Triple-Probiotic-Fermented Goji (Lycium barbarum L.) Ameliorates Metabolic Disorders Associated with Hyperuricemia in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Strain Culture and Preparation of Goji-Fermented Beverage
2.3. Detection of Antioxidant Activity of Probiotic-Fermented Goji Beverage
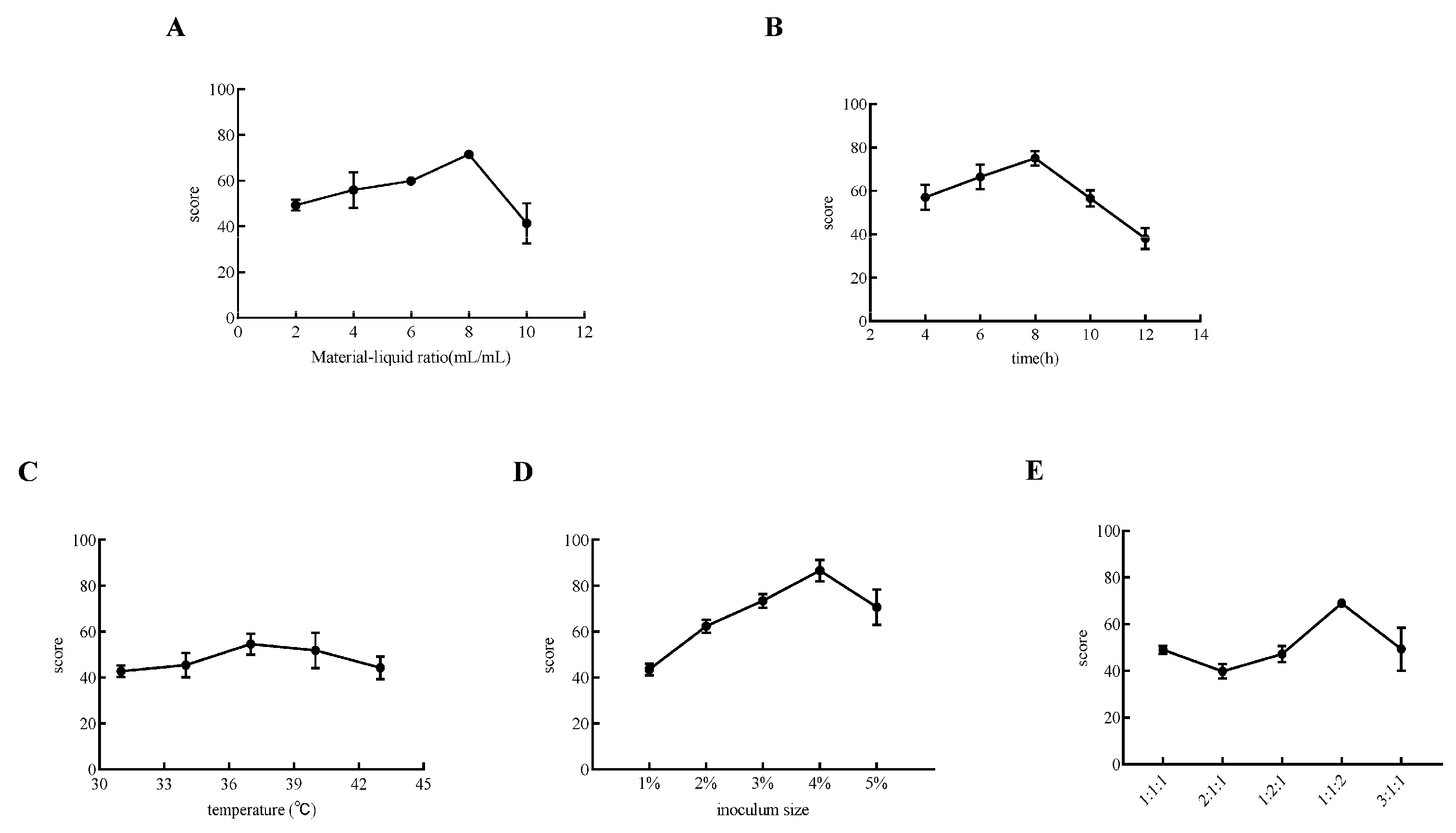
2.4. Single-Factor Experiment on Goji Fermentation
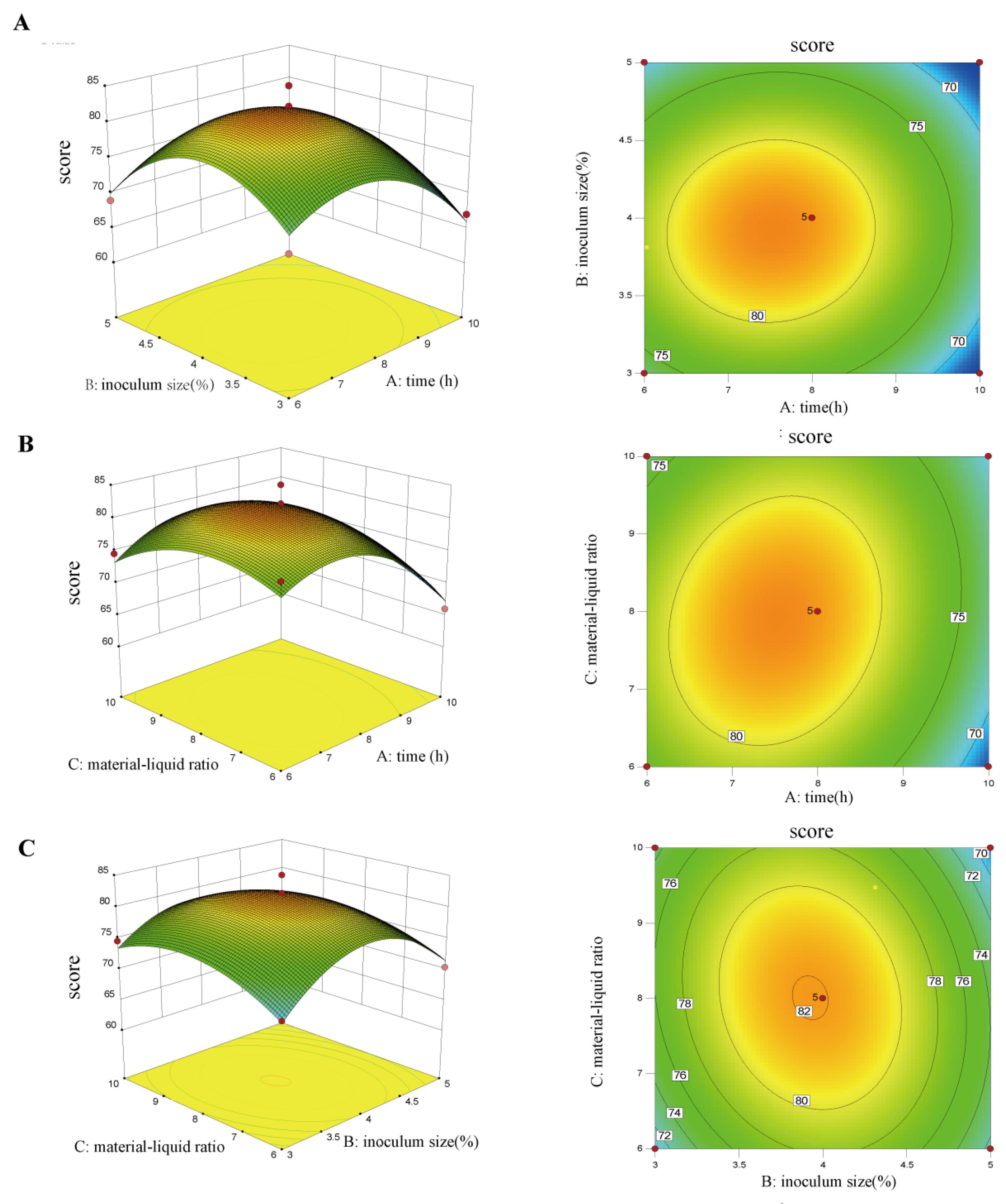
2.5. Optimization of Goji Fermentation Process Using Response Surface Analysis
2.6. Determination of Flavor Substances in Fermented Beverages by SPME-GC/MS
2.7. Cell Viability Assay and Construction of Cellular Models
2.8. Animals and Treatment
2.9. Histological Analysis of Kidney and Small Intestine Tissues
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.11. Gut Microbiota Analysis
2.12. Analysis of SCFAs via Gas Chromatography (GC)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Fermented Goji Beverage by Triple Probiotics
3.2. Analysis of Flavor Substances of Triple-Probiotic-Fermented Goji Beverages
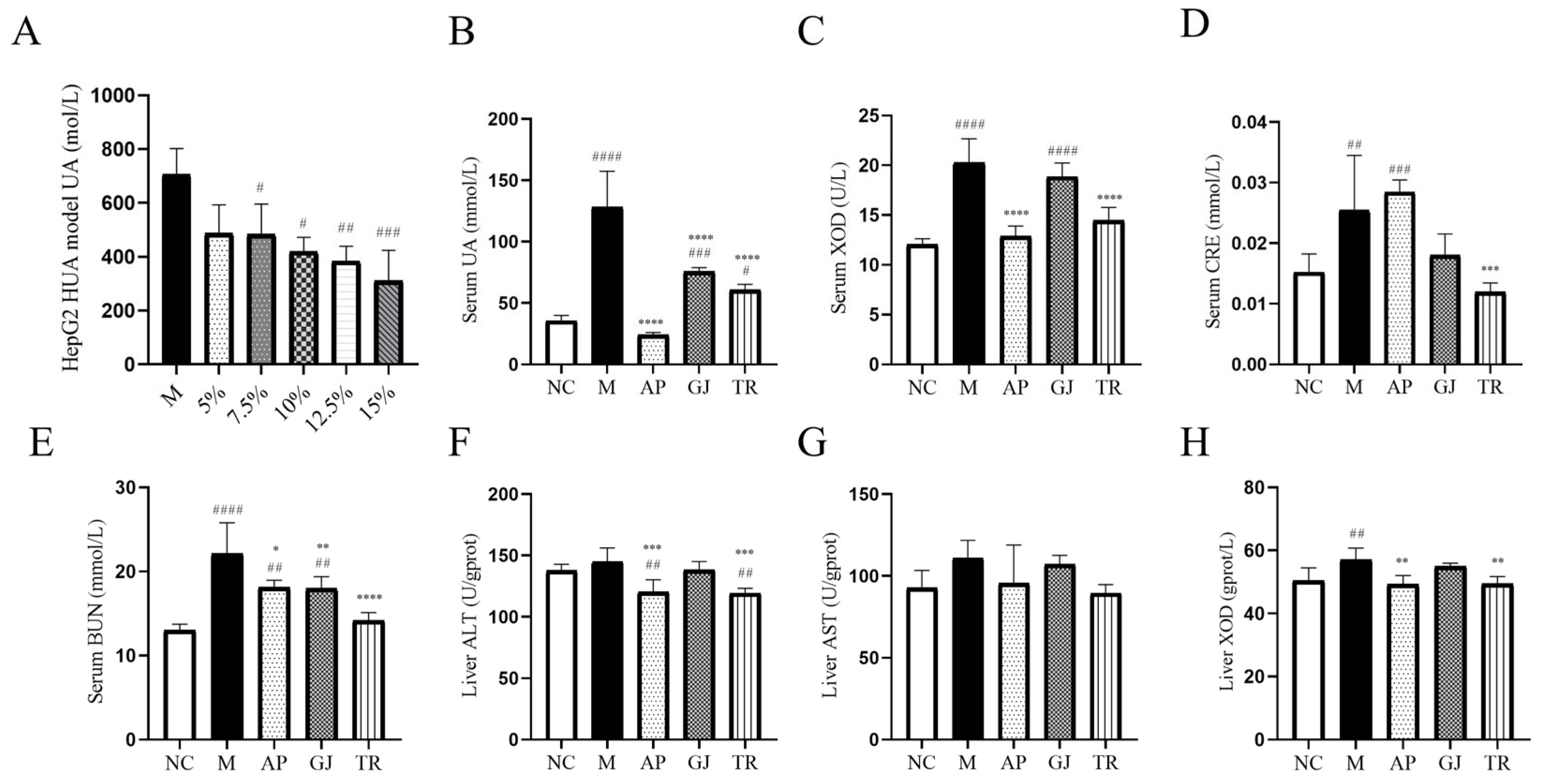
3.3. Effects of Goji Juice and Probiotic-Fermented Goji Beverages on HUA In Vitro and In Vivo
3.4. Probiotic-Fermented Goji Beverage Alleviates HUA-Induced Hepatic, Renal, and Intestinal Damage by Modulating Pathological Changes
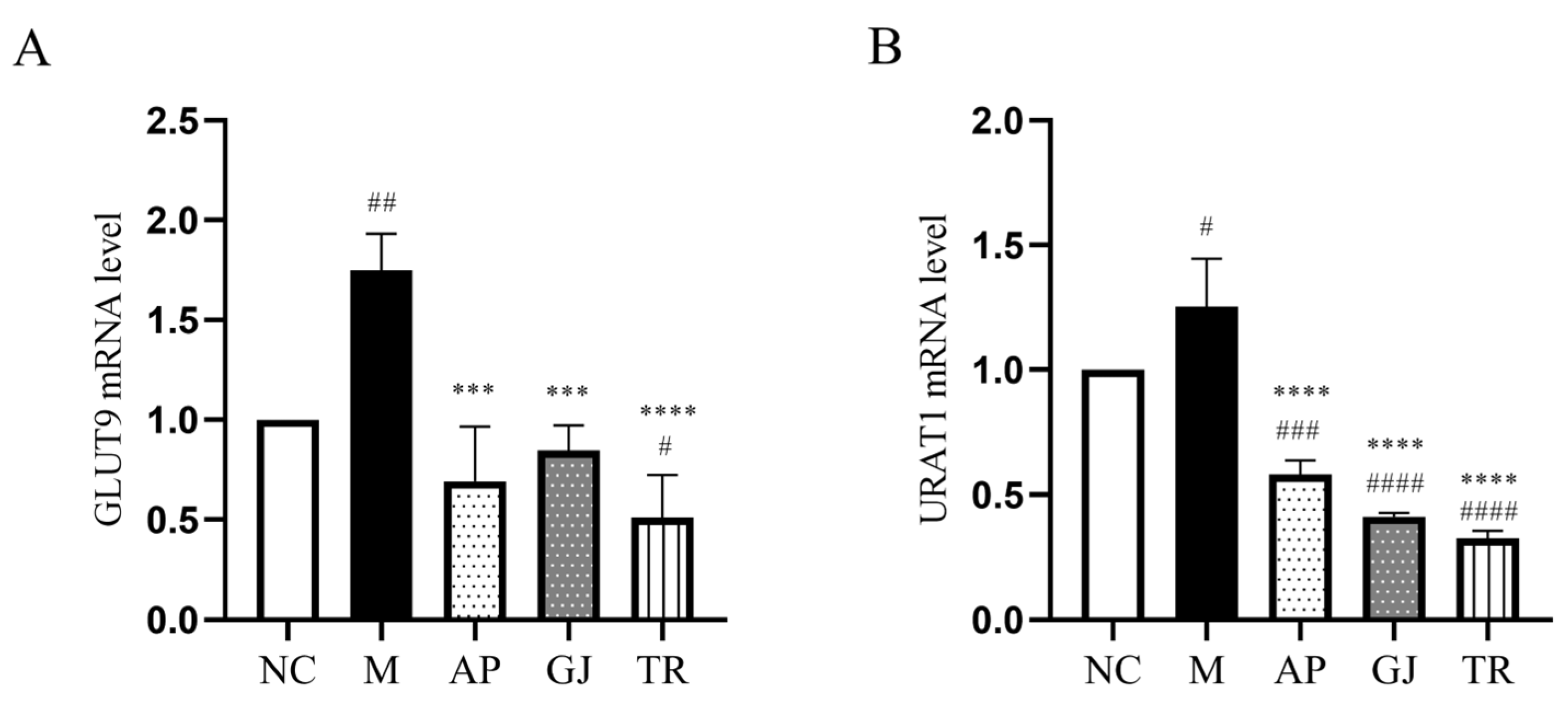
3.5. Probiotic-Fermented Goji Beverage Attenuates UA Reabsorption Through GLUT9 and URAT1 Regulation
3.6. Intestinal Flora Remodeling and Intestinal Barrier Restoration by Probiotic-Fermented Goji Beverage in HUA Mice
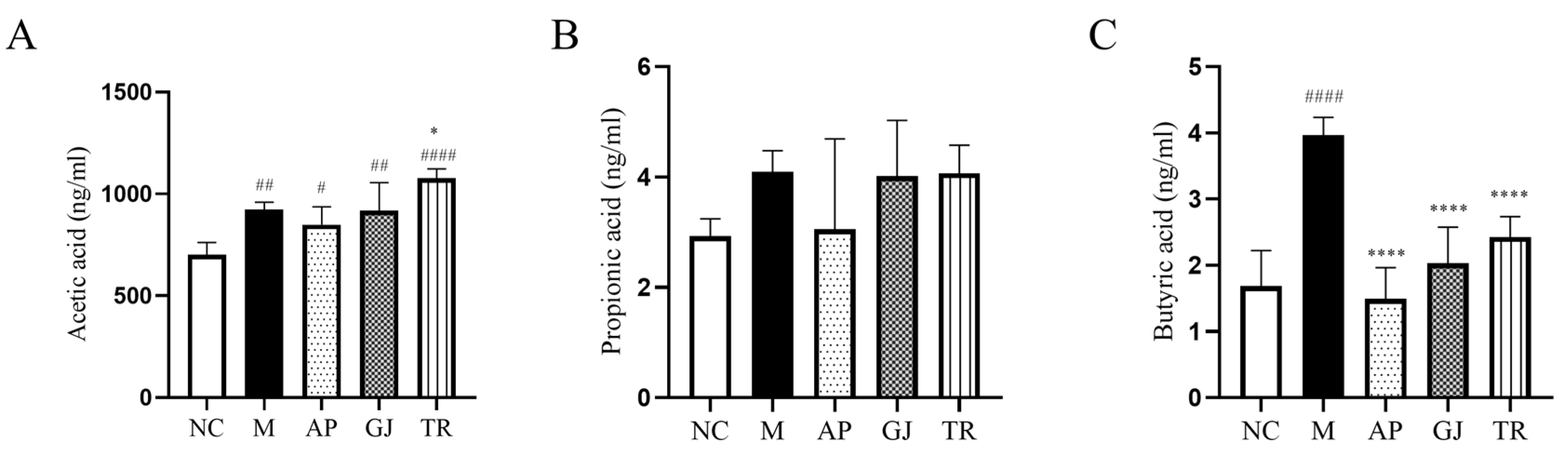
3.7. Probiotic-Fermented Goji Beverage Modulates Gut Microbiota-Derived SCFAs in HUA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound Type | Chemical Compound | CAS | Relative Amount in Goji Juice (%) | Relative Amount in Triple-Probiotic-Fermented Goji Beverage (%) |
|---|---|---|---|---|
| alcohol compound | 3-Methyl-1-butanol | 123-51-3 | 0.44% | 0.33% |
| n-Hexyl alcohol | 111-27-3 | 18.01% | 13.5% | |
| undecan-2-ol | 1653-30-1 | 1.45% | 1.15% | |
| 1-Octanol | 111-87-5 | - | 0.81% | |
| Phenethyl alcohol | 60-12-8 | 0.51% | 0.5% | |
| trans-2-hexen-1-ol | 928-95-0 | 12.42% | 1.03% | |
| 2-Ethyl-1-hexanol | 104-76-7 | 1% | 0.75% | |
| 1-Heptanol | 111-70-6 | 0.19% | 0.34% | |
| 1-Octen-3-ol | 3391-86-4 | 1.63% | 1.86% | |
| acid compounds | Acetic acid | 64-19-7 | - | 5.53% |
| Nonanoic acid | 112-05-0 | 0.8% | 0.38% | |
| ketone compound | 2,3-Butanedione | 431-03-8 | 2.73% | 12.17% |
| Acetyl methyl carbinol | 513-86-0 | 18.29% | 12.4% | |
| 6-Methyl-5-hepten-2-one | 110-93-0 | 1.32% | 1.2% | |
| beta-ionone | 79-77-6 | 1.28% | 0.83% | |
| aldehyde compound | Caproaldehyde | 66-25-1 | 1.07% | 0.54% |
| Benzaldehyde | 100-52-7 | 0.4% | 0.28% | |
| Octyl aldehyde | 124-13-0 | 0.51% | 1.1% | |
| Decanal | 112-31-2 | 1.77% | 4.05% |
References
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Vargas-Santos, A.B.; Neogi, T. Management of Gout and Hyperuricemia in CKD. Am. J. Kidney Dis. 2017, 70, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2020, 79, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jaglan, A.; Sadera, G.; Singh, P.; Singh, B.P.; Goel, G. Probiotic potential of gluten degrading Bacillus tequilensis AJG23 isolated from Indian traditional cereal-fermented foods as determined by Multiple Attribute Decision-Making analysis. Food Res. Int. 2023, 174, 113516. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Song, X.; Xiong, Z.; Zhang, H.; Lai, P.; Wang, S.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 103854. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Bock, H.-J.; Lee, H.-W.; Lee, N.-K.; Paik, H.-D. Probiotic Lactiplantibacillus plantarum KU210152 and its fermented soy milk attenuates oxidative stress in neuroblastoma cells. Food Res. Int. 2024, 177, 113868. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Q.; Ren, H.; Gao, L.; Zhao, P.; Yang, X.; Yang, G.; Xu, D.; Wang, G.; Yang, W.; et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ 2020, 8, e8664. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Chen, M.; Guo, X.; Ding, Z. The antioxidant strain Lactiplantibacillus plantarum AS21 and Clostridium butyricum ameliorate DSS-induced colitis in mice by remodeling the assembly of intestinal microbiota and improving gut functions. Food Funct. 2024, 15, 2022–2037. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Tabrizi, E.; Pourteymour Fard Tabrizi, F.; Mahmoud Khaled, G.; Sestito, M.P.; Jamie, S.; Boone, B.A. Unraveling the gut microbiome’s contribution to pancreatic ductal adenocarcinoma: Mechanistic insights and therapeutic perspectives. Front. Immunol. 2024, 15, 1434771. [Google Scholar] [CrossRef] [PubMed]
- Nandha, M.C.; Shukla, R.M. Exploration of probiotic attributes in lactic acid bacteria isolated from fermented Theobroma cacao L. fruit using in vitro techniques. Front. Microbiol. 2023, 14, 1274636. [Google Scholar] [CrossRef]
- Naito, E.; Yoshida, Y.; Makino, K.; Kounoshi, Y.; Kunihiro, S.; Takahashi, R.; Matsuzaki, T.; Miyazaki, K.; Ishikawa, F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J. Appl. Microbiol. 2011, 110, 650–657. [Google Scholar] [CrossRef]
- Sun, L.; Ni, C.; Zhao, J.; Wang, G.; Chen, W. Probiotics, bioactive compounds and dietary patterns for the effective management of hyperuricemia: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 2016–2031. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Li, X.; Xiao, Y.; Huang, Y.; Zhang, Y.; Li, J.; Li, M.; Ren, Z. Lactiplantibacillus pentosus P2020 protects the hyperuricemia and renal inflammation in mice. Front. Nutr. 2023, 10, 1094483. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Hou, Y.-H.; Wang, C.-S.; Lin, S.-W.; Jhou, B.-Y.; Chen, C.-C.; Chen, Y.-L. Antiobesity and Uric Acid-Lowering Effect of Lactobacillus plantarum GKM3 in High-Fat-Diet-Induced Obese Rats. J. Am. Coll. Nutr. 2019, 38, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, S.; Liu, S.; Prasanthi, H.A.C.; Li, Y.; Cao, J.; Zhong, F.; Guo, L.; Lu, F.; Luo, X. Postbiotic of Pediococcus acidilactici GQ01, a Novel Probiotic Strain Isolated from Natural Fermented Wolfberry, Attenuates Hyperuricaemia in Mice through Modulating Uric Acid Metabolism and Gut Microbiota. Foods 2024, 13, 923. [Google Scholar] [CrossRef]
- Stachelska, M.A.; Karpiński, P.; Kruszewski, B. Health-Promoting and Functional Properties of Fermented Milk Beverages with Probiotic Bacteria in the Prevention of Civilization Diseases. Nutrients 2024, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Li, Z.-X.; Zhuo, J.-L.; Yang, N.; Gao, M.-B.; Qu, Z.-H.; Han, T. Effect of Lycium barbarum polysaccharide on osteoblast proliferation and differentiation in postmenopausal osteoporosis. Int. J. Biol. Macromol. 2024, 271, 132415. [Google Scholar] [CrossRef]
- Feng, X.; Hu, H.; Zhong, F.; Hou, Y.; Li, X.; Qin, Q.; Yang, Y.; Luo, X. Lactiplantibacillus plantarum TCCC11824 exerts hypolipidemic and anti-obesity effects through regulation of NF-κB-HMGCR pathway and gut microbiota in mice and clinical patients. Nutrition 2025, 130, 112598. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, C.; Xu, Q.; Li, Z.; Song, Y.; Zhou, S.; Zhang, T.; Luo, X. Bacterial Diversity and Lactic Acid Bacteria with High Alcohol Tolerance in the Fermented Grains of Soy Sauce Aroma Type Baijiu in North China. Foods 2022, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Do, S.; Kim, Y.; Yim, J.; Lee, K.-G. Analysis of volatile compounds, betaine, and antioxidant effect in goji berry (Lycium barbarum L.) powder extracted by various drying methods and extraction solvents. Curr. Res. Food Sci. 2024, 9, 100798. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-L.; Ma, J.-M.; Fan, Y.-N.; Zhang, Y.-N.; Ge, R.; Tao, X.-J.; Zhang, M.-W.; Gao, Q.-H.; Yang, J.-J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef]
- Zeinali, S.; Natalia Wieczorek, M.; Pawliszyn, J. Free versus droplet-bound aroma compounds in sparkling beverages. Food Chem. 2022, 378, 131985. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Fan, J.; Xie, W.; Ke, H.; Zhang, J.; Wang, J.; Wang, H.; Guo, N.; Bai, Y.; Lei, X. Structural Basis for Inhibition of Urate Reabsorption in URAT1. JACS Au 2025, 5, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef]
- Sato, Y.; Feig, D.I.; Stack, A.G.; Kang, D.-H.; Lanaspa, M.A.; Ejaz, A.A.; Sánchez-Lozada, L.G.; Kuwabara, M.; Borghi, C.; Johnson, R.J. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 2019, 15, 767–775. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Duan, S.; Yuan, X.; Liang, J.; Hou, S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019, 118, 109195. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Author Correction: Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 642. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e1010. [Google Scholar] [CrossRef] [PubMed]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Luo, J.; Jiang, Y.; Li, L.; Chen, Y.; Zhang, L.; Huang, Q.; Cao, Y.; Zhou, P.; et al. Apigenin ameliorates hyperuricemic nephropathy by inhibiting URAT1 and GLUT9 and relieving renal fibrosis via the Wnt/β-catenin pathway. Phytomedicine 2021, 87, 153585. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Miyata, H.; Matsuo, H.; Kassai, H.; Nakao, K.; Nakatochi, M.; Kawamura, Y.; Shimizu, S.; Shinomiya, N.; et al. Identification of GLUT12/SLC2A12 as a urate transporter that regulates the blood urate level in hyperuricemia model mice. Proc. Natl. Acad. Sci. USA 2020, 117, 18175–18177. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2021, 62, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, X.; Yang, X.; Yang, Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren. Fail. 2022, 44, 615–624. [Google Scholar] [CrossRef]
- Fan, X.; Liu, M.; Shi, Z.; Zhang, T.; Du, L.; Wu, Z.; Zeng, X.; Wu, X.; Pan, D. Binary probiotic fermentation promotes signal (cyclic AMP) exchange to increases the number of viable probiotics, anthocyanins and polyphenol content, and the odor scores of wolfberry fermented beverages. Food Chem. 2024, 448, 139085. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Güler, Z.; Gürsoy-Balcı, A.C. Evaluation of volatile compounds and free fatty acids in set types yogurts made of ewes’, goats’ milk and their mixture using two different commercial starter cultures during refrigerated storage. Food Chem. 2011, 127, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, L. Gut microbiota-based translational biomarkers to prevent metabolic syndrome via nutritional modulation. FEMS Microbiol. Ecol. 2014, 87, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Etchart, M.G.; Anderson, L.L.; Ametovski, A.; Jones, P.M.; George, A.M.; Banister, S.D.; Arnold, J.C. In vitro evaluation of the interaction of the cannabis constituents cannabichromene and cannabichromenic acid with ABCG2 and ABCB1 transporters. Eur. J. Pharmacol. 2022, 922, 174836. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, L.; Jin, Y.; Pei, X.; Sun, W.; Wang, M. A Potential Prophylactic Probiotic for Inflammatory Bowel Disease: The Overall Investigation of Clostridium tyrobutyricum ATCC25755 Attenuates LPS-Induced Inflammation via Regulating Intestinal Immune Cells. Mol. Nutr. Food Res. 2021, 65, 2001213. [Google Scholar] [CrossRef]
- Ni, C.; Li, X.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activityviaa short-chain fatty acid-dependent mechanism. Food Funct. 2021, 12, 7054–7067. [Google Scholar] [CrossRef]
- Furci, F.; Cicero, N.; Allegra, A.; Gangemi, S. Microbiota, Diet and Acute Leukaemia: Tips and Tricks on Their Possible Connections. Nutrients 2023, 15, 4253. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Tian, J.; Zheng, F.; Liao, H.; Zhao, Z.; Chen, Y.; Pang, J.; Wu, T. Insoluble Fiber in Barley Leaf Attenuates Hyperuricemic Nephropathy by Modulating Gut Microbiota and Short-Chain Fatty Acids. Foods 2022, 11, 3482. [Google Scholar] [CrossRef]


| Evaluation Indicators and Scores | Evaluation Criteria | Scores |
|---|---|---|
| Viable bacteria count (50) | 1010 CFU/mL < Viable bacteria count ≤ 5 × 1010 CFU/mL | 40–50 |
| 5 × 109 CFU/mL < Viable bacteria count ≤ 1010 CFU/mL | 30–40 | |
| 109 CFU/mL < Viable bacteria count ≤ 5 × 109 CFU/mL | 20–30 | |
| 5 × 108 CFU/mL < Viable bacteria count ≤ 109 CFU/mL | 10–20 | |
| 108 CFU/mL < Viable bacteria count ≤ 5 × 108 CFU/mL | 0–10 | |
| Oxidation resistance (50) | 45% < clearance rate ≤ 50% | 40–50 |
| 40% < clearance rate ≤ 45% | 30–40 | |
| 35% < clearance rate ≤ 40% | 20–30 | |
| 30% < clearance rate ≤ 35% | 10–20 | |
| 25% < clearance rate ≤ 30% | 0–10 |
| Level | Factor | ||
|---|---|---|---|
| A Fermentation Time | B Vaccination Load | C Material–Liquid Ratio | |
| −1 | 6 h | 3% | 6 |
| 0 | 8 h | 4% | 8 |
| 1 | 10 h | 5% | 10 |
| Gene | Sequence (5′→3′) |
|---|---|
| GAPDH | ATGGTGAAGGTCGGTGTGAACGG |
| TGGAACATGTAGACCATGTAGTTGAGG | |
| URAT1 | TCCTGAACTCCTGGACCGAGTG |
| AGTTCTCCAGCATGTTCTGA | |
| GLUT9 | GTGAAAAGAACTCCGCAGAAACCA |
| AGGAAGGAGGACCCGAAGGCTC |
| Serial Number | Factor | |||
|---|---|---|---|---|
| A Fermentation Time (h) | B Inoculation Amount (%) | C Material–Liquid Ratio | Score | |
| 1 | 6 | 3 | 8 | 70.8 |
| 2 | 6 | 5 | 8 | 69 |
| 3 | 8 | 3 | 6 | 71 |
| 4 | 10 | 5 | 8 | 67.2 |
| 5 | 6 | 4 | 10 | 74.6 |
| 6 | 8 | 4 | 8 | 80 |
| 7 | 8 | 5 | 6 | 70.4 |
| 8 | 6 | 4 | 6 | 78.6 |
| 9 | 8 | 3 | 10 | 74.6 |
| 10 | 10 | 3 | 8 | 67 |
| 11 | 10 | 4 | 10 | 68 |
| 12 | 8 | 4 | 8 | 82.2 |
| 13 | 8 | 4 | 8 | 82 |
| 14 | 8 | 4 | 8 | 85 |
| 15 | 10 | 4 | 8 | 66 |
| 16 | 8 | 4 | 8 | 81 |
| 17 | 8 | 5 | 10 | 68.2 |
| Source of Variance | SS | DoF | Variance | F | p | Significance |
|---|---|---|---|---|---|---|
| Model | 602.62 | 9 | 66.96 | 11.10 | 0.0022 | ** (p < 0.01) |
| A | 76.88 | 1 | 76.88 | 12.74 | 0.0091 | ** (p < 0.01) |
| B | 9.24 | 1 | 9.24 | 1.53 | 0.2557 | |
| C | 0.045 | 1 | 0.045 | 7.457 × 10−3 | 0.9336 | |
| AB | 1 | 1 | 1 | 0.17 | 0.6961 | |
| AC | 9 | 1 | 9 | 1.49 | 0.2615 | |
| BC | 8.41 | 1 | 8.41 | 1.39 | 0.2763 | |
| A2 | 172.19 | 1 | 172.19 | 28.53 | 0.0011 | |
| B2 | 214.95 | 1 | 214.95 | 35.62 | 0.0006 | |
| C2 | 62.25 | 1 | 62.25 | 10.32 | 0.0148 | |
| Residuals | 42.24 | 7 | 6.03 | |||
| Ack of fit | 28.21 | 3 | 9.40 | 2.68 | 0.1823 | |
| Pure error | 14.03 | 4 | 3.51 | |||
| Total deviation | 644.86 | 16 |
| Group | Weight (g) | Liver Index (%) | Kidney Index (%) |
|---|---|---|---|
| NC | 35.75 ± 2.92 | 3.58 ± 0.57 | 1.34 ± 0.18 |
| M | 36.05 ± 2.58 | 4.28 ± 0.39 ** | 1.24 ± 0.15 |
| AP | 36.28 ± 2.08 | 4.80 ± 0.64 | 1.37 ± 0.21 |
| GJ | 36.7 ± 1.78 | 4.24 ± 0.21 | 1.10 ± 0.07 |
| TR | 36.6 ± 2.42 | 4.18 ± 0.40 | 1.24 ± 0.11 |
| Graph | Simpson | Shannon | Chao | Ace |
|---|---|---|---|---|
| NC | 0.04 ± 0.01 | 4.18 ± 0.13 | 382.88 ± 19.54 | 384.21 ± 15.29 |
| M | 0.06 ± 0.03 | 3.83 ± 0.36 | 383.42 ± 13.08 | 385.34 ± 9.02 |
| AP | 0.03 ± 0.01 | 4.26 ± 0.11 | 414.63 ± 7.14 | 406.87 ± 8.60 |
| GJ | 0.06 ± 0.01 | 3.81 ± 0.13 | 385.92 ± 4.95 | 380.82 ± 1.39 |
| TR | 0.05 ± 0.01 | 4.07 ± 0.10 | 411.3 ± 45.10 | 404.41 ± 46.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Li, Y.; Liu, S.; Jia, X.; He, H.; Zhong, F.; Lu, F.; Luo, X. Triple-Probiotic-Fermented Goji (Lycium barbarum L.) Ameliorates Metabolic Disorders Associated with Hyperuricemia in Mice. Microorganisms 2025, 13, 1367. https://doi.org/10.3390/microorganisms13061367
Ren L, Li Y, Liu S, Jia X, He H, Zhong F, Lu F, Luo X. Triple-Probiotic-Fermented Goji (Lycium barbarum L.) Ameliorates Metabolic Disorders Associated with Hyperuricemia in Mice. Microorganisms. 2025; 13(6):1367. https://doi.org/10.3390/microorganisms13061367
Chicago/Turabian StyleRen, Lu, Yuechan Li, Shiting Liu, Xiaoke Jia, Hongpeng He, Feiliang Zhong, Fuping Lu, and Xuegang Luo. 2025. "Triple-Probiotic-Fermented Goji (Lycium barbarum L.) Ameliorates Metabolic Disorders Associated with Hyperuricemia in Mice" Microorganisms 13, no. 6: 1367. https://doi.org/10.3390/microorganisms13061367
APA StyleRen, L., Li, Y., Liu, S., Jia, X., He, H., Zhong, F., Lu, F., & Luo, X. (2025). Triple-Probiotic-Fermented Goji (Lycium barbarum L.) Ameliorates Metabolic Disorders Associated with Hyperuricemia in Mice. Microorganisms, 13(6), 1367. https://doi.org/10.3390/microorganisms13061367






