Scholar] [CrossRef]
- Matsumiya, T.; Shiba, Y.; Ding, J.; Kawaguchi, S.; Seya, K.; Imaizumi, T. The double-stranded RNA-dependent protein kinase PKR negatively regulates the protein expression of IFN-β induced by RIG-I signaling. FASEB J. 2023, 37, e22780. [Google Scholar] [CrossRef]
- Brownsword, M.J.; Locker, N. A little less aggregation a little more replication: Viral manipulation of stress granules. Wiley Interdiscip. Rev. RNA 2023, 14, e1741. [Google Scholar] [CrossRef]
- Guo, J.; Huang, R.; Mei, Y.; Lu, S.; Gong, J.; Wang, L.; Ding, L.; Wu, H.; Pan, D.; Liu, W. Application of stress granule core element G3BP1 in various diseases: A review. Int. J. Biol. Macromol. 2024, 282, 137254. [Google Scholar] [CrossRef]
- Guille´n-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e317. [Google Scholar] [CrossRef]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Markmiller, S.; Soltanieh, S.; Server, K.L.; Mak, R.; Jin, W.; Fang, M.Y.; Luo, E.C.; Krach, F.; Yang, D.; Sen, A.; et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 2018, 172, 590–604.e513. [Google Scholar] [CrossRef]
- Zhang, Q.; Xian, W.; Li, Z.; Lu, Q.; Chen, X.; Ge, J.; Tang, Z.; Liu, B.; Chen, Z.; Gao, X.; et al. Shigella induces stress granule formation by ADP-riboxanation of the eIF3 complex. Cell Rep. 2024, 43, 113789. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Gal, J.; Chen, J.; Na, D.Y.; Tichacek, L.; Barnett, K.R.; Zhu, H. The Acetylation of Lysine-376 of G3BP1 Regulates RNA Binding and Stress Granule Dynamics. Mol. Cell. Biol. 2019, 39, e00052-19. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.D.; Kedersha, N.; Schulte, T.; Branca, R.M.; Ivanov, P.; Anderson, P. Phosphorylation of G3BP1-S149 does not influence stress granule assembly. J. Cell Biol. 2019, 218, 2425–2432. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Kang, Y.; Yi, Q.; Wang, T.; Bai, Y.; Liu, Y. Stress granule homeostasis is modulated by TRIM21-mediated ubiquitination of G3BP1 and autophagy-dependent elimination of stress granules. Autophagy 2023, 19, 1934–1951. [Google Scholar] [CrossRef]
- Loughlin, F.E.; West, D.L.; Gunzburg, M.J.; Waris, S.; Crawford, S.A.; Wilce, M.C.J.; Wilce, J.A. Tandem RNA binding sites induce self-association of the stress granule marker protein TIA-1. Nucleic Acids Res. 2021, 49, 2403–2417. [Google Scholar] [CrossRef]
- Sanders, D.W.; Kedersha, N.; Lee, D.S.W.; Strom, A.R.; Drake, V.; Riback, J.A.; Bracha, D.; Eeftens, J.M.; Iwanicki, A.; Wang, A.; et al. Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 2020, 181, 306–324.e328. [Google Scholar] [CrossRef]
- Cirillo, L.; Cieren, A.; Barbieri, S.; Khong, A.; Schwager, F.; Parker, R.; Gotta, M. UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1. Curr. Biol. CB 2020, 30, 698–707.e696. [Google Scholar] [CrossRef]
- Wang, C.; Duan, Y.; Duan, G.; Wang, Q.; Zhang, K.; Deng, X.; Qian, B.; Gu, J.; Ma, Z.; Zhang, S.; et al. Stress Induces Dynamic, Cytotoxicity-Antagonizing TDP-43 Nuclear Bodies via Paraspeckle LncRNA NEAT1-Mediated Liquid-Liquid Phase Separation. Mol. Cell 2020, 79, 443–458.e447. [Google Scholar] [CrossRef]
- Higashi, S.; Kabuta, T.; Nagai, Y.; Tsuchiya, Y.; Akiyama, H.; Wada, K. TDP-43 associates with stalled ribosomes and contributes to cell survival during cellular stress. J. Neurochem. 2013, 126, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jia, R.; Dai, Z.; Zhou, J.; Ruan, J.; Chng, W.; Cai, Z.; Zhang, X. Stress granules in cancer: Adaptive dynamics and therapeutic implications. iScience 2024, 27, 110359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Zhang, Y.; Matthias, P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007, 21, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lo, M.; Chandra, P.; Datta Chaudhuri, R.; De, P.; Dutta, S.; Chawla-Sarkar, M. SARS-CoV-2 nucleocapsid protein promotes self-deacetylation by inducing HDAC6 to facilitate viral replication. Virol. J. 2024, 21, 186. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, S.; Wang, J.; Zhou, L.; Zhang, X.; Billadeau, D.D.; Yang, P.; Zhang, L.; Zhou, F.; Bai, P.; et al. TRIM25 predominately associates with anti-viral stress granules. Nat. Commun. 2024, 15, 4127. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Yao, X.; Wang, G.; Huang, S.; Chen, P.; Tang, M.; Cai, J.; Wu, Z.; Zhang, Y.; et al. Mammalian IRE1α dynamically and functionally coalesces with stress granules. Nat. Cell Biol. 2024, 26, 917–931. [Google Scholar] [CrossRef]
- Poblete-Durán, N.; Prades-Pérez, Y.; Vera-Otarola, J.; Soto-Rifo, R.; Valiente-Echeverría, F. Who Regulates Whom? An Overview of RNA Granules and Viral Infections. Viruses 2016, 8, 180. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Zhou, P. Role(s) of G3BPs in Human Pathogenesis. J. Pharmacol. Exp. Ther. 2023, 387, 100–110. [Google Scholar] [CrossRef]
- Walsh, D.; Mathews, M.B.; Mohr, I. Tinkering with translation: Protein synthesis in virus-infected cells. Cold Spring Harb. Perspect. Biol. 2013, 5, a012351. [Google Scholar] [CrossRef]
- Mars, J.-C.; Culjkovic-Kraljacic, B.; Borden, K.L.B. eIF4E orchestrates mRNA processing, RNA export and translation to modify specific protein production. Nucleus 2024, 15, 2360196. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef] [PubMed]
- McEwen, E.; Kedersha, N.; Song, B.; Scheuner, D.; Gilks, N.; Han, A.; Chen, J.J.; Anderson, P.; Kaufman, R.J. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005, 280, 16925–16933. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Feng, L.; Wek, R.C.; Cigan, A.M.; Donahue, T.F.; Hinnebusch, A.G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 1992, 68, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 2017, 68, 808–820.e805. [Google Scholar] [CrossRef]
- Angel, M.; Fleshler, E.; Atrash, M.K.; Kinor, N.; Benichou, J.I.C.; Shav-Tal, Y. Nuclear RNA-related processes modulate the assembly of cytoplasmic RNA granules. Nucleic Acids Res. 2024, 52, 5356–5375. [Google Scholar] [CrossRef]
- Tsai, W.C.; Lloyd, R.E. Cytoplasmic RNA Granules and Viral Infection. Annu. Rev. Virol. 2014, 1, 147–170. [Google Scholar] [CrossRef]
- Corbet, G.A.; Burke, J.M.; Bublitz, G.R.; Tay, J.W.; Parker, R. dsRNA-induced condensation of antiviral proteins modulates PKR activity. Proc. Natl. Acad. Sci. USA 2022, 119, e2204235119. [Google Scholar] [CrossRef]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef]
- Kedersha, N.; Panas, M.D.; Achorn, C.A.; Lyons, S.; Tisdale, S.; Hickman, T.; Thomas, M.; Lieberman, J.; McInerney, G.M.; Ivanov, P.; et al. G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol. 2016, 212, 845–860. [Google Scholar] [CrossRef]
- Ripin, N.; Macedo de Vasconcelos, L.; Ugay, D.A.; Parker, R. DDX6 modulates P-body and stress granule assembly, composition, and docking. J. Cell Biol. 2024, 223, e202306022. [Google Scholar] [CrossRef]
- Paget, M.; Cadena, C.; Ahmad, S.; Wang, H.T.; Jordan, T.X.; Kim, E.; Koo, B.; Lyons, S.M.; Ivanov, P.; tenOever, B.; et al. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol. Cell 2023, 83, 1180–1196.e1188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Gao, S.; Zhu, C.; Liu, S.; Li, J.; Kang, J.; Wang, Z.; Wang, T. Typical Stress Granule Proteins Interact with the 3′ Untranslated Region of Enterovirus D68 To Inhibit Viral Replication. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.N.; Chong, C.L.; Chou, Y.C.; Huang, C.C.; Wang, Y.L.; Wang, S.W.; Chen, M.L.; Chen, C.H.; Chang, C. Doubly Spliced RNA of Hepatitis B Virus Suppresses Viral Transcription via TATA-Binding Protein and Induces Stress Granule Assembly. J. Virol. 2015, 89, 11406–11419. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Pijlman, G.P. Alphavirus Infection: Host Cell Shut-Off and Inhibition of Antiviral Responses. Viruses 2016, 8, 166. [Google Scholar] [CrossRef]
- Burke, J.M.; Ratnayake, O.C.; Watkins, J.M.; Perera, R.; Parker, R. G3BP1-dependent condensation of translationally inactive viral RNAs antagonizes infection. Sci. Adv. 2024, 10, eadk8152. [Google Scholar] [CrossRef]
- Manjunath, L.; Oh, S.; Ortega, P.; Bouin, A.; Bournique, E.; Sanchez, A.; Martensen, P.M.; Auerbach, A.A.; Becker, J.T.; Seldin, M.; et al. APOBEC3B drives PKR-mediated translation shutdown and protects stress granules in response to viral infection. Nat. Commun. 2023, 14, 820. [Google Scholar] [CrossRef]
- Wylie, K.M.; Schrimpf, J.E.; Morrison, L.A. Increased eIF2alpha phosphorylation attenuates replication of herpes simplex virus 2 vhs mutants in mouse embryonic fibroblasts and correlates with reduced accumulation of the PKR antagonist ICP34.5. J. Virol. 2009, 83, 9151–9162. [Google Scholar] [CrossRef]
- van der Lee, R.; Feng, Q.; Langereis, M.A.; Ter Horst, R.; Szklarczyk, R.; Netea, M.G.; Andeweg, A.C.; van Kuppeveld, F.J.; Huynen, M.A. Integrative Genomics-Based Discovery of Novel Regulators of the Innate Antiviral Response. PLoS Comput. Biol. 2015, 11, e1004553. [Google Scholar] [CrossRef]
- Oh, S.W.; Onomoto, K.; Wakimoto, M.; Onoguchi, K.; Ishidate, F.; Fujiwara, T.; Yoneyama, M.; Kato, H.; Fujita, T. Leader-Containing Uncapped Viral Transcript Activates RIG-I in Antiviral Stress Granules. PLoS Pathog. 2016, 12, e1005444. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, L.; Yu, S.; Wang, X.; Zheng, H.; Zhang, P.; Meng, C.; Zhan, Y.; Tan, L.; Song, C.; et al. Newcastle disease virus induces stable formation of bona fide stress granules to facilitate viral replication through manipulating host protein translation. FASEB J. 2017, 31, 1337–1353. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Deater, M.; Lloyd, R.E. TDRD3 functions as a selective autophagy receptor with dual roles in autophagy and modulation of stress granule stability. bioRxiv Prepr. Serv. Biol. 2024. [CrossRef]
- Wang, Q.; Zhou, L.; Wang, J.; Su, D.; Li, D.; Du, Y.; Yang, G.; Zhang, G.; Chu, B. African Swine Fever Virus K205R Induces ER Stress and Consequently Activates Autophagy and the NF-κB Signaling Pathway. Viruses 2022, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Monahan, Z.; Shewmaker, F.; Pandey, U.B. Stress granules at the intersection of autophagy and ALS. Brain Res. 2016, 1649, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Rubinsztein, D.C. The autophagy of stress granules. FEBS Lett. 2024, 598, 59–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, G.; Tang, Y.; Yan, J.; Han, S.; Yin, J.; Peng, B.; He, X.; Liu, W. HDAC6, A Novel Cargo for Autophagic Clearance of Stress Granules, Mediates the Repression of the Type I Interferon Response During Coxsackievirus A16 Infection. Front. Microbiol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Fujikawa, D.; Nakamura, T.; Yoshioka, D.; Li, Z.; Moriizumi, H.; Taguchi, M.; Tokai-Nishizumi, N.; Kozuka-Hata, H.; Oyama, M.; Takekawa, M. Stress granule formation inhibits stress-induced apoptosis by selectively sequestering executioner caspases. Curr. Biol. CB 2023, 33, 1967–1981.e1968. [Google Scholar] [CrossRef]
- Shi, J.; Li, Z.; Xu, R.; Zhang, J.; Zhou, Q.; Gao, R.; Lu, H.; Lan, Y.; Zhao, K.; He, H.; et al. The PERK/PKR-eIF2α Pathway Negatively Regulates Porcine Hemagglutinating Encephalomyelitis Virus Replication by Attenuating Global Protein Translation and Facilitating Stress Granule Formation. J. Virol. 2022, 96, e0169521. [Google Scholar] [CrossRef]
- Takahashi, M.; Higuchi, M.; Makokha, G.N.; Matsuki, H.; Yoshita, M.; Tanaka, Y.; Fujii, M. HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 2013, 122, 715–725. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.X.; Mao, X.; Fang, H.; Wang, H.; Li, Y.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget 2017, 8, 43201–43217. [Google Scholar] [CrossRef]
- Basu, M.; Courtney, S.C.; Brinton, M.A. Arsenite-induced stress granule formation is inhibited by elevated levels of reduced glutathione in West Nile virus-infected cells. PLoS Pathog. 2017, 13, e1006240. [Google Scholar] [CrossRef]
- Ge, Y.; Jin, J.; Li, J.; Ye, M.; Jin, X. The roles of G3BP1 in human diseases (review). Gene 2022, 821, 146294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.R.; Majerciak, V.; Kruhlak, M.J.; Zheng, Z.M. KSHV inhibits stress granule formation by viral ORF57 blocking PKR activation. PLoS Pathog. 2017, 13, e1006677. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Fung, T.S.; Huang, M.; Fang, S.G.; Zhong, Y.; Liu, D.X. Upregulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. J. Virol. 2013, 87, 8124–8134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhu, Z.; Wang, W.; Wen, W.; Zhang, Z.; Favoreel, H.W.; Li, X. Pseudorabies virus IE180 protein hijacks G3BPs into the nucleus to inhibit stress granule formation. J. Virol. 2025, 99, e0208824. [Google Scholar] [CrossRef]
- Khaperskyy, D.A.; Emara, M.M.; Johnston, B.P.; Anderson, P.; Hatchette, T.F.; McCormick, C. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS Pathog. 2014, 10, e1004217. [Google Scholar] [CrossRef]
- Zhang, P.; Su, C.; Jiang, Z.; Zheng, C. Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via Its RNase Activity. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Gao, P.; Ren, J.; Zhou, Q.; Chen, P.; Zhang, A.; Zhang, Y.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; et al. Pseudorabies virus inhibits the unfolded protein response for viral replication during the late stages of infection. Vet. Microbiol. 2025, 301, 110360. [Google Scholar] [CrossRef]
- Amorim, R.; Temzi, A.; Griffin, B.D.; Mouland, A.J. Zika virus inhibits eIF2α-dependent stress granule assembly. PLoS Neglected Trop. Dis. 2017, 11, e0005775. [Google Scholar] [CrossRef]
- Zhang, F.; Moon, A.; Childs, K.; Goodbourn, S.; Dixon, L.K. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J. Virol. 2010, 84, 10681–10689. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Y.; Yap, P.L.; Png, K.J.; Tam, J.P.; Liu, D.X. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J. Virol. 2009, 83, 12462–12472. [Google Scholar] [CrossRef]
- Xu, S.; Chen, D.; Chen, D.; Hu, Q.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Pseudorabies virus infection inhibits stress granules formation via dephosphorylating eIF2alpha. Vet. Microbiol. 2020, 247, 108786. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Chen, X.; Yu, J.; Wang, Y.; Yang, Y.; Du, M.; Jin, H.; Ma, Y.; He, B.; et al. ICP34.5 protein of herpes simplex virus facilitates the initiation of protein translation by bridging eukaryotic initiation factor 2α (eIF2α) and protein phosphatase 1. J. Biol. Chem. 2011, 286, 24785–24792. [Google Scholar] [CrossRef] [PubMed]
- Balvay, L.; Lopez Lastra, M.; Sargueil, B.; Darlix, J.L.; Ohlmann, T. Translational control of retroviruses. Nat. Rev. Microbiol. 2007, 5, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, Z.; Fan, S.; Zhang, Q.; Zhong, Y.; Guo, D.; Qin, Y.; Chen, M. Picornavirus 2A protease regulates stress granule formation to facilitate viral translation. PLoS Pathog. 2018, 14, e1006901. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, L.; Xu, X.; Han, H.; Li, P.; Zou, D.; Li, X.; Zheng, L.; Cheng, L.; Shen, Y.; et al. Enterovirus 71 inhibits cytoplasmic stress granule formation during the late stage of infection. Virus Res. 2018, 255, 55–67. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Wang, X.; Chen, X.; Zhao, G.; Liu, C.; Bao, M.; Song, J.; Li, J.; Huang, L.; et al. African swine fever virus pS273R antagonizes stress granule formation by cleaving the nucleating protein G3BP1 to facilitate viral replication. J. Biol. Chem. 2023, 299, 104844. [Google Scholar] [CrossRef]
- Visser, L.J.; Medina, G.N.; Rabouw, H.H.; de Groot, R.J.; Langereis, M.A.; de Los Santos, T.; van Kuppeveld, F.J.M. Foot-and-Mouth Disease Virus Leader Protease Cleaves G3BP1 and G3BP2 and Inhibits Stress Granule Formation. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.J.; Jung, Y.S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344. [Google Scholar] [CrossRef]
- Fritzlar, S.; Aktepe, T.E.; Chao, Y.W.; Kenney, N.D.; McAllaster, M.R.; Wilen, C.B.; White, P.A.; Mackenzie, J.M. Mouse Norovirus Infection Arrests Host Cell Translation Uncoupled from the Stress Granule-PKR-eIF2α Axis. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Yang, Z.; Johnson, B.A.; Meliopoulos, V.A.; Ju, X.; Zhang, P.; Hughes, M.P.; Wu, J.; Koreski, K.P.; Clary, J.E.; Chang, T.C.; et al. Interaction between host G3BP and viral nucleocapsid protein regulates SARS-CoV-2 replication and pathogenicity. Cell Rep. 2024, 43, 113965. [Google Scholar] [CrossRef]
- Nelson, E.V.; Schmidt, K.M.; Deflube, L.R.; Doganay, S.; Banadyga, L.; Olejnik, J.; Hume, A.J.; Ryabchikova, E.; Ebihara, H.; Kedersha, N.; et al. Ebola Virus Does Not Induce Stress Granule Formation during Infection and Sequesters Stress Granule Proteins within Viral Inclusions. J. Virol. 2016, 90, 7268–7284. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Kumar, A.; Xu, Z.; Airo, A.M.; Stryapunina, I.; Wong, C.P.; Branton, W.; Tchesnokov, E.; Götte, M.; Power, C.; et al. Zika Virus Hijacks Stress Granule Proteins and Modulates the Host Stress Response. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Domeradzka, N.E.; Baggen, J.; Geertsema, C.; Flipse, J.; Vlak, J.M.; Pijlman, G.P. Chikungunya Virus nsP3 Blocks Stress Granule Assembly by Recruitment of G3BP into Cytoplasmic Foci. J. Virol. 2012, 86, 10873–10879. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhao, R.; Yao, X.; Liu, Q.; Liu, P.; Zhu, Z.; Tu, C.; Gong, W.; Li, X. Getah virus Nsp3 binds G3BP to block formation of bona fide stress granules. Int. J. Biol. Macromol. 2024, 279, 135274. [Google Scholar] [CrossRef]
- Catanzaro, N.; Meng, X.J. Porcine reproductive and respiratory syndrome virus (PRRSV)-induced stress granules are associated with viral replication complexes and suppression of host translation. Virus Res. 2019, 265, 47–56. [Google Scholar] [CrossRef]
- Kim, S.S.; Sze, L.; Liu, C.; Lam, K.P. The stress granule protein G3BP1 binds viral dsRNA and RIG-I to enhance interferon-I response. J. Biol. Chem. 2019, 294, 6430–6438. [Google Scholar] [CrossRef]
- Jayabalan, A.K.; Adivarahan, S.; Koppula, A.; Abraham, R.; Batish, M.; Zenklusen, D.; Griffin, D.E.; Leung, A.K.L. Stress granule formation, disassembly, and composition are regulated by alphavirus ADP-ribosylhydrolase activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2021719118. [Google Scholar] [CrossRef]
- Götte, B.; Panas, M.D.; Hellström, K.; Liu, L.; Samreen, B.; Larsson, O.; Ahola, T.; McInerney, G.M. Separate domains of G3BP promote efficient clustering of alphavirus replication complexes and recruitment of the translation initiation machinery. PLoS Pathog. 2019, 15, e1007842. [Google Scholar] [CrossRef]
- Beckham, C.J.; Parker, R. P bodies, stress granules, and viral life cycles. Cell Host Microbe 2008, 3, 206–212. [Google Scholar] [CrossRef]
- Lloyd, R.E. Regulation of stress granules and P-bodies during RNA virus infection. Wiley Interdiscip. Rev. RNA 2013, 4, 317–331. [Google Scholar] [CrossRef]
- Kershaw, C.J.; Nelson, M.G.; Lui, J.; Bates, C.P.; Jennings, M.D.; Hubbard, S.J.; Ashe, M.P.; Grant, C.M. Integrated multi-omics reveals common properties underlying stress granule and P-body formation. RNA Biol. 2021, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.R.; Zheng, Z.M. RNA Granules in Antiviral Innate Immunity: A Kaposi’s Sarcoma-Associated Herpesvirus Journey. Front. Microbiol. 2021, 12, 794431. [Google Scholar] [CrossRef] [PubMed]
- Dolnik, O.; Gerresheim, G.K.; Biedenkopf, N. New Perspectives on the Biogenesis of Viral Inclusion Bodies in Negative-Sense RNA Virus Infections. Cells 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, D.; Battich, N.; Pelkmans, L. A Systems-Level Study Reveals Regulators of Membrane-less Organelles in Human Cells. Mol. Cell 2018, 72, 1035–1049.e1035. [Google Scholar] [CrossRef]
- Youn, J.-Y.; Dyakov, B.J.A.; Zhang, J.; Knight, J.D.R.; Vernon, R.M.; Forman-Kay, J.D.; Gingras, A.-C. Properties of Stress Granule and P-Body Proteomes. Mol. Cell 2019, 76, 286–294. [Google Scholar] [CrossRef]
- Dhillon, P.; Rao, C.D. Rotavirus Induces Formation of Remodeled Stress Granules and P Bodies and Their Sequestration in Viroplasms To Promote Progeny Virus Production. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Khong, A.; Jan, E. Modulation of stress granules and P bodies during dicistrovirus infection. J. Virol. 2011, 85, 1439–1451. [Google Scholar] [CrossRef]
- Liu, L.; Weiss, E.; Panas, M.D.; Götte, B.; Sellberg, S.; Thaa, B.; McInerney, G.M. RNA processing bodies are disassembled during Old World alphavirus infection. J. Gen. Virol. 2019, 100, 1375–1389. [Google Scholar] [CrossRef]
- Dougherty, J.D.; White, J.P.; Lloyd, R.E. Poliovirus-mediated disruption of cytoplasmic processing bodies. J. Virol. 2011, 85, 64–75. [Google Scholar] [CrossRef]
- Mok, B.W.; Song, W.; Wang, P.; Tai, H.; Chen, Y.; Zheng, M.; Wen, X.; Lau, S.Y.; Wu, W.L.; Matsumoto, K.; et al. The NS1 protein of influenza A virus interacts with cellular processing bodies and stress granules through RNA-associated protein 55 (RAP55) during virus infection. J. Virol. 2012, 86, 12695–12707. [Google Scholar] [CrossRef]
- Galloux, M.; Risso-Ballester, J.; Richard, C.A.; Fix, J.; Rameix-Welti, M.A.; Eléouët, J.F. Minimal Elements Required for the Formation of Respiratory Syncytial Virus Cytoplasmic Inclusion Bodies In Vivo and In Vitro. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Muñoz, N.; Branttie, J.; Slaughter, K.B.; Dutch, R.E. Human Metapneumovirus Induces Formation of Inclusion Bodies for Efficient Genome Replication and Transcription. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Koo, L.Y.; Brown, C.R.; Collins, P.L. p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J. Virol. 2013, 87, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Tang, Q.; Yang, X.; Qin, Y.; Chen, M. Inclusion bodies of human parainfluenza virus type 3 inhibit antiviral stress granule formation by shielding viral RNAs. PLoS Pathog. 2018, 14, e1006948. [Google Scholar] [CrossRef]
- Sarkar, S.; Ganguly, S.; Ganguly, N.K.; Sarkar, D.P.; Sharma, N.R. Chandipura Virus Forms Cytoplasmic Inclusion Bodies through Phase Separation and Proviral Association of Cellular Protein Kinase R and Stress Granule Protein TIA-1. Viruses 2024, 16, 1027. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Li, X. Multi-Faceted Roles of Stress Granules in Viral Infection. Microorganisms 2025, 13, 1434. https://doi.org/10.3390/microorganisms13071434
Zhao R, Li X. Multi-Faceted Roles of Stress Granules in Viral Infection. Microorganisms. 2025; 13(7):1434. https://doi.org/10.3390/microorganisms13071434
Chicago/Turabian StyleZhao, Ruihan, and Xiangdong Li. 2025. "Multi-Faceted Roles of Stress Granules in Viral Infection" Microorganisms 13, no. 7: 1434. https://doi.org/10.3390/microorganisms13071434
APA StyleZhao, R., & Li, X. (2025). Multi-Faceted Roles of Stress Granules in Viral Infection. Microorganisms, 13(7), 1434. https://doi.org/10.3390/microorganisms13071434




