Abstract
Fermenting fruit and vegetable juices with probiotic bacteria is becoming a popular way to create functional drinks, offering an alternative to traditional dairy-based probiotic products. These plant-based juices are naturally rich in nutrients that help support the growth and activity of various probiotic strains. They also meet the rising demand for lactose-free, vegan, and clean-label options. This review looks at the key microbiological, nutritional, and sensory aspects of probiotic fermentation in juice. Common probiotic groups like Lactobacillus, Bifidobacterium, Lactococcus, Bacillus, and Streptococcus show different abilities to adapt to juice environments, affecting properties such as antioxidant levels, shelf life, and taste. The review also explores how factors like pH, sugar levels, heating, and storage can influence fermentation results. New non-thermal processing methods that help maintain probiotic survival are also discussed. Since fermented juices can sometimes develop off-flavors, this paper looks at ways to improve their taste and overall consumer appeal. Finally, future directions are suggested, including personalized nutrition, synbiotic products, and advanced encapsulation technologies. Overall, probiotic fermentation of fruit and vegetable juices shows strong potential for developing a new generation of healthy and appealing functional foods.
1. Introduction
The human gut microbiota plays a fundamental role in host physiology and metabolism. Meanwhile, an increasing number of pieces of clinical evidence support the health benefits of consuming probiotic-enriched products. These benefits extend beyond improving gastrointestinal function and alleviating diarrhea or constipation; they include reducing the risk of allergies and skin conditions, managing immune-related disorders, lowering serum cholesterol levels, and even contributing to the prevention of colorectal cancer [1,2,3,4].
The past few decades have witnessed a marked rise in consumer demand for probiotic-enriched food products [5,6,7,8]. This surge has stimulated both scientific inquiry and innovation in the food industry, driving the development of functional food components and new formulations that incorporate live microbial cultures with demonstrated health benefits [9].
The global market for probiotic products is expanding rapidly and is projected to grow from USD 69.8 billion in 2024 to USD 102.1 billion by the end of 2029 [10].
The health-promoting properties of probiotics depend primarily on their concentration in food products and their ability to survive the harsh conditions of the gastrointestinal tract. Despite the growing commercial interest in probiotic strains, many currently available probiotics are sensitive to environmental stressors and tend to lose viability during processing and storage. It has been established that probiotic viability should remain at a minimum of 107 CFU/mL throughout a product’s shelf life, regardless of the strain used [11,12,13,14]. Consequently, selecting an appropriate food matrix for probiotic delivery is critical.
Among the most straightforward and consumer-preferred delivery systems for probiotics are beverages, which provide an effective medium for the fermentation of probiotic bacteria and the transport of both nutritional and bioactive compounds to the host [15]. Depending on the raw materials used, probiotic beverages can be classified into dairy-based, fruit- and vegetable-based, legume-based, cereal-based, and fermented-tea- or coffee-based categories.
At the start of the 21st century, approximately 74% of probiotic foods on the market were dairy-based [16]. However, rising prevalence of lactose intolerance, the adoption of vegan diets, and increased consumer interest in cholesterol-lowering diets [7,17] have catalyzed a global search for plant-based alternatives to traditional dairy matrices.
In the last decade, fruit and vegetable juices—either individually or in blends—have gained traction as promising carriers for probiotics under gastrointestinal stress conditions. These juices provide essential nutrients that support probiotic viability [9,18,19,20,21] and are broadly acceptable across all demographic groups [21,22]. Fermented fruit and vegetable juices are rich in vitamins, minerals, and antioxidants, and the fermentation process can enhance the bioavailability of these compounds. Juices fermented using probiotics are therefore emerging as increasingly popular functional beverages [23].
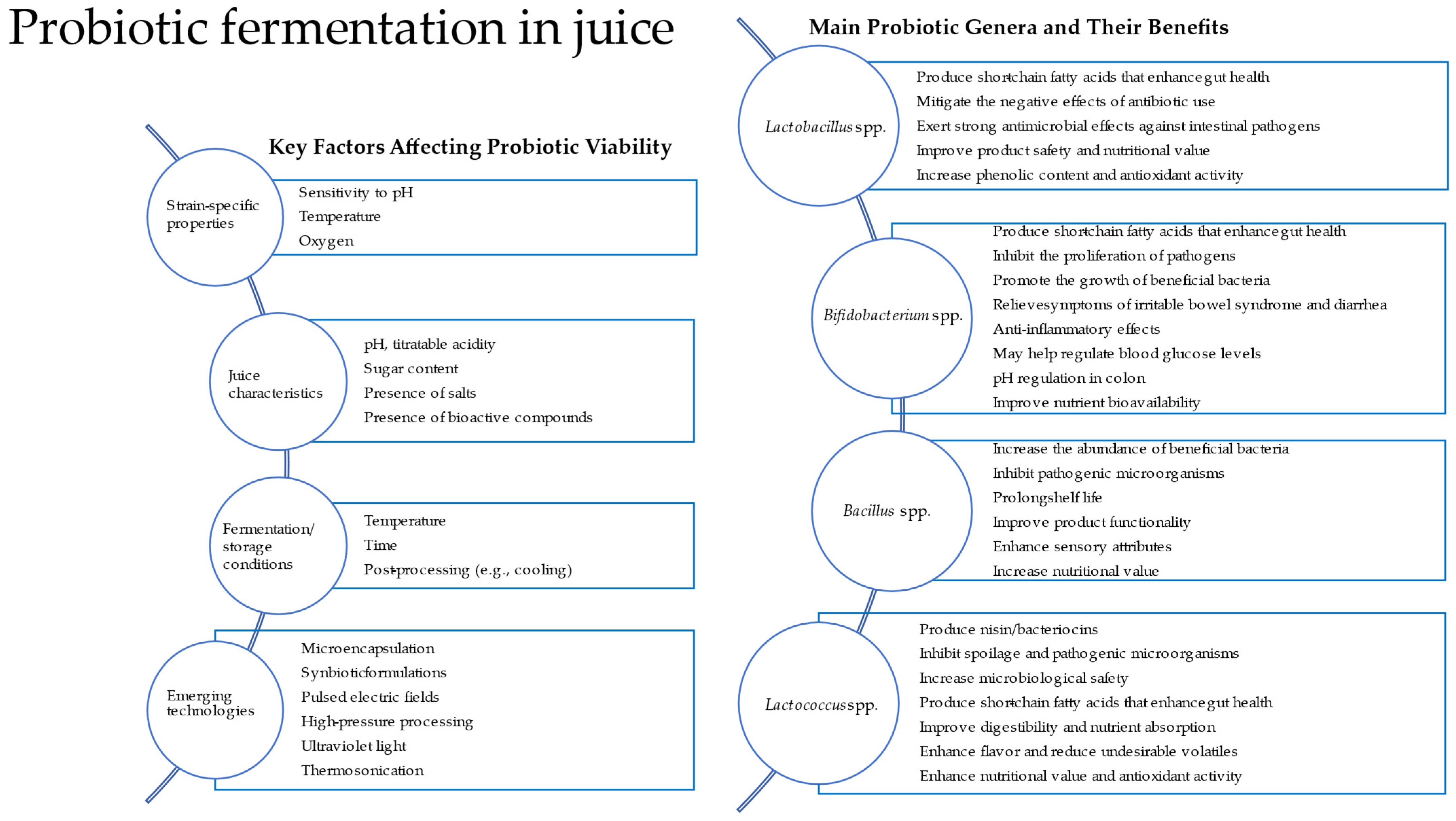
Traditionally, probiotics such as Lactobacillus and Bifidobacterium have been associated with fermented dairy products. However, recent research has demonstrated their viability and metabolic activity in plant-based substrates. This review summarizes the probiotic strains commonly used in the fermentation of fruit and vegetable juices, the criteria for selecting such strains, the suitability of juices as fermentation substrates, and the influence of fermentation on product characteristics and bacterial viability. A conceptual diagram (Figure 1) shows key factors affecting probiotic viability, the main probiotic genera used, and their benefits, and emerging technologies can be applied in probiotic juice fermentation.
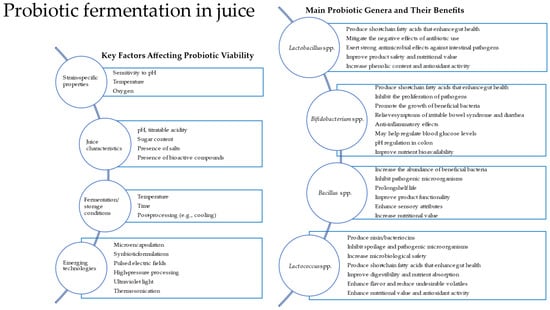
Figure 1.
Diagram showing key factors affecting probiotic viability and the main probiotic genera used and their benefits. Emerging technologies can be applied in probiotic juice fermentation.
2. Factors Influencing Probiotic Fermentation in Fruit and Vegetable Juices
Fruit and vegetable juices serve as nutrient-rich substrates that support the growth and metabolic activity of probiotic microorganisms. These juices are natural sources of fermentable sugars, essential vitamins, minerals, dietary fibers, and antioxidants, which collectively create a favorable environment for probiotic colonization and activity [24,25,26,27]. Furthermore, bioactive compounds such as polyphenols and flavonoids found in these matrices offer additional health benefits by mitigating chronic diseases [28]. Some juices also naturally contain prebiotics, which further enhance the viability and longevity of probiotics [29,30,31]. Notably, many fruit and berry juices exhibit antimicrobial activity against pathogenic microorganisms [32,33,34,35].
Despite their favorable composition, several interrelated factors critically influence the viability and functionality of probiotics during fermentation and subsequent storage.
- Strain-Specific Characteristics: Different probiotic strains confer distinct metabolic profiles and sensory outcomes in fermented beverages. Therefore, strain selection is a fundamental determinant of fermentation success.
- pH and Acidity: The initial and dynamic pH values of juices play a pivotal role. Many fruit juices are inherently acidic, and fermentation further lowers pH levels due to organic acid production, potentially compromising probiotic survival. Titratable acidity, water activity, and the presence of salts, sugars, and other compounds also modulate the microbial environment [36].
- Processing and Fermentation Conditions: The survival of probiotics is also affected by juice pre-treatment and fermentation parameters such as temperature, duration, and cooling rate after fermentation [37].
These factors must be carefully optimized to ensure effective fermentation and stable probiotic delivery through juice-based functional beverages.
3. Key Probiotic Strains Used in Juice Fermentation
The choice of probiotic strains for juice fermentation significantly influences the physicochemical and functional characteristics of the final product. Selected strains must demonstrate resilience under processing, storage, and gastrointestinal conditions, while also contributing positively to the organoleptic profile of the product [38,39,40,41].
In plant-based beverages, strain selection is guided by the ability to ferment complex carbohydrates and produce desirable sensory attributes [42]. Moreover, strains must not generate undesirable metabolites, off-flavors, or biogenic amines during fermentation [43,44,45,46,47].
The most frequently utilized probiotic microorganisms include species from the genera Lactobacillus, Bifidobacterium, Enterococcus, Lactococcus, Streptococcus, and heat-resistant spore-formers like Bacillus. Some strains from Escherichia and Saccharomyces are also explored for specific applications. Most widely used are heterofermentative lactic acid bacteria (LAB), particularly Lactobacillus and Enterococcus, along with bifidobacteria [48].
3.1. Application of Lactobacillus Strains in Juice Fermentation
Species within the Lactobacillus genus are Gram-positive, non-motile, catalase-negative, rod-shaped bacteria that are either anaerobic or facultatively anaerobic. They thrive in environments enriched with 5–10% CO2 and require specific nutritional components such as amino acids, peptides, fatty acids, vitamins, carbohydrates, and nucleotide derivatives. Their optimal growth temperature ranges between 30 and 40 °C, though they can proliferate in a broader range from 5 °C to 53 °C. The ideal pH for their growth lies between 5.5 and 5.8 [49].
Among the most frequently used Lactobacillus strains in juice fermentation are L. plantarum, L. acidophilus, and L. casei, all of which are valued for their robust fermentation performance and potential health benefits.
L. acidophilus is one of the most important strains within the Lactobacillus genus. It primarily colonizes the small intestine, where it helps maintain the integrity of the intestinal barrier, ensuring efficient nutrient absorption and proper digestive function. This strain is known for its ability to mitigate the negative effects of antibiotic use, resist bile acids, and exert strong antimicrobial effects against intestinal pathogens, including coliform strains of E. coli [50].
L. acidophilus remains viable at pH 2.0 for up to 2 h of incubation and tolerates bile salt concentrations of 0.3%. It can be produced on an industrial scale and remains viable and stable both in food products and within the intestinal microbiota. L. acidophilus meets all essential FAO/WHO criteria for classification as a probiotic bacterium [2,51].
Studies have confirmed that Lactobacillus strains remain viable in fermented fruit juices. For example, L. paracasei demonstrated promising viability during cold fermentation of chokeberry juice [52]. Other strains, including L. rhamnosus, L. acidophilus, and L. fermentum, have been shown to significantly increase the probiotic count in fermented juices and maintain viable counts exceeding 10⁷ CFU/mL [53,54]. L. plantarum-fermented pineapple juice also exhibited high probiotic viability and enhanced antioxidant properties, confirming its suitability as a non-dairy probiotic beverage [55].
Apple juice has been successfully used as a substrate for probiotic fermentation. L. plantarum was able to reach the viable cell concentrations required for health benefits [56]. Additionally, apple juice fermented with L. acidophilus and L. plantarum showed potential in reducing contaminants such as mycotoxins. These probiotic strains were shown to remove patulin, a common contaminant in apple juice, thereby improving product safety and nutritional value [57]. This added value further strengthens the role of probiotics, not only in fermentation, but also in ensuring food safety.
The fermentation of pear juice with various Lactobacillus strains increased antioxidant activity and modified phenolic compound profiles, highlighting the enhancement of health-promoting properties through fermentation [53,55,58].
Similarly, the fermentation of strawberry juice with Lactobacillus strains improved radical scavenging capacity, associated with increased phenolic content during fermentation [59,60]. These results underscore the beneficial impact of fermentation on juice quality and health attributes.
Lactobacillus strains are also known to produce short-chain fatty acids (SCFAs) during fermentation, compounds that support gut health. SCFA production helps maintain microbiome balance and reduce intestinal inflammation, reinforcing the role of fermented fruit and vegetable juices as functional foods [61,62]. Additional studies have shown that vegetable juices such as cabbage or spinach fermented with LAB may improve calcium bioavailability due to enzymatic activity [63].
3.2. Bacillus Genus and Its Role in Fermented Juices
The genus Bacillus includes Gram-positive, rod-shaped, spore-forming bacteria that are typically aerobic or facultatively anaerobic. While commonly associated with soil microbiota, certain non-pathogenic Bacillus species have been successfully applied as probiotics [64]. These strains are excellent candidates for use in probiotic food products, particularly due to their ability to maintain viability at elevated temperatures [65,66]. Optimal growth for Bacillus spp. occurs between 35 and 50 °C, with a preferred pH range of 5.5–6.5.
Bacillus coagulans is particularly noteworthy due to its capacity to form heat-resistant spores, making it suitable for application in high-temperature environments [64,67,68,69]. Another advantage of B. coagulans is its high biomass yield in bioreactor cultures, surpassing that of other probiotic strains such as L. rhamnosus, L. salivarius, L. plantarum, and L. lactis [70].
B. coagulans has been recognized as safe for human consumption by the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). It is included in both the GRAS (Generally Recognized as Safe) and QPS (Qualified Presumption of Safety) lists and is currently used in numerous commercial probiotic formulations [71].
Its ability to remain viable in acidic conditions reinforces its suitability for application in probiotic juice fermentation. For instance, Ebrahimi et al. (2021) found that B. coagulans-enriched fruit juices increased the levels of immunoglobulins and lymphocytes in athletes [72]. Similarly, Almada-Érix et al. (2021) demonstrated that orange juice supplemented with B. coagulans altered the gut microbiota in Wistar rats by increasing the abundance of beneficial bacteria such as Lactobacillus and Bacillus spp. [73]. These results emphasize the potential of Bacillus strains to modulate gut flora in a health-promoting direction.
The fermentation of fruit and vegetable juices with Bacillus probiotics not only improves product functionality but also enhances sensory attributes and inhibits pathogenic microorganisms. Flavor and aroma profiles of fermented juices are often altered positively due to the metabolic activity of Bacillus spp. Sireswar et al. (2018) demonstrated that the synergistic effect between Bacillus strains and phenolic compounds in fruit juices significantly suppressed foodborne pathogens while enhancing overall flavor acceptability [74].
Further support for these findings comes from Maia et al. (2023), who showed that incorporating Bacillus strains in juice fermentation altered both volatile and non-volatile compound profiles [60]. This modification led to improved sensory perception and consumer appeal.
Recent literature by Saud et al. (2024) underscores that probiotic fermentation with Bacillus not only prolongs shelf life but also increases nutritional value by enhancing levels of beneficial compounds while inhibiting spoilage organisms [75]. These benefits position Bacillus-fermented juices as promising functional beverages with extended stability, enhanced safety, and superior consumer appeal.
3.3. Bifidobacterium Genus and Its Application in Juice Fermentation
The Bifidobacterium genus currently comprises over 30 recognized bacterial species, many of which are considered essential components of the human gut microbiota [76]. These bacteria produce lactic and acetic acids, contributing up to 70% of the energy required by the colonic epithelium [77]. This activity enhances the intestinal barrier function and supports the maintenance of a balanced gastrointestinal environment.
The production of lactic acid by Bifidobacterium strains helps maintain an optimal colonic pH, inhibiting the proliferation of pathogenic microorganisms while promoting the growth of beneficial bacteria [78]. Important species with well-documented probiotic properties include B. lactis, B. bifidum, B. longum, B. infantis, and B. breve. However, the abundance of bifidobacteria tends to decrease with age, stress, and poor dietary habits [79].
Among these, B. longum, B. breve, and B. infantis are frequently highlighted not only for their beneficial effects on health but also for their potential in fermentation processes. B. longum is capable of metabolizing a broad spectrum of carbohydrates, producing lactic acid and short-chain fatty acids (SCFAs) that contribute to gut health [80]. Its ability to ferment polysaccharides improves the nutritional quality of fermented juices by breaking down complex carbohydrates into simpler, more bioavailable sugars for both the host and the microbial community [81].
B. breve has demonstrated probiotic properties that enhance gastrointestinal health and systemic wellness. It has been associated with improved immune responses in the gut and symptom alleviation in conditions such as irritable bowel syndrome (IBS) and diarrhea [82]. SCFA production by B. breve is particularly important, as these metabolites contribute to the integrity of the intestinal barrier and have anti-inflammatory effects [83].
The selection of appropriate Bifidobacterium strains for fruit and vegetable juice fermentation significantly affects the sensory and functional properties of the final product. In a recent study by Güler et al. (2024), new Bifidobacterium strains isolated from infant feces were evaluated for their probiotic potential in juice matrices [84]. The selected strains exhibited favorable characteristics that could enhance the health-promoting value of fermented fruit and vegetable juices.
Juices enriched with oligosaccharides—naturally present in many fruits—offer a conducive environment for Bifidobacterium growth. Fermentation under these conditions not only improves probiotic activity but also results in the formation of bioactive compounds, increasing the nutritional value of the final product [85]. Moreover, the ability of Bifidobacterium strains to outcompete harmful intestinal pathogens contributes to both the safety and shelf life of fermented juices [82,83].
There is also evidence that the postprandial consumption of Bifidobacterium-fermented juices may help regulate blood glucose levels, suggesting potential applications in functional foods for diabetes management [86].
Genomic analyses of Bifidobacterium strains have revealed considerable diversity in metabolic profiles, which may be exploited to optimize fermentation processes for specific juice types [81,87]. These metabolic adaptations are crucial for defining the final flavor, aroma, and health benefits of the fermented product.
3.4. Lactococcus Genus, Health Benefits, and Functional Potential
Among the various probiotic strains applied in juice fermentation, Lactococcus lactis stands out for its widespread use and recognized safety. It has been granted GRAS (Generally Recognized as Safe) status and has a long history of safe use in the production of dairy products such as cheese and fermented milk [88,89].
L. lactis primarily metabolizes lactose into lactic acid, thereby contributing to pH reduction and inhibition of spoilage and pathogenic microorganisms. This acidification process not only preserves the structural and microbiological integrity of fermented products but also generates a range of flavor-enhancing metabolites [90,91]. Additionally, L. lactis is known to produce antimicrobial peptides, such as nisin and bacteriocins, which further enhance food safety and extend shelf life [90,92].
Several studies have confirmed the antimicrobial efficacy of L. lactis in fermented juices. For instance, Özdogan et al. (2012) and Boumaiza et al. (2018) demonstrated that L. lactis effectively inhibits foodborne pathogens, increasing the microbiological safety of probiotic beverages [93,94].
Beyond its antimicrobial properties, L. lactis contributes positively to gut health. It produces short-chain fatty acids (SCFAs), which are essential for maintaining intestinal barrier function, modulating immune responses, and reducing inflammation [95,96]. Although L. lactis is traditionally associated with dairy fermentation, its performance in fruit and vegetable juice fermentation has gained increasing attention. For example, Siroli et al. (2019) reported that the fermentation of carrot juice with L. lactis not only reduced undesirable volatile compounds but also enhanced the nutritional value of the final product [92]. The study showed that L. lactis fermentation reduced the levels of potentially harmful substances and improved the overall safety and acceptability of the juice.
These findings are consistent with other studies suggesting that lactic acid fermentation can neutralize antinutritional factors present in raw plant-based juices, thus improving their bioavailability and health benefits [97]. L. lactis also contributes to improved digestibility and nutrient absorption through the breakdown of complex carbohydrates and the release of bioactive peptides, thus providing a functional advantage to consumers looking for health-oriented choices [98].
Fermentation trials using L. lactis have also been extended to non-traditional plant-based substrates. Aloe vera juice, for instance, demonstrated enhanced antioxidant activity when fermented with L. lactis owing to the improved bioavailability of phenolic compounds [99]. This suggests that L. lactis not only ferments juices but also increases their health benefits. Similarly, a coconut-based beverage fermented with L. lactis showed probiotic functionality and enhanced nutritional characteristics [100].
These examples highlight the adaptability of L. lactis to a range of juice matrices, underscoring its potential as a versatile probiotic in the development of innovative plant-based functional beverages.
3.5. Streptococcus thermophilus: Its Comparative Performance, Limitations, and Recommendations
Streptococcus thermophilus is a homofermentative lactic acid bacterium best known for its central role in the production of yogurt and cheese. Most of the existing literature regarding this species pertains to its application in dairy fermentations [101]. However, due to its ability to produce exopolysaccharides (EPS) and its potential health benefits, S. thermophilus has attracted interest as a candidate for the development of functional non-dairy beverages [102,103].
Recent research indicates that S. thermophilus can be used in plant-based fermentations. It has been shown to dominate the microbial population throughout the fermentation of vegetable juices, thanks to its strong acidification ability and competitive fitness in certain substrate conditions [104,105,106]. This is because heterofermentative lactic acid cultures dominate only in the early stages of fermentation and cannot maintain their dominance throughout the process, so their dominance is quickly replaced by homofermentative ones [105,107]. However, if only homofermentative bacteria dominate the starter, the sensory properties of the product may deteriorate, an overly sour off-flavor may appear, and the texture of the product may change. It has been found that the fresh and pleasant taste and high sensory quality of kimchi are determined by the properties of heterofermentative lactic acid bacteria, and they are considered better candidates for starters than homofermentative ones [108,109]. This was confirmed by a comparative study by Cai et al. [110], which examined the fermentation of jujube juice using S. thermophilus and L. plantarum. The findings revealed that S. thermophilus-fermented juice had significantly poorer sensory properties compared with that fermented with L. plantarum [110].
Given these observations, S. thermophilus may not be the optimal choice for the standalone fermentation of fruit and vegetable juices, especially in formulations where sensory quality is a priority. However, it may still serve as a useful component in multi-strain probiotic mixtures or in substrates where its fast acidification and EPS production offer technological advantages, such as viscosity improvement or texture stabilization.
To achieve balanced flavor and functional outcomes, combinations of S. thermophilus with heterofermentative bacteria or yeasts may provide better results, particularly for consumer-acceptable probiotic beverages.
3.6. Use of Multi-Strain Probiotic Mixtures
Scientific evidence suggests that combining multiple probiotic strains may offer enhanced health benefits compared with single-strain applications. This is particularly relevant for combating gastrointestinal pathogens, where synergistic interactions between strains may lead to broader antimicrobial activity. For example, multi-strain probiotic formulations have been found to be more effective against pathogens such as Escherichia coli, Campylobacter jejuni, and Shigella [111,112].
Accordingly, mixed cultures are sometimes employed in juice fermentation to achieve improved functionality, product safety, and probiotic efficacy. Commonly used probiotic combinations include strains such as L. acidophilus, L. rhamnosus GG, Saccharomyces boulardii, B. bifidum, and B. coagulans [113].
3.6.1. Considerations for Strain Compatibility and Technological Viability
When designing multi-strain formulations for juice fermentation, several factors, such as strain compatibility, product characteristics, temperature regime, bacteriophage risk, and raw material variability, must be taken into account. Microorganisms must not inhibit one another’s growth or function. The biochemical composition of the juice may affect probiotic stability and metabolic activity. Fermentation and storage temperatures must support the viability of all included strains. Mixed cultures may be vulnerable to phage attack, which can disrupt fermentation. Probiotic resistance to variations in juice composition is essential for consistent product quality. Mesophilic strains are more suitable for fermentation processes at 20–30 °C, while thermophilic strains are preferred for processes conducted at 40–45 °C [114].
3.6.2. Safety Considerations
Although most probiotics—especially those from the Lactobacillus and Bifidobacterium genera—are considered safe due to their long history of use, strain-specific safety assessments remain essential. Some strains may pose risks if not properly evaluated, particularly in immunocompromised individuals [115,116].
The selection of multi-strain consortia for probiotic juice products must therefore be guided by rigorous safety, efficacy, and stability evaluations to ensure consumer protection and product performance.
4. Environmental Parameters: pH and Related Factors for Viability
The pH level of fruit and vegetable juices plays a critical role in determining the survival and stability of probiotic bacteria during fermentation and subsequent storage. These juices are naturally rich in organic acids, resulting in an inherently low pH that may create unfavorable conditions for microbial viability. Acidic environments and the associated antimicrobial effects of organic acids are considered key stressors limiting probiotic persistence in juice-based systems.
Probiotic bacteria generally prefer a pH range close to neutral. Numerous studies have demonstrated that pH changes during fermentation have a direct impact on probiotic viability [24,36,117,118,119,120]. Furthermore, although sugars in fruit juices serve as a useful energy source for probiotics, their metabolism during fermentation leads to the production of additional organic acids, thereby further decreasing the pH [55,121].
Viability losses may also occur during later stages of storage due to continued acidification, autolysis, and enzymatic degradation of dead cells [122]. This highlights the importance of not only the initial pH but also its evolution over time as a determinant of probiotic stability. Interestingly, some strains—particularly within the Lactobacillus genus—are better adapted to acidic conditions. These bacteria have demonstrated the ability to survive in juices with pH values ranging from 3.7 to 4.3, making them suitable candidates for acidic substrates [12].
4.1. Strategies to Enhance Probiotic Viability in Acidic Conditions
Several approaches have been explored to improve the survival of probiotics in low-pH environments.
4.2. Microencapsulation
Encapsulating probiotic cells in protective matrices has proven to be one of the most effective methods for shielding them from acidic stress. Studies have shown that encapsulated probiotics exhibit higher survival rates during fermentation and storage compared with free cells [122,123,124,125,126].
4.3. Sensory Acceptability
In addition to improving viability, encapsulation may also enhance the overall sensory acceptability of the product by mitigating the negative effects of acid-induced cell death and metabolite release [127].
4.4. Strain Selection
Selecting acid-tolerant lactic acid bacteria can significantly increase probiotic survival under acidic conditions [128].
4.5. Inclusion of Antioxidants
Certain antioxidant compounds have been shown to support probiotic viability, possibly by modulating the fermentation environment or reducing oxidative stress [129,130].
4.6. Storage Conditions
Maintaining appropriate storage temperatures is crucial. Higher storage temperatures can accelerate metabolic processes and exacerbate pH fluctuations, negatively affecting probiotic stability [126,131].
5. Thermal Processing of Juices
Fruit and vegetable juices are typically preserved using physical, chemical, or biological methods, all aimed at ensuring microbiological safety, shelf life, and quality. The primary objective of these processing techniques is to eliminate pathogenic microorganisms while preserving key attributes essential for both consumer health and probiotic functionality.
The effectiveness of thermal processing is influenced by several factors, including the juice’s composition, microbial diversity, and selected processing parameters. Among the most commonly used approaches prior to fermentation are thermal treatments of varying intensities.
Pasteurization remains the standard thermal treatment method for ensuring microbial safety and prolonging the shelf life of juices. This process involves the application of elevated temperatures to inactivate spoilage microorganisms and undesirable enzymes. For example, high-temperature short-time (HTST) pasteurization has been shown to effectively reduce microbial loads in various juices, but it may also degrade heat-sensitive nutrients and negatively impact sensory attributes [132,133]. Recent studies emphasize the need to balance microbial inactivation efficacy with the preservation of nutritional quality, suggesting that mild heat treatments—such as low-intensity thermal processing or thermosonication—can maintain essential quality attributes while achieving microbial reduction [134,135].
5.1. Emerging Thermal Technologies
Innovative thermal processing techniques such as ohmic heating and thermosonication have gained popularity due to their ability to deliver more controlled heat transfer while minimizing the degradation of bioactive compounds. Studies show that thermosonicated juices exhibit improved color, retention of bioactive compounds, and enhanced sensory characteristics compared with their conventionally pasteurized counterparts [134]. This method effectively reduces microbial populations while preserving both sensory and nutritional properties—an essential consideration for subsequent probiotic fermentation.
Moreover, as previously discussed, changes in juice pH during processing can complement thermal treatment strategies. Fruit juices, typically acidic in nature, enhance microbial inactivation due to the increased heat sensitivity of spoilage microorganisms under low-pH conditions. A combination of moderate thermal treatment and juice acidification creates a synergistic effect. Modeling studies confirm that the interplay between pH and temperature significantly influences the thermal resistance of Alicyclobacillus acidoterrestris, a common spoilage microorganism in fruit juices [136,137].
5.2. Non-Thermal Alternatives
A range of non-thermal methods have been investigated as either alternatives or complements to thermal processing. Technologies such as pulsed electric fields (PEFs), high-pressure processing (HPP), and ultraviolet (UV) light have been evaluated for their ability to inactivate microorganisms without compromising the nutritional integrity of juices [135,138]. These techniques preserve heat-sensitive micronutrients while achieving microbial safety, making them particularly attractive for probiotic juice applications [139].
The thermal treatment and stabilization of fruit and vegetable juices play a critical role in preparing substrates for probiotic fermentation. A combination of conventional thermal processing with emerging technologies—such as thermosonication or non-thermal methods—can ensure microbiological safety while preserving the sensory and nutritional quality of the juice. These attributes are essential for the successful development of health-promoting probiotic beverages.
6. Impact of Probiotic Fermentation on the Physicochemical and Sensory Properties of Juices
One of the key challenges in developing probiotic-enriched fruit and vegetable juices is preserving their original physicochemical and sensory characteristics. Thus, it is essential to evaluate the metabolic activity of different probiotic microorganisms within juice matrices and their capacity to alter product sensory attributes.
The application of various Lactococcus species in fruit and vegetable juice fermentation has yielded promising results, particularly in enhancing the sensory quality of the final products. Studies of volatile compound profiles in juices fermented with Lactococcus lactis demonstrate that specific strains can generate complex flavor and texture characteristics, potentially increasing consumer acceptance [140]. Metabolites produced during fermentation contribute to unique flavor profiles, making the beverages more appealing while retaining their health-promoting properties [91]. Successful fermentations of apple and grape juices with L. lactis have led to improved flavor, aroma, and shelf-life stability [97,141]. Additionally, fermented apple juice has shown increased concentrations of bioactive compounds and antioxidants, further enhancing its health benefits [142].
Other investigations have demonstrated that the antioxidant activity of apple juice increases after fermentation, linked to the bioconversion of phenolic compounds. This suggests that fermentation can enhance the overall nutritional profile of the juice [143]. Fermentation with Lactiplantibacillus plantarum has been associated with high esterase activity, significantly influencing aroma development, while maintaining probiotic viability above recommended levels [144].
Recent studies also indicate that fermentation with Lactobacillus strains can intensify juice color and the concentration of antioxidant compounds, yielding probiotic beverages that are both nutritionally valuable and visually appealing [59,145]. These findings support earlier results by Hossain et al. (2020), which showed that LAB fermentation improves both the biochemical and sensory qualities of fruit juices, enhancing their health value and market potential [55].
Tomato juice has likewise shown substantial improvement through fermentation with lactic acid bacteria (LAB), including increased probiotic counts and enhanced antioxidant activity [146,147]. Fermentation with different Lactobacillus strains improved both the antioxidant profile and the sensory acceptability of tomato juice [148,149]. L. acidophilus was reported to induce substantial flavor modifications and prolong shelf life due to its pronounced acidification capabilities [150]. Similarly, L. plantarum strains isolated from tomatoes improved the sensory profile and probiotic attributes of fermented tomato juice, while also reducing pH levels [151].
Pumpkin juice fermentation resulted in desirable sensory properties and sustained high levels of probiotic viability [152]. Carrot juice has been identified as a promising delivery medium for probiotics, yielding a naturally sweet flavor that enhances consumer acceptance [153].
Fermentation of carrot and beetroot juices has also proven suitable for the growth of L. plantarum, L. acidophilus, and L. casei. During fermentation, these substrates exhibited reduced sugar content and pH values alongside increased acidity—indicators of a successful metabolic activity that promoted probiotic growth [119]. The properties of beetroot juice further support its efficacy as a fermentation substrate, offering favorable flavor and nutritional benefits [43,154]. The probiotic fermentation of beetroot juice not only ensured excellent bacterial viability but also conferred antimicrobial properties to the final beverage [43].
In the case of pomegranate juice, despite its initially low pH (~3.09), LAB such as L. plantarum were able to thrive, indicating high acid tolerance—an essential trait for successful fermentation in acidic matrices [155]. Fermentation improved the antioxidant activity and modified sugar and organic acid profiles, thereby enhancing the health-promoting characteristics of the final product [25].
Pineapple juice fermented with various LAB strains also exhibited improved antioxidant capacity [156], reinforcing the idea that sugar-rich juices are excellent substrates for supporting probiotic viability and metabolic activity. Studies have identified L. casei, L. rhamnosus, L. paracasei, and L. reuteri as particularly well suited to fermenting pineapple juice [45].
Multiple studies have confirmed that most probiotics can successfully grow in fruit and vegetable juices or their blends [28,56,117,131,155,156,157,158,159,160,161,162,163,164,165]. Typically, the fermentation process increases lactic acid concentrations in juice, improving both flavor and nutritional value. A summary of probiotic strains used in juice fermentation and their advantages and limitations is provided in Table 1.

Table 1.
Summary of probiotic strains used in juice fermentation: advantages and limitations.
7. Future Perspectives and Conclusions
The regulation of non-dairy probiotics varies significantly across regions, reflecting diverse approaches to food safety, public health, and market regulation. These regulatory differences have a substantial influence on the development and commercialization of probiotic enriched products.
In Europe, probiotics are subject to stringent rules shaped by both EU and member state-specific legislation, particularly to comply with the EU Nutrition and Health Claims Regulation (NHCR) [166]. In the United States, the Food and Drug Administration (FDA) oversees the use of the term “probiotic” and its associated health claims. However, regulatory overlaps with pharmaceutical laws can sometimes lead to the misclassification of food products, thereby affecting product development and public understanding [167].
In Southeast Asia, regulations are tailored to local markets and cultural contexts, with specific definitions and guidelines for probiotic products [168]. These varying frameworks influence consumer behavior, especially when considered alongside regional dietary practices. Moreover, the complexity of regulatory environments may hinder market entry for new products and contribute to reduced consumer confidence.
Despite these challenges, interest in probiotic-rich functional foods is growing around the world, as more people recognize the link between diet, gut health, and overall well-being. Fruit and vegetable juices are becoming a strong alternative to dairy-based probiotic products, especially for those with lactose intolerance, dairy allergies, or those following vegan or cholesterol-conscious diets. As research moves forward, fermented plant-based drinks are being seen as multifunctional systems—not only supporting probiotic survival but also delivering health-promoting compounds with antioxidant, anti-inflammatory, and antimicrobial effects.
Studies show that a wide range of probiotic species—especially from the Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus, and Bacillus genera—can successfully ferment fruit and vegetable juices. These probiotics survive and stay active in acidic juice environments, while also improving the nutritional value, taste, and safety of the final product. Fermentation usually boosts the levels of beneficial compounds like organic acids, phenolics, and antioxidants, which support the health benefits of the beverage.
However, some challenges remain before these products can be widely commercialized. Key issues include keeping probiotics alive during storage, avoiding unwanted changes in taste, coping with low pH conditions, and ensuring each strain remains safe and effective. Success in this field depends on carefully choosing the right juice bases, fermentation cultures, and processing methods—including how the product is treated and preserved after fermentation.
Looking into future perspectives, new screening techniques and genomic tools can help identify strong, acid-resistant probiotic strains that are well suited for specific juice types. Additionally, protective technologies like microencapsulation can help maintain probiotic viability during storage and ensure they reach the gut alive. Mixing probiotics with prebiotics (to form synbiotics), plant polyphenols, or other health-boosting compounds may improve both the health effects and the taste of the product. Innovative methods like high-pressure processing (HPP), pulsed electric fields (PEFs), and thermosonication may improve safety while preserving sensitive nutrients and live probiotics. Research that combines sensory science, consumer studies, and nutrition can help develop probiotic drinks that are both effective and enjoyable for a wide range of people.
Integrating probiotics into fruit and vegetable juice matrices provides a new and versatile platform to deliver health-promoting microorganisms to diverse consumer groups. While dairy-based functional products have long dominated the probiotic market, non-dairy alternatives are increasingly gaining consumer interest due to their natural bioactive compounds, broad demographic compatibility, and favorable regulatory perceptions.
In conclusion, the development of probiotic-fermented fruit and vegetable juices is a future-oriented and sustainable direction for functional food innovation. With continued scientific advances in microbiology, food technology, and systems biology, it is expected that the next generation of probiotic beverages will not only meet consumer expectations for taste and well-being, but also contribute to the global shift towards personalized nutrition and preventive healthcare.
Author Contributions
Conceptualization, D.Č. and R.Ž.; methodology, D.Č., V.J. and R.Ž.; formal analysis, D.Č., L.B. and R.Ž.; investigation, D.Č., L.B., V.J. and R.Ž.; data curation, D.Č. and R.Ž.; writing—original draft preparation, D.Č., V.J. and R.Ž.; writing—review and editing, D.Č., L.B. and R.Ž.; supervision, D.Č. and R.Ž. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential Non-Dairy Probiotic Products—A Healthy Approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Athmaselvi, K.A. Stress Tolerance and Physicochemical Properties of Encapsulation Processes for Lactobacillus rhamnosus in Pomegranate (Punica granatum L.) Fruit Juice. Food Sci. Biotechnol. 2016, 25, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, M.; Chandrasekaran, S.; Mehra, A.; Prakash, S.; Agarwal, A.; Ethiraj, S.; Vaithiyanathan, S. Fermentation of Beet Juice Using Lactic Acid Bacteria and Its Cytotoxic Activity Against Human Liver Cancer Cell Lines HepG2. Curr. Bioact. Compd. 2016, 12, 258–263. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in Non-Dairy Probiotic Beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Sinderen, D. Van. Fermented Functional Foods Based on Probiotics and Their Biogenic Metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef]
- Agrawal, R. Probiotics: An Emerging Food Supplement with Health Benefits. Food Biotechnol. 2005, 19, 227–246. [Google Scholar] [CrossRef]
- Naseem, Z.; Mir, S.A.; Wani, S.M.; Rouf, M.A.; Bashir, I.; Zehra, A. Probiotic-Fortified Fruit Juices: Health Benefits, Challenges, and Future Perspective. Nutrition 2023, 115, 112154. [Google Scholar] [CrossRef]
- Probiotics in Food, Beverages, Dietary Supplements and Animal Feed. Available online: https://www.globenewswire.com/news-release/2025/01/15/3009865/28124/en/Probiotics-in-Food-Beverages-Dietary-Supplements-and-Animal-Feed-Markets-A-102-Billion-Opportunity-by-2029.html (accessed on 27 May 2025).
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of Food Matrix on the Viability of Probiotic Bacteria: A Review Based on Dairy and Non-Dairy Beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of Probiotics in Functional Foods during Shelf Life. In Food Quality and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–233. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the Total Antioxidant Capacity and Total Polyphenol Content of 23 Commercially Available Vegetable Juices before and after in Vitro Digestion Measured by FRAP, DPPH, ABTS and Folin–Ciocalteu Methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Hernandez, E.; Pandiella, S. Development of Probiotics and Prebiotics. 2013, pp. 21–60. Available online: https://zenodo.org/records/11237311 (accessed on 27 May 2025). [CrossRef]
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in Dairy and Non-Dairy Probiotic Products—A Review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S. Survival of Human Derived Lactobacillus Plantarum in Fermented Cereal Extracts during Refrigerated Storage. LWT-Food Sci. Technol. 2010, 43, 431–435. [Google Scholar] [CrossRef]
- Kumar, S.; Rattu, G.; Mitharwal, S.; Chandra, A.; Kumar, S.; Kaushik, A.; Mishra, V.; Nema, P.K. Trends in Non-dairy-based Probiotic Food Products: Advances and Challenges. J. Food Process. Preserv. 2022, 46, e16578. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of Vegetable Origin: A New Alternative for the Consumption of Probiotic Bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Klososki, S.J.; Rosset, M.; Barão, C.E.; Marcolino, V.A. Fruit Juices as Probiotic Foods. In Sports and Energy Drinks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–513. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Potentially Probiotic Lactic Acid Bacteria on the Physicochemical Composition and Acceptance of Fermented Cereal Beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Patel, A. Probiotic Fruit and Vegetable Juices-Recent Advances and Future Perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Saarela, M.H.; Alakomi, H.-L.; Puhakka, A.; Mättö, J. Effect of the Fermentation PH on the Storage Stability of Lactobacillus rhamnosus Preparations and Suitability of In Vitro Analyses of Cell Physiological Functions to Predict It. J. Appl. Microbiol. 2009, 106, 1204–1212. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of Fermentation of Pomegranate Juice by Lactobacillus plantarum and Lactobacillus acidophilus on the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar] [CrossRef]
- Kumar, B.V.; Sreedharamurthy, M.; Reddy, O.V.S. Probiotication of Mango and Sapota Juices Using Lactobacillus plantarum NCDC LP 20. Nutrafoods 2015, 14, 97–106. [Google Scholar] [CrossRef]
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in Fruit and Vegetable Matrices: Opportunities for Nondairy Consumers. LWT 2021, 151, 112106. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Madrona, G.S.; Garcia, S.; Prudencio, S.H. Probiotic Viability, Physicochemical Characteristics and Acceptability during Refrigerated Storage of Clarified Apple Juice Supplemented with Lactobacillus paracasei ssp. paracasei and Oligofructose in Different Package Type. LWT-Food Sci. Technol. 2015, 63, 415–422. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Sada, A.; Orlando, P. Synbiotic Potential of Carrot Juice Supplemented with Lactobacillus Spp. and Inulin or Fructooligosaccharides. J. Sci. Food Agric. 2008, 88, 2271–2276. [Google Scholar] [CrossRef]
- White, J.; Hekmat, S. Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber. Fermentation 2018, 4, 27. [Google Scholar] [CrossRef]
- de Andrade, R.M.; Silva, S.; Costa, C.M.d.S.F.; Veiga, M.; Costa, E.; Ferreira, M.S.L.; Gonçalves, E.C.B.d.A.; Pintado, M.E. Potential Prebiotic Effect of Fruit and Vegetable Byproducts Flour Using in Vitro Gastrointestinal Digestion. Food Res. Int. 2020, 137, 109354. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Wu, V.C.H. The Potential of Berries to Serve as Selective Inhibitors of Pathogens and Promoters of Beneficial Microorganisms. Food Qual. Saf. 2017, 1, 3–12. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Barat, A.; Ozcan, T. Growth of Probiotic Bacteria and Characteristics of Fermented Milk Containing Fruit Matrices. Int. J. Dairy Technol. 2018, 71 (Suppl. S1), 120–129. [Google Scholar] [CrossRef]
- Agirman, B.; Yildiz, I.; Polat, S.; Erten, H. The Evaluation of Black Carrot, Green Cabbage, Grape, and Apple Juices as Substrates for the Production of Functional Water Kefir-like Beverages. Food Sci. Nutr. 2024, 12, 6595–6611. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; M’hir, S.; Hamdi, M. Microbiological, Biochemical, and Functional Aspects of Fermented Vegetable and Fruit Beverages. J. Chem. 2020, 2020, 5790432. [Google Scholar] [CrossRef]
- Skryplonek, K.; Jasińska, M. Fermented Probiotic Beverages Based on Acid Whey. Acta Sci. Pol. Technol. Aliment. 2015, 14, 397–405. [Google Scholar] [CrossRef]
- Garcia, C.; Remize, F. Lactic Acid Fermentation of Fruit and Vegetable Juices and Smoothies: Innovation and Health Aspects. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–46. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- da Silva Vale, A.; Venturim, B.C.; da Silva Rocha, A.R.F.; Martin, J.G.P.; Maske, B.L.; Balla, G.; De Dea Lindner, J.; Soccol, C.R.; de Melo Pereira, G.V. Exploring Microbial Diversity of Non-Dairy Fermented Beverages with a Focus on Functional Probiotic Microorganisms. Fermentation 2023, 9, 496. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Fermentation of Beet Juice by Beneficial Lactic Acid Bacteria. LWT-Food Sci. Technol. 2005, 38, 73–75. [Google Scholar] [CrossRef]
- Buruleanu, L.; Nicolescu, C.L.; Avram, D.; Bratu, M.G.; Manea, I. Survival of Probiotic Bacteria during Lactic Acid Fermentation of Vegetable Juices. J. Agroaliment. Process. Technol. 2009, 15, 132–139. [Google Scholar]
- do Espírito Santo, A.P.; Perego, P.; Converti, A.; Oliveira, M.N. Influence of Food Matrices on Probiotic Viability—A Review Focusing on the Fruity Bases. Trends Food Sci. Technol. 2011, 22, 377–385. [Google Scholar] [CrossRef]
- Fessard, A.; Bourdon, E.; Payet, B.; Remize, F. Identification, Stress Tolerance, and Antioxidant Activity of Lactic Acid Bacteria Isolated from Tropically Grown Fruits and Leaves. Can. J. Microbiol. 2016, 62, 550–561. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and Technological Characterization of Lactic Acid Bacteria Isolated from Tropically Grown Fruits and Vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An Overview of Beneficial Effects. In Lactic Acid Bacteria: Genetics, Metabolism and Applications; Springer: Dordrecht, The Netherlands, 2002; pp. 279–289. [Google Scholar] [CrossRef]
- Giraffa, G. Lactobacillus Helveticus: Importance in Food and Health. Front. Microbiol. 2014, 5, 338. [Google Scholar] [CrossRef]
- Ahmed, Z.; Vohra, M.S.; Khan, M.N.; Ahmed, A.; Khan, T.A. Antimicrobial Role of Lactobacillus Species as Potential Probiotics against Enteropathogenic Bacteria in Chickens. J. Infect. Dev. Ctries. 2019, 13, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Scourboutakos, M.; Franco-Arellano, B.; Murphy, S.; Norsen, S.; Comelli, E.; L’Abbé, M. Mismatch between Probiotic Benefits in Trials versus Food Products. Nutrients 2017, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 °C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Lei, H. Phenolics Profile, Antioxidant Activity and Flavor Volatiles of Pear Juice: Influence of Lactic Acid Fermentation Using Three Lactobacillus Strains in Monoculture and Binary Mixture. Foods 2021, 11, 11. [Google Scholar] [CrossRef]
- Özcan, T.; Yilmaz Ersan, L.; Akpinar Bayizit, A.; Delikanli Kiyak, B.; Keser, G.; Ciniviz, M.; Barat, A. Probiotic Fermentation and Organic Acid Profile in Milk Based Lactic Beverages Containing Potential Prebiotic Apple Constituents. J. Agric. Sci. 2023, 29, 734–742. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hoque, M.M.; Hossain, M.M.; Kabir, M.H.; Yasin, M.; Islam, M.A. Biochemical, Microbiological and Organoleptic Properties of Probiotic Pineapple Juice Developed by Lactic Acid Bacteria. J. Sci. Res. 2020, 12, 743–750. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple Juice as a Medium for Fermentation by the Probiotic Lactobacillus plantarum PCS 26 Strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H.; Alavi, S.A. Effect of Probiotics on Patulin Removal from Synbiotic Apple Juice. J. Sci. Food Agric. 2017, 97, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy Apple Juice Fermented by Lactobacillus Prevents Obesity via Modulating Gut Microbiota and Protecting Intestinal Tract Health. Nutrients 2021, 13, 971. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in Color Expression and Antioxidant Activity of Strawberry Juice Fermented with Lactic Acid Bacteria: A Phenolic-Based Research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.S.; Domingos, M.M.; de São José, J.F.B. Viability of Probiotic Microorganisms and the Effect of Their Addition to Fruit and Vegetable Juices. Microorganisms 2023, 11, 1335. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Guo, C.; Li, W.; Gao, Z. Lactobacillus Co-Fermentation of Cerasus humilis Juice Alters Chemical Properties, Enhances Antioxidant Activity, and Improves Gut Microbiota. Food Funct. 2023, 14, 8248–8260. [Google Scholar] [CrossRef]
- Marnpae, M.; Balmori, V.; Kamonsuwan, K.; Nungarlee, U.; Charoensiddhi, S.; Thilavech, T.; Suantawee, T.; Sivapornnukul, P.; Chanchaem, P.; Payungporn, S.; et al. Modulation of the Gut Microbiota and Short-Chain Fatty Acid Production by Gac Fruit Juice and Its Fermentation in in Vitro Colonic Fermentation. Food Funct. 2024, 15, 3640–3652. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, H.; Na, G.; Jung, H.; Kim, D.-G.; Shin, S.-I.; Jung, S.-E.; Choi, I.; Lee, J.-H.; Sim, J.-H.; et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules 2020, 10, 725. [Google Scholar] [CrossRef]
- Hyronimus, B.; Le Marrec, C.; Hadj Sassi, A.; Deschamps, A. Acid and Bile Tolerance of Spore-Forming Lactic Acid Bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Menga, V.; Martina, A.; Pellegrini, N.; Scazzina, F.; Torriani, S. Nutritional Profile and Cooking Quality of a New Functional Pasta Naturally Enriched in Phenolic Acids, Added with β-Glucan and Bacillus Coagulans GBI-30, 6086. J. Cereal. Sci. 2015, 65, 260–266. [Google Scholar] [CrossRef]
- Pilevar, Z.; Hosseini, H. Effects of Starter Cultures on the Properties of Meat Products: A Review. Annu. Res. Rev. Biol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus Coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Matei lațiu, M.C.; Buza, V.; Chirilă, F.; Boros, Z.; Lațiu, C.; Szakacs, A.R.; Ștefănuț, L.C. In Vitro Qualitative Assessment of Tolerance to Simulated Gastric Juice, Bile, Fructose, Glucose and Lactose for Different Probiotic Bacteria. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2022, 79. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Natarajan, S.; Arumugam, S.; Ali, F.; Pande, A.; Karri, S.K. Evaluation of Anti-Diarrhoeal Activity of Bacillus Coagulans MTCC 5856 and Its Effect on Gastrointestinal Motility in Wistar Rats. Int. J. Pharma. Bio. Sci. 2016, 7, 311–316. [Google Scholar]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Golshahi, M.; Yazdi, S.; Mehdi Pirnia, M. Evaluation of the Effect of Fruit Juice Containing Bacillus Coagulans Probiotic Supplement on the Level of Immunoglobulins A, M and Lymphocytes in Two-Speed Athletes. In Functional Foods—Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Almada-Érix, C.N.; Almada, C.N.; Cabral, L.; Barros de Medeiros, V.P.; Roquetto, A.R.; Santos-Junior, V.A.; Fontes, M.; Gonçalves, A.E.S.S.; dos Santos, A.; Lollo, P.C.; et al. Orange Juice and Yogurt Carrying Probiotic Bacillus coagulans GBI-30 6086: Impact of Intake on Wistar Male Rats Health Parameters and Gut Bacterial Diversity. Front. Microbiol. 2021, 12, 623951. [Google Scholar] [CrossRef]
- Sireswar, S.; Montet, D.; Dey, G. Principal Component Analysis for Clustering Probiotic-Fortified Beverage Matrices Efficient in Elimination of Shigella Sp. Fermentation 2018, 4, 34. [Google Scholar] [CrossRef]
- Saud, S.; Xiaojuan, T.; Fahad, S. The Consequences of Fermentation Metabolism on the Qualitative Qualities and Biological Activity of Fermented Fruit and Vegetable Juices. Food Chem. X 2024, 21, 101209. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.; Chauhan, R.P.; Zhao, J.-Y.; Chu, K.-K.; Han, P.-Y.; Yang, Z.; Tang, Y.; Kong, W.; Long, Y.; Zong, L.-D.; et al. Academic Editors: Michelle Detection of Coronaviruses and Genomic Characterization of Gammacoronaviruses from Overwintering Black-Headed Gulls (Chroicocephalus ridibundus) in Yunnan Province, China. Microorganisms 2025, 13, 874. [Google Scholar] [CrossRef]
- Serpa, J.; Caiado, F.; Carvalho, T.; Torre, C.; Gonçalves, L.G.; Casalou, C.; Lamosa, P.; Rodrigues, M.; Zhu, Z.; Lam, E.W.F.; et al. Butyrate-Rich Colonic Microenvironment Is a Relevant Selection Factor for Metabolically Adapted Tumor Cells. J. Biol. Chem. 2010, 285, 39211–39223. [Google Scholar] [CrossRef]
- Matera, M. Bifidobacteria, Lactobacilli… When, How and Why to Use Them. Glob. Pediatr. 2024, 8, 100139. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; La Rosa, S.L.; Roger, L.C.; Pope, P.B.; Hoyles, L.; McCartney, A.L.; Hall, L.J. Succession of Bifidobacterium longum Strains in Response to a Changing Early Life Nutritional Environment Reveals Dietary Substrate Adaptations. iScience 2020, 23, 101368. [Google Scholar] [CrossRef]
- Kaktcham, P.M.; Kujawska, M.; Kouam, E.M.F.; Piame, L.T.; Tientcheu, M.L.T.; Mueller, J.; Felsl, A.; Truppel, B.-A.; Ngoufack, F.Z.; Hall, L.J. Genomic Insights into the Beneficial Potential of Bifidobacterium and Enterococcus Strains Isolated from Cameroonian Infants. Microb. Genom. 2025, 11, 001354. [Google Scholar] [CrossRef] [PubMed]
- Michalik, M.; Podbielska-Kubera, A.; Basińska, A.M.; Szewc, M.; Gałęcka, M.; Schwiertz, A. Alteration of Indicator Gut Microbiota in Patients with Chronic Sinusitis. Immun. Inflamm. Dis. 2023, 11, e996. [Google Scholar] [CrossRef]
- Oh, S.; Yap, G.C.; Hong, P.-Y.; Huang, C.-H.; Aw, M.M.; Shek, L.P.-C.; Liu, W.-T.; Lee, B.W. Immune-Modulatory Genomic Properties Differentiate Gut Microbiota of Infants with and without Eczema. PLoS ONE 2017, 12, e0184955. [Google Scholar] [CrossRef]
- Güler, M.A.; Çetin, B.; Albayrak, B.; Meral-Aktaş, H.; Tekgündüz, K.Ş.; Kara, M.; Işlek, A. Isolation, Identification, and in Vitro Probiotic Characterization of Forty Novel Bifidobacterium Strains from Neonatal Feces in Erzurum Province, Türkiye. J. Sci. Food Agric. 2024, 104, 4165–4175. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.Y.; Zuo, F.L.; Yu, R.; Zhang, Y.; Ma, H.Q.; Chen, S.W. Bifidobacteria Possess Inhibitory Activity against Dipeptidyl Peptidase-IV. Lett. Appl. Microbiol. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Simamora, A.; Santoso, A.W.; Timotius, K.H. α-Glucosidase Inhibitory Effect of Fermented Fruit Juice of Morinda citrifolia L and Combination Effect with Acarbose. Curr. Res. Nutr. Food Sci. J. 2019, 7, 218–226. [Google Scholar] [CrossRef]
- Odamaki, T.; Horigome, A.; Sugahara, H.; Hashikura, N.; Minami, J.; Xiao, J.; Abe, F. Comparative Genomics Revealed Genetic Diversity and Species/Strain-Level Differences in Carbohydrate Metabolism of Three Probiotic Bifidobacterial Species. Int. J. Genom. 2015, 2015, 567809. [Google Scholar] [CrossRef]
- Tenea, G.N. Metabiotics Signature through Genome Sequencing and In Vitro Inhibitory Assessment of a Novel Lactococcus Lactis Strain UTNCys6-1 Isolated from Amazonian Camu-Camu Fruits. Int. J. Mol. Sci. 2023, 24, 6127. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Liu, F.; Wan, X.; Qiao, Y.; Li, R.; Wu, Z.; Saris, P.E.J.; Xu, H.; Qiao, M. Genomic Features and Construction of Streamlined Genome Chassis of Nisin Z Producer Lactococcus Lactis N8. Microorganisms 2021, 10, 47. [Google Scholar] [CrossRef]
- Purk, L.; Kitsiou, M.; Ioannou, C.; El Kadri, H.; Costello, K.M.; Gutierrez Merino, J.; Klymenko, O.; Velliou, E.G. Unravelling the Impact of Fat Content on the Microbial Dynamics and Spatial Distribution of Foodborne Bacteria in Tri-Phasic Viscoelastic 3D Models. Sci. Rep. 2023, 13, 21811. [Google Scholar] [CrossRef]
- Biswas, P.; Khan, A.; Mallick, A.I. Targeted Bioimaging of Microencapsulated Recombinant LAB Vector Expressing Fluorescent Reporter Protein: A Non-Invasive Approach for Microbial Tracking. ACS Biomater. Sci. Eng. 2024, 10, 5210–5225. [Google Scholar] [CrossRef]
- Siroli, L.; Camprini, L.; Pisano, M.B.; Patrignani, F.; Lanciotti, R. Volatile Molecule Profiles and Anti-Listeria monocytogenes Activity of Nisin Producers Lactococcus lactis Strains in Vegetable Drinks. Front. Microbiol. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Ozdogan, D.K.; Akcelik, N.; Aslim, B.; Suludere, Z.; Akcelik, M. Probiotic and Antioxidative Properties of L. Lactis LL27 Isolated from Milk. Biotechnol. Biotechnol. Equip. 2012, 26, 2750–2758. [Google Scholar] [CrossRef]
- Boumaiza, M.; Colarusso, A.; Parrilli, E.; Garcia-Fruitós, E.; Casillo, A.; Arís, A.; Corsaro, M.M.; Picone, D.; Leone, S.; Tutino, M.L. Getting Value from the Waste: Recombinant Production of a Sweet Protein by Lactococcus lactis Grown on Cheese Whey. Microb. Cell Fact. 2018, 17, 126. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yeom, Y.; Kim, Y.-S.; Kim, E.; Shin, J.-H.; Seok, P.R.; Woo, M.J.; Kim, Y. Effect of Vitamin C on Azoxymethane (AOM)/Dextran Sulfate Sodium (DSS)-Induced Colitis-Associated Early Colon Cancer in Mice. Nutr. Res. Pract. 2018, 12, 101. [Google Scholar] [CrossRef]
- Gaudu, P.; Yamamoto, Y.; Jensen, P.R.; Hammer, K.; Lechardeur, D.; Gruss, A. Genetics of Lactococci. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Li, W.; Ren, M.; Duo, L.; Li, J.; Wang, S.; Sun, Y.; Li, M.; Ren, W.; Hou, Q.; Yu, J.; et al. Fermentation Characteristics of Lactococcus lactis Subsp. Lactis Isolated From Naturally Fermented Dairy Products and Screening of Potential Starter Isolates. Front. Microbiol. 2020, 11, 1794. [Google Scholar] [CrossRef]
- Saizen, A.; Stipkovits, L.; Muto, Y.; Serventi, L. Fermentation of Peanut Slurry with Lactococcus lactis Species, Leuconostoc and Propionibacterium freudenreichii Subsp. Globosum Enhanced Protein Digestibility. Foods 2023, 12, 3447. [Google Scholar] [CrossRef] [PubMed]
- Cuvas-Limon, R.B.; Ferreira-Santos, P.; Cruz, M.; Teixeira, J.A.; Belmares, R.; Nobre, C. Effect of Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds and Antioxidant Activity of Fermented Aloe Vera Juices. Antioxidants 2022, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Olaide, A.O.; Lihan, S.; Husain, A.A.S.A.; Saat, R.; Mohammad, F.S.; Adewale, I.A.; Abideen, W.A. Use of the Lactococcus lactis IO-1 for Developing a Novel Functional Beverage from Coconut Water. Ann. Univ. Dunarea Jos Galati Fascicle VI-Food Technol. 2020, 44, 118–131. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Z.; Liang, J.; Dou, J.; Guo, F.; Xu, Z.; Wang, T. Advances in Genetic Tools and Their Application in Streptococcus thermophilus. Foods 2023, 12, 3119. [Google Scholar] [CrossRef]
- Martinović, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus Thermophilus: To Survive, or Not to Survive the Gastrointestinal Tract, That Is the Question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, X.; Hao, M.; Qu, X.; Hu, T. New Advances in Exopolysaccharides Production of Streptococcus thermophilus. Arch. Microbiol. 2017, 199, 799–809. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Jiménez-Díaz, R. A Novel Lactobacillus Pentosus-Paired Starter Culture for Spanish-Style Green Olive Fermentation. Food Microbiol. 2012, 30, 253–259. [Google Scholar] [CrossRef]
- Wouters, D.; Bernaert, N.; Anno, N.; Van Droogenbroeck, B.; De Loose, M.; Van Bockstaele, E.; De Vuyst, L. Application’ and Validation of Autochthonous Lactic Acid Bacteria Starter Cultures for Controlled Leek Fermentations and Their Influence on the Antioxidant Properties of Leek. Int. J. Food Microbiol. 2013, 165, 121–133. [Google Scholar] [CrossRef]
- Moon, S.H.; Moon, J.S.; Chang, H.C. Rapid Manufacture and Quality Evaluation of Long-Term Fermented Kimchi (Mukeunji) Using Lactobacillus Sakei SC1. Food Sci. Biotechnol. 2015, 24, 1797–1804. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter Culture Fermentation of Chinese Sauerkraut: Growth, Acidification and Metabolic Analyses. Food Control 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.-Y.; Park, W.-S.; Jeon, C.O. Effects of Leuconostoc Mesenteroides Starter Cultures on Microbial Communities and Metabolites during Kimchi Fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of Combining Two Lactic Acid Bacteria as a Starter Culture on Model Kimchi Fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Zhao, X.; Guo, Z.; Zhang, Z.; Dong, Y.; Shan, C. Different Lactic Acid Bacteria Strains Affecting the Flavor Profile of Fermented Jujube Juice. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- McFarland, L.V. Meta-Analysis of Probiotics for the Prevention of Traveler’s Diarrhea. Travel. Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef]
- Doron, S.I.; Hibberd, P.L.; Gorbach, S.L. Probiotics for Prevention of Antibiotic-Associated Diarrhea. J. Clin. Gastroenterol. 2008, 42 (Suppl. S2), S58–S63. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic Acid Fermentation of Apricot Juice by Mono- and Mixed Cultures of Probiotic Lactobacillus and Bifidobacterium Strains. Food Sci. Biotechnol. 2017, 27, 547–554. [Google Scholar] [CrossRef]
- Johnson, M.E. Mesophilic and Thermophilic Cultures Used in Traditional Cheesemaking. Microbiol. Spectr. 2013, 1. [Google Scholar] [CrossRef]
- Liu, X.; Guo, W.; Cui, S.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. A Comprehensive Assessment of the Safety of Blautia Producta DSM 2950. Microorganisms 2021, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Zawistowska-Rojek, A.; Tyski, S. Are Probiotic Really Safe for Humans? Pol. J. Microbiol. 2018, 67, 251–258. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Salmeron, I.; Charalampopoulos, D. Investigation of the Factors Influencing the Survival of Bifidobacterium longum in Model Acidic Solutions and Fruit Juices. Food Chem. 2011, 129, 1037–1044. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Malik, M.; Bora, J.; Sharma, V. Growth Studies of Potentially Probiotic Lactic Acid Bacteria (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus casei) in Carrot and Beetroot Juice Substrates. J. Food Process. Preserv. 2019, 43, e14214. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving Survival of Probiotic Bacteria Using Bacterial Poly-γ-Glutamic Acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of Encapsulated Probiotics in Pasteurized Grape Juice and Evaluation of Their Properties during Storage. Food Sci. Technol. Int. 2019, 25, 120–129. [Google Scholar] [CrossRef]
- Sohail, A.; Turner, M.S.; Prabawati, E.K.; Coombes, A.G.A.; Bhandari, B. Evaluation of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM Encapsulated Using a Novel Impinging Aerosol Method in Fruit Food Products. Int. J. Food Microbiol. 2012, 157, 162–166. [Google Scholar] [CrossRef]
- Marcelli, B.; Karsens, H.; Nijland, M.; Oudshoorn, R.; Kuipers, O.P.; Kok, J. Employing Lytic Phage-Mediated Horizontal Gene Transfer in Lactococcus Lactis. PLoS ONE 2020, 15, e0238988. [Google Scholar] [CrossRef]
- Mahmud, S.; Khan, S.; Khan, M.R.; Islam, J.; Sarker, U.K.; Hasan, G.M.M.A.; Ahmed, M. Viability and Stability of Microencapsulated Probiotic Bacteria by Freeze-drying under in Vitro Gastrointestinal Conditions. J. Food Process. Preserv. 2022, 46, e17123. [Google Scholar] [CrossRef]
- Rahman, M.; Emon, D.; Toma, M.; Nupur, A.; Karmoker, P.; Iqbal, A.; Aziz, M.; Alim, M. Recent Advances in Probiotication of Fruit and Vegetable Juices. J. Adv. Vet. Anim. Res. 2023, 10, 522. [Google Scholar] [CrossRef]
- King, V.A.E.; Huang, H.Y.; Tsen, J.H. Fermentation of Tomato Juice by Cell Immobilized Lactobacillus acidophilus. Mid-Taiwan J. Med. 2007, 12, 1–7. [Google Scholar]
- Osaro-Matthew, R.C.; Itaman, V.O.; Ughala, E.; Obike-Martins, V.; Igwe, G. Viability of Probiotic Lactic Acid Bacteria in Malay Apple (Syzygium malaccense) during Fermentation and Storage. Bio-Research 2024, 22, 2255–2263. [Google Scholar] [CrossRef]
- Alsharafani, M.A.M.; Abdullah, T.; Jabur, Z.A.; Hassan, A.A.; Alhendi, A.S.; Abdulmawjood, A.; Khan, I.U.H. Assessing Synergistic Effect of Jerusalem Artichoke Juice and Antioxidant Compounds on Enhanced Viability and Persistence of Bifidobacterium Species, Palatability, and Shelf Life. Food Sci. Nutr. 2022, 10, 1994–2008. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Xiao, G.; Xu, Y.; Tang, D.; Chen, Y.; Zhang, Y. Combined Effect of Dimethyl Dicarbonate (DMDC) and Nisin on Indigenous Microorganisms of Litchi Juice and Its Microbial Shelf Life. J. Food Sci. 2013, 78, M1236–M1241. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic Beverage from Cashew Apple Juice Fermented with Lactobacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Raju, S.; Deka, S.C. Influence of Thermosonication Treatments on Bioactive Compounds and Sensory Quality of Fruit (Haematocarpus validus) Juice. J. Food Process. Preserv. 2018, 42, e13701. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Xu, B.; Khan, S.; Shukat, R.; Ahmad, N.; Imran, M.; Rehman, A.; Karrar, E.; Aadil, R.M.; Korma, S.A. Impact of High-Intensity Thermosonication Treatment on Spinach Juice: Bioactive Compounds, Rheological, Microbial, and Enzymatic Activities. Ultrason. Sonochem 2021, 78, 105740. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, R.; Pradhan, D.; Deka, S.C. Effects of Thermosonication Process on Inactivation of Escherichia coli and Saccharomyces cerevisiae and Its Survival Kinetics Modeling in Khoonphal (Haematocarpus validus) Juice to Extend Its Shelf Life. J. Food Process. Preserv. 2019, 43, e14220. [Google Scholar] [CrossRef]
- Vignali, G.; Gozzi, M.; Pelacci, M.; Stefanini, R. Non-Conventional Stabilization for Fruit and Vegetable Juices: Overview, Technological Constraints, and Energy Cost Comparison. Food Bioproc. Technol. 2022, 15, 1729–1747. [Google Scholar] [CrossRef]
- Silva, L.P.; Gonzales-Barron, U.; Cadavez, V.; Sant’Ana, A.S. Modeling the Effects of Temperature and PH on the Resistance of Alicyclobacillus acidoterrestris in Conventional Heat-Treated Fruit Beverages through a Meta-Analysis Approach. Food Microbiol. 2015, 46, 541–552. [Google Scholar] [CrossRef]
- de Castro Oliveira, W.; Hungaro, H.M.; Paiva, A.D.; Mantovani, H.C. Modeling Heat-Resistance of Alicyclobacillus acidoterrestris in Different Fruit Juices: Combined Effects of PH and Temperature. Braz. Arch. Biol. Technol. 2023, 66, e23220566. [Google Scholar] [CrossRef]
- Chandra, R.D.; Prihastyanti, M.N.U.; Lukitasari, D.M. Effects of PH, High Pressure Processing, and Ultraviolet Light on Carotenoids, Chlorophylls, and Anthocyanins of Fresh Fruit and Vegetable Juices. eFood 2021, 2, 113–124. [Google Scholar] [CrossRef]
- Dogan, K.; Akman, P.K.; Tornuk, F. Role of Non-thermal Treatments and Fermentation with Probiotic Lactobacillus plantarum on in Vitro Bioaccessibility of Bioactives from Vegetable Juice. J. Sci. Food Agric. 2021, 101, 4779–4788. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, W.; Dong, G. Dung Beetle Optimizer Algorithm and Machine Learning-Based Genome Analysis of Lactococcus lactis: Predicting Electronic Sensory Properties of Fermented Milk. Foods 2024, 13, 1958. [Google Scholar] [CrossRef]
- Fusieger, A.; Martins, M.C.F.; de Freitas, R.; Nero, L.A.; de Carvalho, A.F. Technological Properties of Lactococcus lactis Subsp. Lactis Bv. Diacetylactis Obtained from Dairy and Non-Dairy Niches. Braz. J. Microbiol. 2020, 51, 313–321. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Q.; Luo, J.; Wu, J.; Wang, S.; Wang, Z.; Gong, S.; Zhang, W.; Lan, X. Intracellular Expression of Antifreeze Peptides in Food Grade Lactococcus lactis and Evaluation of Their Cryoprotective Activity. J. Food Sci. 2018, 83, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus Plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus Plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef]
- Plessas, S.; Mantzourani, I.; Terpou, A.; Bekatorou, A. Assessment of the Physicochemical, Antioxidant, Microbial, and Sensory Attributes of Yogurt-Style Products Enriched with Probiotic-Fermented Aronia melanocarpa Berry Juice. Foods 2023, 13, 111. [Google Scholar] [CrossRef]
- Koh, J.; Kim, Y.; Oh, J. Chemical Characterization of Tomato Juice Fermented with Bifidobacteria. J. Food Sci. 2010, 75, C428–C432. [Google Scholar] [CrossRef]
- Dunkley, K.E.; Hekmat, S. Development of Probiotic Vegetable Juice Using Lactobacillus rhamnosus GR-1. Nutr. Food Sci. 2019, 50, 955–968. [Google Scholar] [CrossRef]
- Chukwuemeka, I.C.; Odinakachi, O.H.; Kalu, A.D. Bio-Preservation of Processed Watermelon (Citrullus lanatus) Juice Using Lactobacillus Species Isolated from Processed Fermented Maize (Akamu). GSC Biol. Pharm. Sci. 2020, 10, 126–136. [Google Scholar] [CrossRef]
- Goderska, K.; Dombhare, K.; Radziejewska-Kubzdela, E. Probiotics in Vegetable Juices: Tomato (Solanum Lycopersicum), Carrot (Daucus Carota, Subsp. Sativus) and Beetroot Juice (Beta Vulgaris). 29 September 2021. Available online: https://www.researchsquare.com/article/rs-907042/v1 (accessed on 27 May 2025). [CrossRef]
- Orike, E.L.; Olajugbagbe, T.E.; Omafuvbe, B.O.; Animasahun, T.O. Effect of Storage on Probiotic Viability, Physicochemical and Sensory Properties of Probiotic-Enriched Orange Juice. Eur. J. Nutr. Food Saf. 2024, 16, 304–312. [Google Scholar] [CrossRef]
- Tuerhong, N.; Wang, L.; Cui, J.; Shataer, D.; Yan, H.; Dong, X.; Gao, Z.; Zhang, M.; Qin, Y.; Lu, J. Targeted Screening of Lactiplantibacillus Plantarum Strains Isolated from Tomatoes and Its Application in Tomato Fermented Juice. Foods 2024, 13, 3569. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Dimitrovska-Vetadjoka, M.; Hristov, H.; Doneva-Shapceska, D. Developing Probiotic Pumpkin Juice by Fermentation with Commercial Probiotic Strain Lactobacillus casei 431. J. Food Process. Preserv. 2021, 45, e15245. [Google Scholar] [CrossRef]
- Tamminen, M.; Salminen, S.; Ouwehand, A.C. Fermentation of Carrot Juice by Probiotics: Viability and Preservation of Adhesion. Int. J. Biotechnol. Wellness Ind. 2013, 2, 10. [Google Scholar] [CrossRef]
- Foss, K.; Starowicz, M.; Kłębukowska, L.; Sawicki, T. Effect of Lactic Acid Fermentation of Red Beetroot Juice on Volatile Compounds Profile and Content. Eur. Food Res. Technol. 2023, 249, 2401–2418. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of Pomegranate Juice by Probiotic Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic Beverage from Pineapple Juice Fermented with Lactobacillus and Bifidobacterium Strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gardner, N.J. Effect of Storage in a Fruit Drink on Subsequent Survival of Probiotic Lactobacilli to Gastro-Intestinal Stresses. Food Res. Int. 2008, 41, 539–543. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Rodrigues, S. Optimization of the Fermentation of Cantaloupe Juice by Lactobacillus casei NRRL B-442. Food Bioproc. Tech. 2012, 5, 2819–2826. [Google Scholar] [CrossRef]
- de Souza Neves Ellendersen, L.; Granato, D.; Bigetti Guergoletto, K.; Wosiacki, G. Development and Sensory Profile of a Probiotic Beverage from Apple Fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical Analysis and Flavor Properties of Blended Orange, Carrot, Apple and Chinese Jujube Juice Fermented by Selenium-Enriched Probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- da Costa, G.M.; de Carvalho Silva, J.V.; Mingotti, J.D.; Barão, C.E.; Klososki, S.J.; Pimentel, T.C. Effect of Ascorbic Acid or Oligofructose Supplementation on L. Paracasei Viability, Physicochemical Characteristics and Acceptance of Probiotic Orange Juice. LWT 2017, 75, 195–201. [Google Scholar] [CrossRef]
- Pakbin, B.; Razavi, S.H.; Mahmoudi, R.; Gajarbeygi, P. Producing Probiotic Peach Juice. Biotechnol. Health Sci. 2014, 1, e24683. [Google Scholar] [CrossRef]
- Nguyen, T.T.G.; Nguyen, T.K.; Tran, N.N.; Dong, T.A.D.; Nguyen, P.M. Cashew Apple Juice Anacardium Occidentale L Probiotic Fermented from Lactobacillus acidophilus. Eur. J. Sustain. Dev. 2013, 2, 99–109. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Corbo, M.R.; Maddalena, L.; Sinigaglia, M. Suitability of Bifidobacterium Spp. and Lactobacillus plantarum as Probiotics Intended for Fruit Juices Containing Citrus Extracts. J. Food Sci. 2013, 78, M1764–M1771. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M. Fortification and Fermentation of Fruit Juices with Probiotic Lactobacilli. Ann. Microbiol. 2012, 62, 1573–1578. [Google Scholar] [CrossRef]
- Jost, S.; Herzig, C.; Birringer, M. A Balancing Act—20 Years of Nutrition and Health Claims Regulation in Europe: A Historical Perspective and Reflection. Foods 2025, 14, 1651. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Tee, E.S.; Hardinsyah; Au, C.S.S. Status of Probiotic Regulations in Southeast Asia Countries. Malays. J. Nutr. 2021, 27, 507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).