A Pilot Study on Single-Cell Raman Spectroscopy Combined with Machine Learning for Phenotypic Characterization of Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
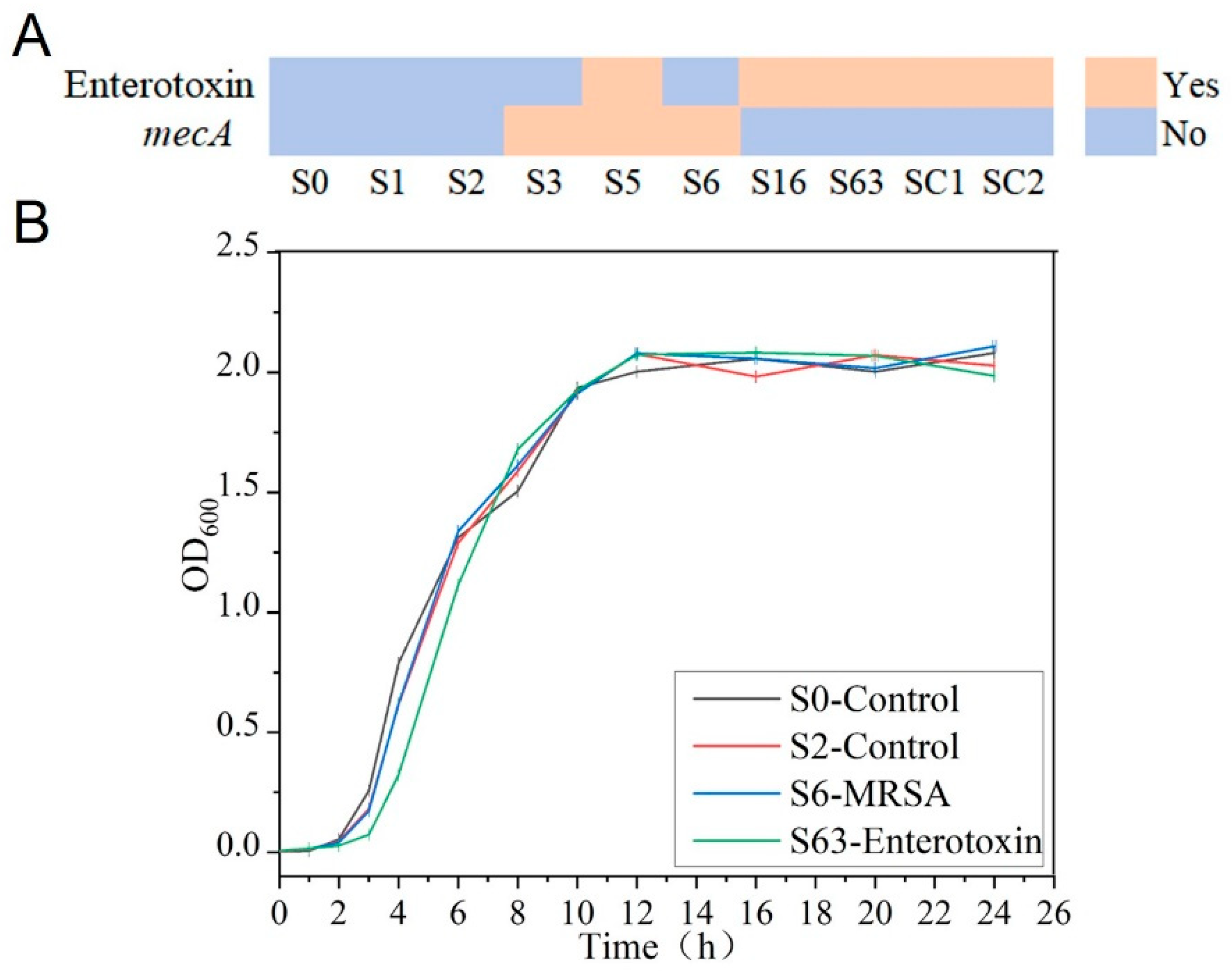
2.1. Experimental Strains
2.2. Enterotoxin Identification Experiment
2.3. Susceptibility Testing
2.4. Measurement of Experimental Strain Growth Curve
2.5. Single-Cell Raman Spectrum Acquisition
2.6. Pretreatment and Modeling of Single-Cell Raman Spectra
3. Result
3.1. Phenotypic Characteristics of the Experimental Strains
3.2. Construction of Raman Spectral Database of S. aureus
3.3. Raman Spectral Identification and Analysis of Enterotoxin-Producing and Non-Enterotoxin-Producing S. aureus
3.4. Raman Spectral Identification and Analysis of MRSA and MSSA Strains
3.5. Raman Spectral Identification and Analysis of S. aureus at Different Growth Stages
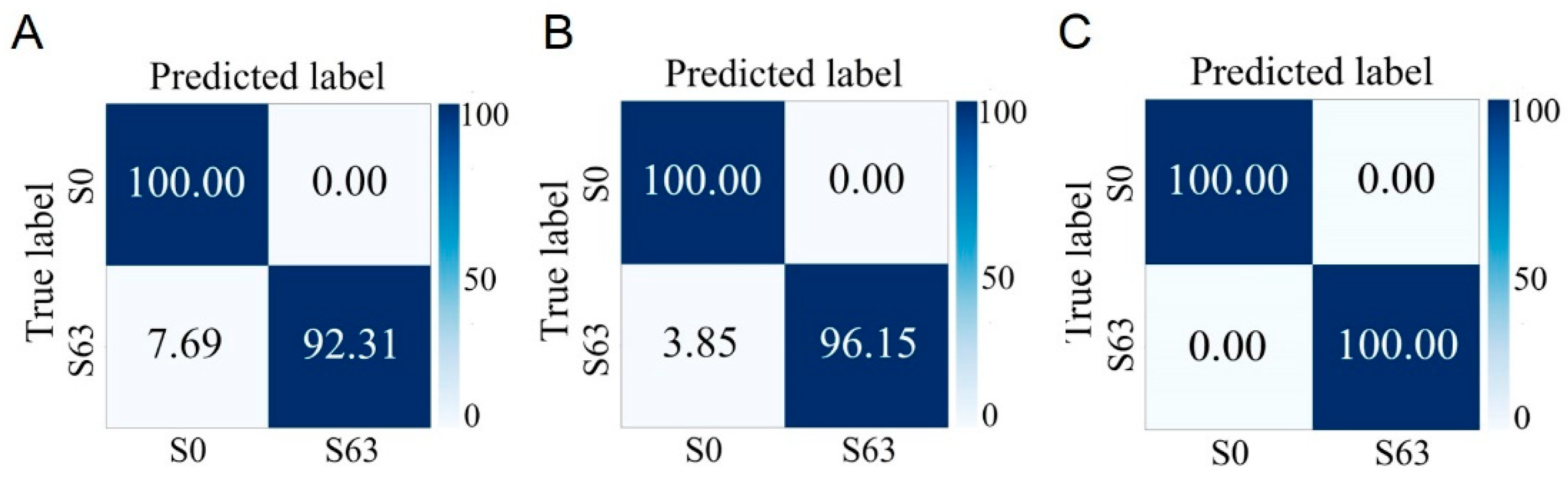
3.6. Identification of Different Phenotypes of S. aureus Cultured for Different Durations
4. Discussion
4.1. Analysis and Discussion of Enterotoxin-Producing and Non-Enterotoxin-Producing S. aureus
4.2. Analysis and discussion of MRSA and MSSA
4.3. Analysis and Discussion of S. aureus at Different Growth Stages
4.4. Analysis and Discussion on the Phenotypic Identification of S. aureus Cultured for Different Durations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Wang, J.; Jin, J.; Li, X.; Zhang, H.; Shi, X.; Zhao, C. Prevalence, Antibiotic Resistance, and Enterotoxin Genes of Staphylococcus aureus Isolated from Milk and Dairy Products Worldwide: A Systematic Review and Meta-Analysis. Food Res. Int. 2022, 162, 111969. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.A.C.; Ferreira, F.A.; Rubio Cieza, M.Y.; Silva, N.C.C.; Miotto, M.; Carvalho, M.M.; Bazzo, B.R.; Botelho, L.A.B.; Dias, R.S.; De Dea Lindner, J. Staphylococcus aureus Isolated from Traditional Artisanal Raw Milk Cheese from Southern Brazil: Diversity, Virulence, and Antimicrobial Resistance Profile. J. Food Prot. 2024, 87, 100285. [Google Scholar] [CrossRef] [PubMed]
- Cieza, M.Y.R.; Bonsaglia, E.C.R.; Rall, V.L.M.; dos Santos, M.V.; Silva, N.C.C. Staphylococcal Enterotoxins: Description and Importance in Food. Pathogens 2024, 13, 676. [Google Scholar] [CrossRef]
- Chen, W.; You, C.; Liu, Z. Progress in Research on Staphylococcus aureus Exotoxin. Dairy Sci. Technol. 2015, 38, 31–37. [Google Scholar] [CrossRef]
- Burillo, A.; Bouza, E. Community-Acquired Methicillin-Resistant Staphylococcus aureus: Is It Still a Significant Pathogen for Skin and Soft Tissue Infections? A 30-Year Overview. Curr. Opin. Infect. Dis. 2025, 38, 78–91. [Google Scholar] [CrossRef]
- Cui, K. Study on the Mechanism of Tanreqing Injection and Its Effective Components Synergistically Inhibiting Methicillin-Resistant Staphylococcus aureus with Antibiotics. Ph.D. Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2023. [Google Scholar]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qin, D.; Zhu, J.; Yang, X.; Lu, Z.; Ye, S.; Zhang, Y.; Yang, H.; Wang, Z.; Shen, J.; et al. Development and Validation of an ELISA Kit for the Detection of Staphylococcus aureus Enterotoxin A, B, C1, C2, C3, D, E from Food Samples. Food Control. 2024, 166, 110630. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, X.; Liu, Y.; Zhao, H.; Wang, G. Burden of Infectious Diseases and Bacterial Antimicrobial Resistance in China: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Reg. Health West. Pac. 2023, 43, 100972. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Leung, V.; Raybardhan, S.; Lo, J.; Kan, T.; Leung, F.; Westwood, D.; Daneman, N.; MacFadden, D.R.; et al. Predictors and Microbiology of Respiratory and Bloodstream Bacterial Infection in Patients with COVID-19: Living Rapid Review Update and Meta-Regression. Clin. Microbiol. Infect. 2022, 28, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Chauhan, A.; Kumar, A.; Kumar, M.; Kanchan, K. Synchronization of Mycobacterium Life Cycle: A Possible Novel Mechanism of Antimycobacterial Drug Resistance Evolution and Its Manipulation. Life Sci. 2024, 346, 122632. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, Z.; Tang, J.; Wang, A.; Zhao, D.; Yang, Y. Early Antimicrobial Evaluation of Nanostructured Surfaces Based on Bacterial Biological Properties. ACS Biomater. Sci. Eng. 2022, 8, 4976–4986. [Google Scholar] [CrossRef]
- Frempong, S.B.; Salbreiter, M.; Mostafapour, S.; Pistiki, A.; Bocklitz, T.W.; Rösch, P.; Popp, J. Illuminating the Tiny World: A Navigation Guide for Proper Raman Studies on Microorganisms. Molecules 2024, 29, 1077. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Kim, S.; Kim, S.-G.; Lee, J.; Lee, D.-G.; Jang, J.; Jeong, Y.-S.; Song, D.-H.; Min, J.-K.; Park, J.-G.; et al. A Sensitive Immunodetection Assay Using Antibodies Specific to Staphylococcal Enterotoxin B Produced by Baculovirus Expression. Biosensors 2022, 12, 787. [Google Scholar] [CrossRef]
- Le, N.T.; Hoang, P.H.; Van Dang, C.; Ho, T.H.; Le Hoang, P.; Truong, D.Q.; Thanh Nguyen, H.T.; Van Le, C.; Tinh Truong, T.T.; Tran, P.N.; et al. Antibiotic and Colistin Resistance Pattern of Salmonella Spp. Isolated from Pediatric Patients with Diarrhea in the Southern Region of Vietnam. New Microbes New Infect. 2025, 65, 101576. [Google Scholar] [CrossRef]
- Schönenbrücher, V.; Mallinson, E.T.; Bülte, M. A Comparison of Standard Cultural Methods for the Detection of Foodborne Salmonella Species Including Three New Chromogenic Plating Media. Int. J. Food. Microbiol. 2008, 123, 61–66. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Ke, Z.; Kan, N.; Zheng, E.; Qiu, Y.; Huang, M. Absolute Quantification of Viable Vibrio Cholerae in Seawater Samples Using Multiplex Droplet Digital PCR Combined with Propidium Monoazide. Front. Microbiol. 2023, 14, 1149981. [Google Scholar] [CrossRef]
- Guo, J.; Fan, F.; Wang, W.; Wan, M.; Li, Y. Development of PMA-qPCR Assay to Accurately and Reproducible Quantify Viable Bacteria of Paenibacillus polymyxa. Lett. Appl. Microbiol. 2023, 76, ovad127. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Xu, H.; Aguilar, Z.P.; Shah, N.P.; Wei, H. Propidium Monoazide Combined with Real-Time PCR for Selective Detection of Viable Staphylococcus aureus in Milk Powder and Meat Products. J. Dairy Sci. 2015, 98, 1625–1633. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, H.-H.; Chen, C.-H.; Tseng, K.-H.; Hsu, P.-C.; Wu, Y.-L.; Chang, W.-C.; Liao, N.-S.; Chou, Y.-F.; Hsu, C.-Y.; et al. Identification of Staphylococcus aureus, Enterococcus Faecium, Klebsiella Pneumoniae, Pseudomonas Aeruginosa and Acinetobacter Baumannii from Raman Spectra by Artificial Intelligent Raman Detection and Identification System (AIRDIS) with Machine Learning. J. Microbiol. Immunol. Infect. 2025, 58, 77–85. [Google Scholar] [CrossRef]
- Lu, W.; Li, H.; Qiu, H.; Wang, L.; Feng, J.; Fu, Y.V. Identification of Pathogens and Detection of Antibiotic Susceptibility at Single-Cell Resolution by Raman Spectroscopy Combined with Machine Learning. Front. Microbiol. 2023, 13, 1076965. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, X.; Lu, S.; Du, J. Shining a Light on Environmental Science: Recent Advances in SERS Technology for Rapid Detection of Persistent Toxic Substances. J. Environ. Sci. 2025, 153, 251–263. [Google Scholar] [CrossRef]
- Yan, S.; Wang, S.; Qiu, J.; Li, M.; Li, D.; Xu, D.; Li, D.; Liu, Q. Raman Spectroscopy Combined with Machine Learning for Rapid Detection of Food-Borne Pathogens at the Single-Cell Level. Talanta 2021, 226, 122195. [Google Scholar] [CrossRef]
- Xu, G.; Bao, Y.; Zhang, Y.; Xiang, X.; Luo, H.; Guo, X. Applying Machine Learning and SERS for Precise Typing of DNA Secondary Structures. Anal. Chem. 2024, 96, 17109–17117. [Google Scholar] [CrossRef] [PubMed]
- Colniță, A.; Dina, N.E.; Leopold, N.; Vodnar, D.C.; Bogdan, D.; Porav, S.A.; David, L. Characterization and Discrimination of Gram-Positive Bacteria Using Raman Spectroscopy with the Aid of Principal Component Analysis. Nanomaterials 2017, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.H.; Ciloglu, F.U.; Yilmaz, U.; Simsek, E.; Aydin, O. Discrimination of Waterborne Pathogens, Cryptosporidium Parvum Oocysts and Bacteria Using Surface-Enhanced Raman Spectroscopy Coupled with Principal Component Analysis and Hierarchical Clustering. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120475. [Google Scholar] [CrossRef]
- Kang, H.; Wang, Z.; Sun, J.; Song, S.; Cheng, L.; Sun, Y.; Pan, X.; Wu, C.; Gong, P.; Li, H. Rapid Identification of Bloodstream Infection Pathogens and Drug Resistance Using Raman Spectroscopy Enhanced by Convolutional Neural Networks. Front. Microbiol. 2024, 15, 1428304. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-W.; Zhang, X.D.; Tang, J.-W.; Zhao, Y.-H.; Liu, S.-L.; Zhao, Y.; Zhang, N.; Wang, D.; Ye, L.; Chen, X.-L.; et al. Rapid Prediction of Multidrug-Resistant Klebsiella Pneumoniae through Deep Learning Analysis of SERS Spectra. Microbiol. Spectrum 2023, 11, e04126-22. [Google Scholar] [CrossRef]
- GB 4789.10-2016; National Food Safety Standard Food Microbiological Examination for Staphylococcus aureus. China Food and Drug Administration: Beijing, China, 2016.
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing, 34th ed. CLSI: Wayne, PA, USA, 2024.
- Lu, W.; Chen, X.; Wang, L.; Li, H.; Fu, Y.V. Combination of an Artificial Intelligence Approach and Laser Tweezers Raman Spectroscopy for Microbial Identification. Anal. Chem. 2020, 92, 6288–6296. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.J.; Kim, J.H.; Chang, B.; Park, H.-K. Label-Free Detection for a DNA Methylation Assay Using Raman Spectroscopy. Chin. Med. J. 2017, 130, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Macgregor-Fairlie, M.; De Gomes, P.; Weston, D.; Rickard, J.J.S.; Goldberg Oppenheimer, P. Hybrid Use of Raman Spectroscopy and Artificial Neural Networks to Discriminate Mycobacterium Bovis BCG and Other Mycobacteriales. PLoS ONE 2023, 18, e0293093. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Balaji, S.A.; Bn, V.K.; Ariese, F.; Umapathy, S.; Rangarajan, A. Different Phases of Breast Cancer Cells: Raman Study of Immortalized, Transformed, and Invasive Cells. Biosensors 2016, 6, 57. [Google Scholar] [CrossRef]
- Rusciano, G.; Capriglione, P.; Pesce, G.; Del Prete, S.; Cennamo, G.; Di Cave, D.; Cerulli, L.; Sasso, A. Raman Microspectroscopy Analysis in the Treatment of Acanthamoeba Keratitis. PLoS ONE 2013, 8, e72127. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Li, M.; Jarvis, R.M.; Goodacre, R.; Banwart, S.A. Shining Light on the Microbial World the Application of Raman Microspectroscopy. Adv. Appl. Microbiol. 2010, 70, 153–186. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Raman Spectroscopy of Microbial Pigments. Appl. Environ. Microbiol. 2014, 80, 3286–3295. [Google Scholar] [CrossRef]
- Ayala, O.D.; Wakeman, C.A.; Pence, I.J.; Gaddy, J.A.; Slaughter, J.C.; Skaar, E.P.; Mahadevan-Jansen, A. Drug-Resistant Staphylococcus aureus Strains Reveal Distinct Biochemical Features with Raman Microspectroscopy. ACS Infect. Dis. 2018, 4, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Hu, Z.; Mustapha, A.; Lin, M. Rapid Detection of Food- and Waterborne Bacteria Using Surface-Enhanced Raman Spectroscopy Coupled with Silver Nanosubstrates. Appl. Microbiol. Biotechnol. 2011, 92, 1053–1061. [Google Scholar] [CrossRef]
- Sundaram, J.; Park, B.; Hinton, A.; Lawrence, K.C.; Kwon, Y. Detection and Differentiation of Salmonella Serotypes Using Surface Enhanced Raman Scattering (SERS) Technique. Food Meas. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Germond, A.; Ichimura, T.; Horinouchi, T.; Fujita, H.; Furusawa, C.; Watanabe, T.M. Raman Spectral Signature Reflects Transcriptomic Features of Antibiotic Resistance in Escherichia Coli. Commun. Biol. 2018, 1, 85. [Google Scholar] [CrossRef]
- Dong, P.; Mohammad, H.; Hui, J.; Leanse, L.G.; Li, J.; Liang, L.; Dai, T.; Seleem, M.N.; Cheng, J. Photolysis of Staphyloxanthin in Methicillin-resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species. Adv. Sci. 2019, 6, 1900030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xue, Y.; Yang, C.; Li, B.; Zhang, Y. Rapid Identification and Drug Resistance Screening of Respiratory Pathogens Based on Single-Cell Raman Spectroscopy. Front Microbiol 2023, 14, 1065173. [Google Scholar] [CrossRef] [PubMed]
- Strola, S.A.; Baritaux, J.-C.; Schultz, E.; Simon, A.C.; Allier, C.; Espagnon, I.; Jary, D.; Dinten, J.-M. Single Bacteria Identification by Raman Spectroscopy. J. Biomed. Opt. 2014, 19, 111610. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Kong, L.; Wang, Y.; Zhu, Y.; Zhang, C. In Situ Identification of Environmental Microorganisms with Raman Spectroscopy. Environ. Sci. Ecotechnol. 2022, 11, 100187. [Google Scholar] [CrossRef]
- Dantas, S.T.A.; Silva, L.B.B.; Takume, L.T.S.; Rossi, B.F.; Bonsaglia, E.C.R.; Fernandes Júnior, A.; Pantoja, J.C.F.; Dos Santos, M.V.; Gonçalves, J.L.; Ribon, A.O.B.; et al. Diversity of Staphylococcus Aureus Enterotoxin Genes and Its Potential Impact on Severity of Mastitis in Dairy Cows. Microb. Pathogen. 2025, 198, 107119. [Google Scholar] [CrossRef]
- Jin, L.; Mu, Q.; Zhang, Q.; Li, K.; Wang, Y.; Jiang, Z.; Yan, Y.; He, D.; Zhu, L.; Li, M.; et al. Temperature-Enhanced Purine Metabolism-Based Versatile SERS Platform for Rapid Clinical Pathogens Diagnosis and Drug-Resistant Assessment. Anal. Chem. 2025, 97, 2754–2761. [Google Scholar] [CrossRef]
- Ogunlade, B.; Tadesse, L.F.; Li, H.; Vu, N.; Banaei, N.; Barczak, A.K.; Saleh, A.A.E.; Prakash, M.; Dionne, J.A. Rapid, Antibiotic Incubation-Free Determination of Tuberculosis Drug Resistance Using Machine Learning and Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2315670121. [Google Scholar] [CrossRef]
- Tahseen, H.; Ul Huda, N.; Nawaz, H.; Majeed, M.I.; Alwadie, N.; Rashid, N.; Aslam, M.A.; Zafar, N.; Asghar, M.; Anwar, A.; et al. Surface-Enhanced Raman Spectroscopy for Comparison of Biochemical Profile of Bacteriophage Sensitive and Resistant Methicillin-Resistant Staphylococcus aureus (MRSA) Strains. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123968. [Google Scholar] [CrossRef]
- Ho, C.-S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid Identification of Pathogenic Bacteria Using Raman Spectroscopy and Deep Learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef]
- Wulf, M.W.H.; Willemse-Erix, D.; Verduin, C.M.; Puppels, G.; van Belkum, A.; Maquelin, K. The Use of Raman Spectroscopy in the Epidemiology of Methicillin-Resistant Staphylococcus aureus of Human- and Animal-Related Clonal Lineages. Clin. Microbiol. Infect. 2012, 18, 147–152. [Google Scholar] [CrossRef]
- Ciloglu, F.U.; Caliskan, A.; Saridag, A.M.; Kilic, I.H.; Tokmakci, M.; Kahraman, M.; Aydin, O. Drug-Resistant Staphylococcus aureus Bacteria Detection by Combining Surface-Enhanced Raman Spectroscopy (SERS) and Deep Learning Techniques. Sci. Rep. 2021, 11, 18444. [Google Scholar] [CrossRef] [PubMed]
- Aros-Calt, S.; Muller, B.H.; Boudah, S.; Ducruix, C.; Gervasi, G.; Junot, C.; Fenaille, F. Annotation of the Staphylococcus aureus Metabolome Using Liquid Chromatography Coupled to High-Resolution Mass Spectrometry and Application to the Study of Methicillin Resistance. J. Proteome Res. 2015, 14, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Sachdeva, M. Binding of Beta-Lactam Antibiotics to Penicillin-Binding Proteins in Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 1990, 161, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.S.; Goto, M.; Hammer, K.D.P. Evaluating the Performance of the Alere PBP2a SA Culture Colony Test with the Vitek 2 Antimicrobial Susceptibility Test Card System as Reference Standard in Coagulase-Negative Staphylococcus Species. Infect. Med. 2024, 3, 100126. [Google Scholar] [CrossRef]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and Genomic Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Exposed to Manuka Honey in Vitro Demonstrated down-Regulation of Virulence Markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef]
- Busche, T.; Hillion, M.; Van Loi, V.; Berg, D.; Walther, B.; Semmler, T.; Strommenger, B.; Witte, W.; Cuny, C.; Mellmann, A.; et al. Comparative Secretome Analyses of Human and Zoonotic Staphylococcus aureus Isolates CC8, CC22, and CC398. Mol. Cell. Proteomics. 2018, 17, 2412–2433. [Google Scholar] [CrossRef]
- Pistiki, A.; Monecke, S.; Shen, H.; Ryabchykov, O.; Bocklitz, T.W.; Rösch, P.; Ehricht, R.; Popp, J. Comparison of Different Label-Free Raman Spectroscopy Approaches for the Discrimination of Clinical MRSA and MSSA Isolates. Microbiol. Spectr. 2022, 10, e0076322. [Google Scholar] [CrossRef]
- Lorenz, B.; Wichmann, C.; Stöckel, S.; Rösch, P.; Popp, J. Cultivation-Free Raman Spectroscopic Investigations of Bacteria. Trends Microbiol. 2017, 25, 413–424. [Google Scholar] [CrossRef]
- Ren, Y.; Ji, Y.; Teng, L.; Zhang, H. Using Raman Spectroscopy and Chemometrics to Identify the Growth Phase of Lactobacillus Casei Zhang during Batch Culture at the Single-Cell Level. Microb. Cell Fact. 2017, 16, 233. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Huang, J.; Wu, Q.; Zhang, J.; Dai, J.; Zeng, H.; Yang, X.; Chen, M.; Pang, R.; et al. Phenotypic and Genotypic Characterization of PVL-Positive Staphylococcus aureus Isolated from Retail Foods in China. Int. J. Food Microbiol. 2019, 304, 119–126. [Google Scholar] [CrossRef]
- Allen, D.M.; Einarsson, G.G.; Tunney, M.M.; Bell, S.E.J. Characterization of Bacteria Using Surface-Enhanced Raman Spectroscopy (SERS): Influence of Microbiological Factors on the SERS Spectra. Anal. Chem. 2022, 94, 9327–9335. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.K.; Baral, R.; Van Vlack, E.R.; Gil-Marqués, M.L.; Lenhart, T.; Hooper, D.C.; Kahne, D.; Losick, R.; Bradshaw, N. Biofilm Formation by Staphylococcus aureus Is Triggered by a Drop in the Levels of a Cyclic Dinucleotide. Proc. Natl. Acad. Sci. USA 2024, 121, e2417323121. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, L.; Wang, P.; Meng, G. Structural Basis of Host Recognition and Biofilm Formation by Salmonella Saf Pili. eLife 2017, 6, e28619. [Google Scholar] [CrossRef] [PubMed]
- Moritz, T.J.; Taylor, D.S.; Polage, C.R.; Krol, D.M.; Lane, S.M.; Chan, J.W. Effect of Cefazolin Treatment on the Nonresonant Raman Signatures of the Metabolic State of Individual Escherichia Coli Cells. Anal. Chem. 2010, 82, 2703–2710. [Google Scholar] [CrossRef]


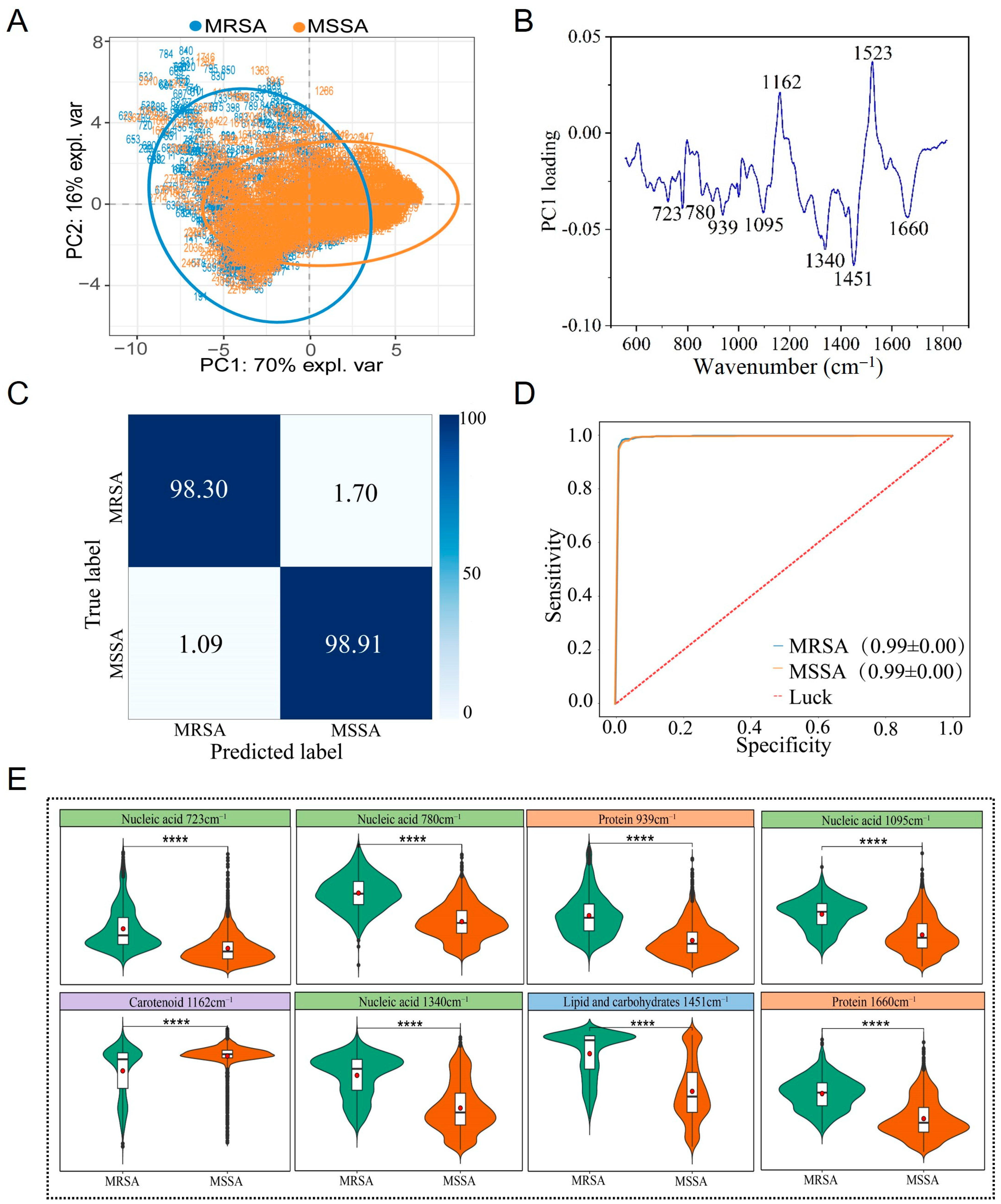
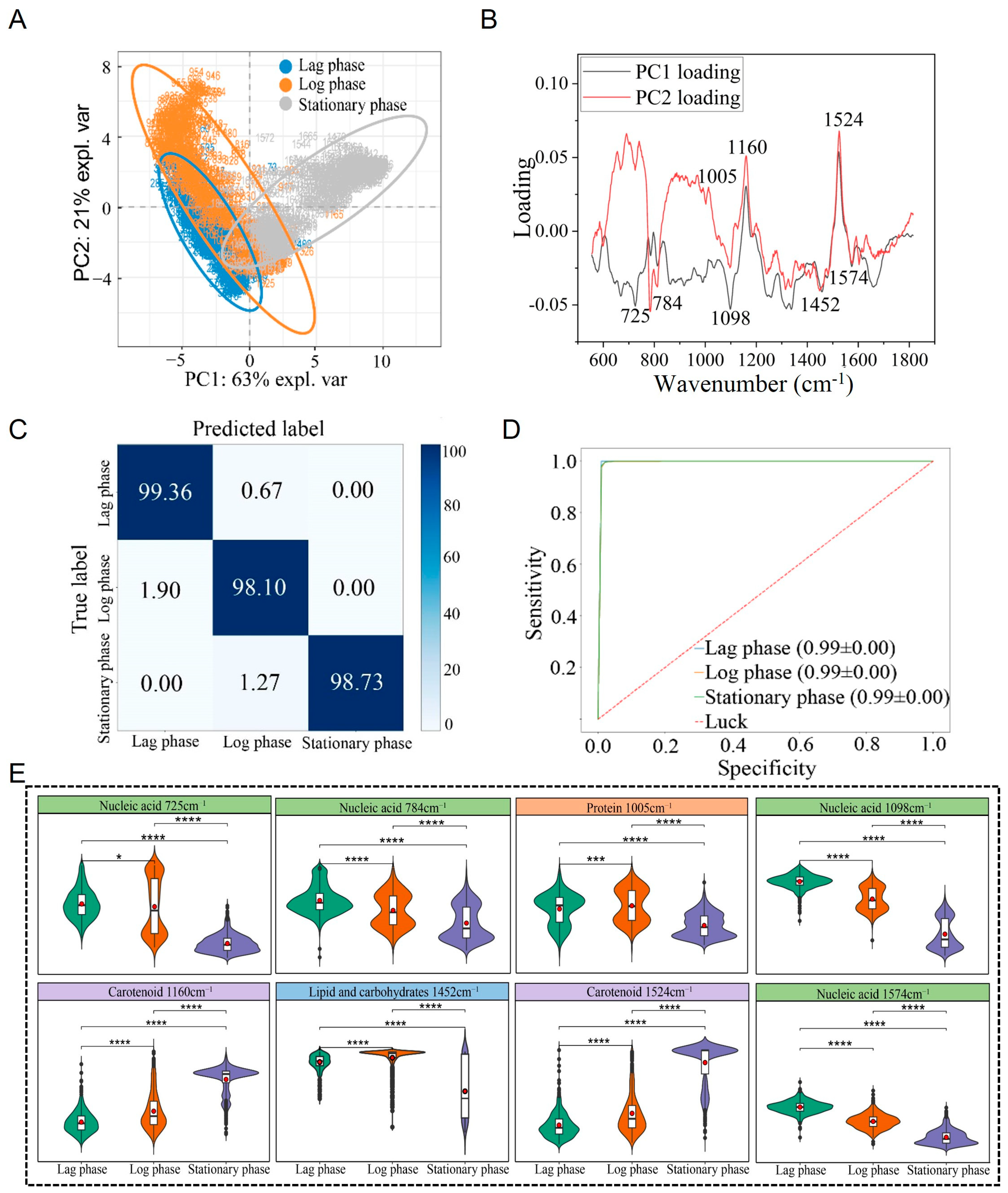

| Wavenumber (cm−1) | Component/Wavenumber (cm−1) | Reference |
|---|---|---|
| 723/725 | Adenine ring breathing, nucleic acid (724) | [34] |
| 780/781/784 | Uracil-based ring breathing, thymine (780), DNA (783), nucleic acid (785) | [34,35] |
| 939 | Protein (936) | [36,37] |
| 1005 | Protein | [38,39] |
| 1095/1098 | Nucleic acid (1095) | [36] |
| 1160–1162 | Carotenoid (1159) | [40] |
| 1337/1340 | Guanine, nucleic acid (1336) | [34] |
| 1451/1452 | Lipid and carbohydrates | [41,42,43] |
| 1523–1525 | Carotenoid (1523) | [40,44] |
| 1574 | Guanine, adenine of nucleic acid (1573/1575) | [34,45,46] |
| 1660 | Amide I, protein | [35,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Xue, J.; Song, Y.; Zhan, T.; Liu, Y.; Song, X.; Mei, L.; Wang, D.; Fu, Y.V.; Wei, Q. A Pilot Study on Single-Cell Raman Spectroscopy Combined with Machine Learning for Phenotypic Characterization of Staphylococcus aureus. Microorganisms 2025, 13, 1333. https://doi.org/10.3390/microorganisms13061333
Liu L, Xue J, Song Y, Zhan T, Liu Y, Song X, Mei L, Wang D, Fu YV, Wei Q. A Pilot Study on Single-Cell Raman Spectroscopy Combined with Machine Learning for Phenotypic Characterization of Staphylococcus aureus. Microorganisms. 2025; 13(6):1333. https://doi.org/10.3390/microorganisms13061333
Chicago/Turabian StyleLiu, Li, Junjing Xue, Yang Song, Taijie Zhan, Yang Liu, Xiaohui Song, Li Mei, Duochun Wang, Yu Vincent Fu, and Qiang Wei. 2025. "A Pilot Study on Single-Cell Raman Spectroscopy Combined with Machine Learning for Phenotypic Characterization of Staphylococcus aureus" Microorganisms 13, no. 6: 1333. https://doi.org/10.3390/microorganisms13061333
APA StyleLiu, L., Xue, J., Song, Y., Zhan, T., Liu, Y., Song, X., Mei, L., Wang, D., Fu, Y. V., & Wei, Q. (2025). A Pilot Study on Single-Cell Raman Spectroscopy Combined with Machine Learning for Phenotypic Characterization of Staphylococcus aureus. Microorganisms, 13(6), 1333. https://doi.org/10.3390/microorganisms13061333








