The Application and Challenges of Brain Organoids in Exploring the Mechanism of Arbovirus Infection
Abstract
1. Introduction
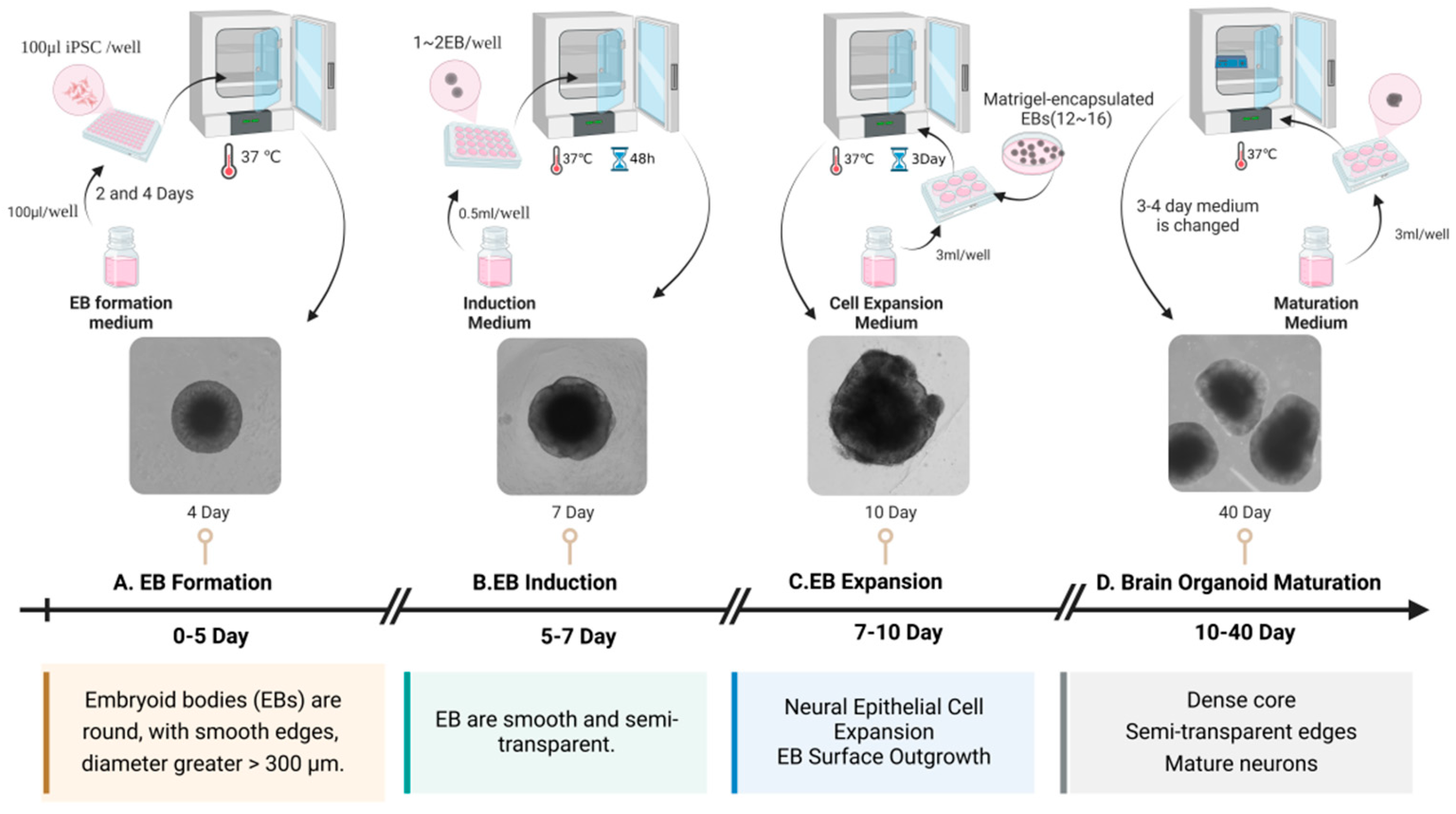
2. Development of Brain Organoids
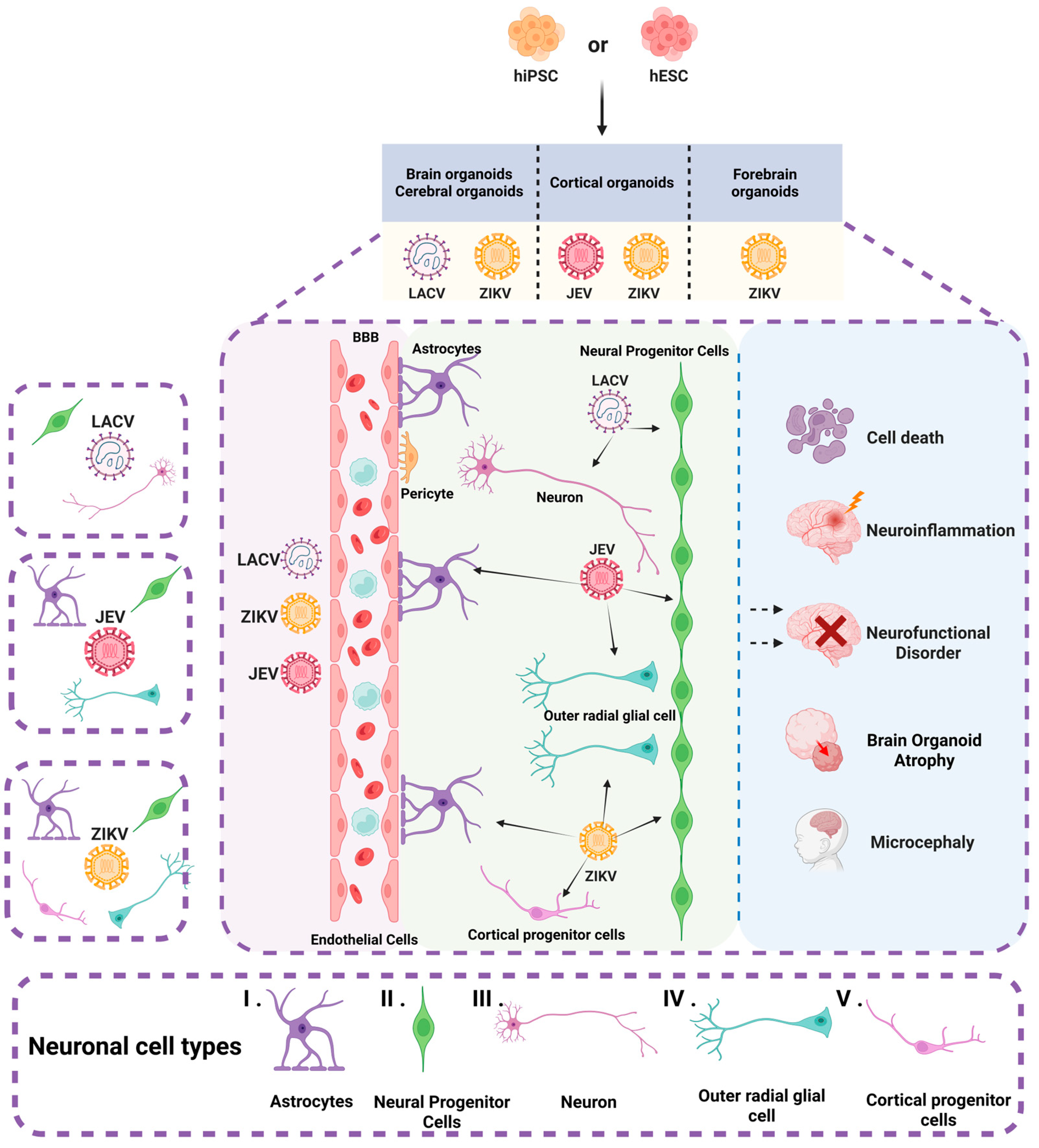
3. Application of Brain Organoids in the Study of Arbovirus Infection Mechanism
3.1. ZIKV Infection Model
3.2. JEV Infection Model
3.3. LACV Infection Model
4. The Limitation of Brain Organoids in Arbovirus Research: Challenges and Implications
4.1. Heterogeneity of Brain Organoids
4.2. Immunodeficiency in Brain Organoids
4.3. Short Lifespan of Brain Organoids
4.4. Epistemological Limitations
4.5. Ethical Controversy
5. Future Directions
5.1. Establish a Standardized Culture System
5.2. Engineering the Immune Microenvironment and Modeling the BBB
5.3. Constructing Vascularized Brain Organoids
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Liu, K.; Guo, D.; Lv, Y.; Wang, X. Arbovirus Infections and Epigenetic Mechanisms; a Potential Therapeutic Target. Rev. Med. Virol. 2025, 35, e70033. [Google Scholar] [CrossRef] [PubMed]
- Karabatsos, N. International Catalogue of Arboviruses: Including Certain Other Viruses of Vertebrates, 3rd ed.; Published for the Subcommittee on Information Exchange of the American Committee on Arthropod-borne Viruses; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Kang, L.; Li, Y.; Cheng, G.; Cui, F.; Zhang, L.; Zhang, X.; Wu, Y.; Zhao, W.; Huo, Y.; Du, H.; et al. Infection, Transmission, and Cross-kingdom immune Adaption of Arboviruses. Bull. Natl. Nat. Sci. Found. China 2023, 37, 1011–1020. [Google Scholar] [CrossRef]
- WHO. Japanese Encephalitis. Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 6 August 2024).
- Bangoura, S.T.; Keita, A.K.; Diaby, M.; Sidibé, S.; Le-Marcis, F.; Camara, S.C.; Maltais, S.; Kadio, K.; D’Ortenzio, E.; Camara, A.; et al. Arbovirus Epidemics as Global Health Imperative, Africa, 2023. Emerg. Infect. Dis. 2025, 31, 1–8. [Google Scholar] [CrossRef]
- Salimi, H.; Cain, M.D.; Klein, R.S. Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis. Neurotherapeutics 2016, 13, 514–534. [Google Scholar] [CrossRef]
- Rust, R.S.; Thompson, W.H.; Matthews, C.G.; Beaty, B.J.; Chun, R.W. La Crosse and other forms of California encephalitis. J. Child Neurol. 1999, 14, 1–14. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Faye, O.; Diallo, M.; Diop, D.; Bezeid, O.E.; Bâ, H.; Niang, M.; Dia, I.; Mohamed, S.A.; Ndiaye, K.; Diallo, D.; et al. Rift Valley fever outbreak with East-Central African virus lineage in Mauritania, 2003. Emerg. Infect. Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Casel, M.A.; Park, S.J.; Choi, Y.K. Severe fever with thrombocytopenia syndrome virus: Emerging novel phlebovirus and their control strategy. Exp. Mol. Med. 2021, 53, 713–722. [Google Scholar] [CrossRef]
- van de Beek, D.; Brouwer, M.C. 2016, the year of Zika virus. Nat. Rev. Neurol. 2017, 13, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.M.; Abreu, F.V.S.d.; Santos, A.A.C.d.; Mello, I.S.d.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Miranda, R.M.d.; Castro, M.G.d.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016–2017 Brazilian outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef]
- Shoemaker, T.R.; Nyakarahuka, L.; Balinandi, S.; Ojwang, J.; Tumusiime, A.; Mulei, S.; Kyondo, J.; Lubwama, B.; Sekamatte, M.; Namutebi, A.; et al. First Laboratory-Confirmed Outbreak of Human and Animal Rift Valley Fever Virus in Uganda in 48 Years. Am. J. Trop. Med. Hyg. 2019, 100, 659–671. [Google Scholar] [CrossRef]
- Nguyen, W.; Gyawali, N.; Stewart, R.; Tang, B.; Cox, A.L.; Yan, K.; Larcher, T.; Bishop, C.R.; Wood, N.; Devine, G.J.; et al. Characterisation of a Japanese Encephalitis virus genotype 4 isolate from the 2022 Australian outbreak. Npj Viruses 2024, 2, 15. [Google Scholar] [CrossRef]
- WHO. Dengue—Global Situation.pdf. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 21 December 2023).
- WHO. Oropouche Virus Disease—Region of the Americas. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON530 (accessed on 23 August 2024).
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020, 182, 734–743.e5. [Google Scholar] [CrossRef]
- Wagar, L.E.; DiFazio, R.M.; Davis, M.M. Advanced model systems and tools for basic and translational human immunology. Genome Med. 2018, 10, 73. [Google Scholar] [CrossRef]
- Yu, D.; Cao, H.; Wang, X. Advances and applications of organoids: A review. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2021, 37, 3961–3974. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-–bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19, 258–265. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Wernig, M.; Duncan, I.D.; Brüstle, O.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kamiya, D.; Nishiyama, A.; Katayama, T.; Nozaki, S.; Kawasaki, H.; Watanabe, Y.; Mizuseki, K.; Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005, 8, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Zhou, T.; Tan, L.; Cederquist, G.Y.; Fan, Y.; Hartley, B.J.; Mukherjee, S.; Tomishima, M.; Brennand, K.J.; Zhang, Q.; Schwartz, R.E.; et al. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell Stem Cell 2017, 21, 274–283.e5. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Xu, Y.; Mo, F.; Mi, T.; Shen, Q.S.; Li, C.; Li, Y.; Liu, J.; Wu, Y.; et al. Differential antiviral immunity to Japanese encephalitis virus in developing cortical organoids. Cell Death Dis. 2018, 9, 719. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e5. [Google Scholar] [CrossRef]
- Gumbs, S.B.H.; Berdenis van Berlekom, A.; Kübler, R.; Schipper, P.J.; Gharu, L.; Boks, M.P.; Ormel, P.R.; Wensing, A.M.J.; de Witte, L.D.; Nijhuis, M. Characterization of HIV-1 Infection in Microglia-Containing Human Cerebral Organoids. Viruses 2022, 14, 829. [Google Scholar] [CrossRef]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.Y.; Zhang, N.N.; Watanabe, M.; Dong, H.L.; Liu, P.; et al. 25-–Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Ayala-Nunez, N.V.; Follain, G.; Delalande, F.; Hirschler, A.; Partiot, E.; Hale, G.L.; Bollweg, B.C.; Roels, J.; Chazal, M.; Bakoa, F.; et al. Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells. Nat. Commun. 2019, 10, 4430. [Google Scholar] [CrossRef]
- Sacramento, C.Q.; de Melo, G.R.; de Freitas, C.S.; Rocha, N.; Hoelz, L.V.; Miranda, M.; Fintelman-Rodrigues, N.; Marttorelli, A.; Ferreira, A.C.; Barbosa-Lima, G.; et al. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci. Rep. 2017, 7, 40920. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, C.; Souza, L.R.Q.; Gomes, T.A.; de Lima, C.V.F.; Ledur, P.F.; Karmirian, K.; Barbeito-Andres, J.; Costa, M.D.N.; Higa, L.M.; Rossi, Á.D.; et al. The cyanobacterial saxitoxin exacerbates neural cell death and brain malformations induced by Zika virus. PLoS Neglected Trop. Dis. 2020, 14, e0008060. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Schotsaert, M.; Karnik, R.; Balasubramaniam, V.; Dejosez, M.; Meissner, A.; García-Sastre, A.; Zwaka, T.P. Zika Virus Alters DNA Methylation of Neural Genes in an Organoid Model of the Developing Human Brain. mSystems 2018, 3, e00219-17. [Google Scholar] [CrossRef] [PubMed]
- Salick, M.R.; Wells, M.F.; Eggan, K.; Kaykas, A. Modelling Zika Virus Infection of the Developing Human Brain In Vitro Using Stem Cell Derived Cerebral Organoids. J. Vis. Exp. 2017, 127, 56404. [Google Scholar] [CrossRef]
- Xu, Y.P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.R.; et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-–Buzhala, G.; Krut, O.; Peters, F.; Nikolic, M.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 2017, 20, 397–406.e5. [Google Scholar] [CrossRef]
- Slonchak, A.; Chaggar, H.; Aguado, J.; Wolvetang, E.; Khromykh, A.A. Noncoding RNA of Zika Virus Affects Interplay between Wnt-Signaling and Pro-Apoptotic Pathways in the Developing Brain Tissue. Viruses 2023, 15, 1062. [Google Scholar] [CrossRef]
- Cavalcante, B.R.R.; Aragão-França, L.S.; Sampaio, G.L.A.; Nonaka, C.K.V.; Oliveira, M.S.; Campos, G.S.; Sardi, S.I.; Dias, B.R.S.; Menezes, J.P.B.; Rocha, V.P.C.; et al. Betulinic Acid Exerts Cytoprotective Activity on Zika Virus-Infected Neural Progenitor Cells. Front. Cell. Infect. Microbiol. 2020, 10, 558324. [Google Scholar] [CrossRef]
- Winkler, C.W.; Woods, T.A.; Groveman, B.R.; Carmody, A.B.; Speranza, E.E.; Martens, C.A.; Best, S.M.; Haigh, C.L.; Peterson, K.E. Neuronal maturation reduces the type I IFN response to orthobunyavirus infection and leads to increased apoptosis of human neurons. J. Neuroinflammation 2019, 16, 229. [Google Scholar] [CrossRef]
- Wells, M.F.; Salick, M.R.; Wiskow, O.; Ho, D.J.; Worringer, K.A.; Ihry, R.J.; Kommineni, S.; Bilican, B.; Klim, J.R.; Hill, E.J.; et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 2016, 19, 703–708. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S.; Pierson, T.C. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91, e00009-17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Calegari, F.; Haubensak, W.; Haffner, C.; Huttner, W.B. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 6533–6538. [Google Scholar] [CrossRef]
- Gabriel, E.; Wason, A.; Ramani, A.; Gooi, L.M.; Keller, P.; Pozniakovsky, A.; Poser, I.; Noack, F.; Telugu, N.S.; Calegari, F.; et al. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 2016, 35, 803–819. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Krenn, V.; Bosone, C.; Burkard, T.R.; Spanier, J.; Kalinke, U.; Calistri, A.; Salata, C.; Rilo Christoff, R.; Pestana Garcez, P.; Mirazimi, A.; et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 2021, 28, 1362–1379.e7. [Google Scholar] [CrossRef]
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Furlong, M.; Adamu, A.M.; Hoskins, A.; Russell, T.L.; Gummow, B.; Golchin, M.; Hickson, R.I.; Horwood, P.F. Japanese Encephalitis Enzootic and Epidemic Risks across Australia. Viruses 2023, 15, 450. [Google Scholar] [CrossRef]
- Khan, U.M.; Gudlavalleti, A. La Crosse Encephalitis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562248/ (accessed on 8 August 2023).
- McCarter, M.; Self, S.C.W.; Li, H.; Ewing, J.A.; Gual-–Gonzalez, L.; Kanyangarara, M.; Nolan, M.S. Validating a Bayesian Spatio-Temporal Model to Predict La Crosse Virus Human Incidence in the Appalachian Mountain Region, USA. Microorganisms 2025, 13, 812. [Google Scholar] [CrossRef]
- Narasipura, S.D.; Zayas, J.P.; Ash, M.K.; Reyes, A.F.; Shull, T.; Gambut, S.; Szczerkowski, J.L.A.; McKee, C.; Schneider, J.R.; Lorenzo-Redondo, R.; et al. Inflammatory responses revealed through HIV infection of microglia-containing cerebral organoids. J. Neuroinflammation 2025, 22, 36. [Google Scholar] [CrossRef]
- Dos Reis, R.S.; Sant, S.; Keeney, H.; Wagner, M.C.E.; Ayyavoo, V. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci. Rep. 2020, 10, 15209. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, R.S.; Wagner, M.C.E.; McKenna, S.; Ayyavoo, V. Neuroinflammation driven by human immunodeficiency virus-1 (HIV-1) directs the expression of long noncoding RNA RP11-677M14.2 resulting in dysregulation of neurogranin in vivo and in vitro. J. Neuroinflammation 2024, 21, 107. [Google Scholar] [CrossRef]
- Song, L.; Yan, Y.; Marzano, M.; Li, Y. Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration. Cells 2019, 8, 299. [Google Scholar] [CrossRef]
- Werner, J.M.; Gillis, J. Meta-analysis of single-cell RNA sequencing co-expression in human neural organoids reveals their high variability in recapitulating primary tissue. PLoS Biol. 2024, 22, e3002912. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Feng, L.; Tian, E.; Shi, Y. Human iPSC-derived microglial cells protect neurons from neurodegeneration in long-term cultured adhesion brain organoids. Commun. Biol. 2025, 8, 30. [Google Scholar] [CrossRef]
- Popova, G.; Soliman, S.S.; Kim, C.N.; Keefe, M.G.; Hennick, K.M.; Jain, S.; Li, T.; Tejera, D.; Shin, D.; Chhun, B.B.; et al. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell 2021, 28, 2153–2166.e6. [Google Scholar] [CrossRef]
- Lin, W.; Shiomoto, S.; Yamada, S.; Watanabe, H.; Kawashima, Y.; Eguchi, Y.; Muramatsu, K.; Sekino, Y. Dendritic spine formation and synapse maturation in transcription factor-induced human iPSC-derived neurons. iScience 2023, 26, 106285. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Berishvili, E.; Fonseca, L.M.; Lebreton, F.; Bellofatto, K.; Bignard, J.; Seissler, J.; Buerck, L.W.-v.; Honarpisheh, M.; et al. Organoids: A systematic review of ethical issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Hyun, I.; Scharf-Deering, J.C.; Lunshof, J.E. Ethical issues related to brain organoid research. Brain Res. 2020, 1732, 146653. [Google Scholar] [CrossRef]
- Arnason, G.; Pichl, A.; Ranisch, R. Ethical Issues in Cerebral Organoid Research. Camb. Q. Healthc. Ethics 2023, 32, 515–517. [Google Scholar] [CrossRef]
- Hartung, T.; Morales Pantoja, I.E.; Smirnova, L. Brain organoids and organoid intelligence from ethical, legal, and social points of view. Front. Artif. Intell. 2024, 6, 1307613. [Google Scholar] [CrossRef] [PubMed]
- Ethics need to keep up with human brain organoid research. Nat. Rev. Bioeng. 2024, 2, 711. [CrossRef]
- Di Stefano, J.; Di Marco, F.; Cicalini, I.; FitzGerald, U.; Pieragostino, D.; Verhoye, M.; Ponsaerts, P.; Van Breedam, E. Generation, interrogation, and future applications of microglia-containing brain organoids. Neural Regen. Res. 2025, 20, 3448–3460. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.Y.; Koo, D.J.; Yu, W.; et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Kübler, R.; Missall, R.; De Jong, R.; Chan, S.; Haage, V.; Wendt, S.; Lin, A.J.; Mattei, D.; Graziani, M.; et al. A microglia-containing cerebral organoid model to study early life immune challenges. bioRxiv, 2024; Preprint. [Google Scholar] [CrossRef]
- Xiang, D.; He, A.; Zhou, R.; Wang, Y.; Xiao, X.; Gong, T.; Kang, W.; Lin, X.; Wang, X.; Liu, L.; et al. Building consensus on the application of organoid-based drug sensitivity testing in cancer precision medicine and drug development. Theranostics 2024, 14, 3300–3316. [Google Scholar] [CrossRef]
- FDA. FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs. Available online: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs (accessed on 10 April 2025).

| Virus | Disease | Origin | Types of Brain Organoids | Infected Cells | Key Factors | Key Findings | Limitation of Brain Organoids | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZIKV | Microcephaly | hESC | Brain organoids * | Neural progenitor cells (NPCs) Outer radial glial cells (oRGCs) | Toll-like receptor 3 (TLR3) | Inhibition of TLR3 activity attenuates neuronal apoptosis brain-like organ contraction | II, III | [24] |
| ZIKV | Microcephaly | hESC | Brain organoids * | Cortical progenitor cells | / | ZIKV damages cortical areas of brain-like organs by infecting cortical progenitor cells, impairing neurodevelopment and leading to microcephaly | I, II, III | [32] |
| ZIKV | Microcephaly | hESC | Cortical organoids | / | 25-hydroxycholesterol (25HC) | 25HC inhibits ZIKV infection and protects human cortical organoid mouse embryonic brain tissue | II, III | [33] |
| ZIKV | Microcephaly | hESC | Cerebral organoids * | / | Monocytes | ZIKV induces monocyte migration and facilitates virus dissemination in neuronal cells | I, III | [34] |
| ZIKV | Microcephaly | hiPSC | Forebrain-specific organoids | NPCs oRGCs | / | ZIKV infection causes NPC death and reduced neuronal layer thickness | I, II, III | [23] |
| ZIKV | Microcephaly | hiPSC | Fetal-like forebrain organoids | NPCs | Hippeastrine hydrobromide (HH) Amodiaquinedihydr -ochloride dihydrate (AQ) | HH and AQ inhibit ZIKV infection and HH repairs ZIKV-induced Fetal-like forebrain organoid growth defects and differentiation | I, II | [28] |
| ZIKV | Microcephaly | hiPSC | Brain organoids * | / | Sofosbuvir | Sofosbuvir inhibition of ZIKV RNA polymerase attenuates replication in brain-like organs | I, II, III | [35] |
| ZIKV | Microcephaly | hiPSC | Brain organoids * | NPCs | Saxitoxin (STX) | STX promotes ZIKV infection of brain organoids and increases NPC death | I, II, III | [36] |
| ZIKV | Microcephaly | hESC | Brain organoids * | / | / | ZIKV infection alters DNA methylation in neural precursor cells, astrocytes, and differentiated neurons in human brain organoids | I, II, III | [37] |
| ZIKV | Microcephaly | hESC/hiPSC | Cerebral organoids * | NPCs | Severe cell death after ZIKV infection | II, III | [38] | |
| ZIKV | Microcephaly | hESC | Brain organoids * | NPCs | RNA interference (RNAi) Enoxacin | RNAi mechanism inhibits ZIKV replication in hNPCs Enoxacin inhibits the ZIKV invasion of brain organoids | II, III | [39] |
| ZIKV | Microcephaly | hiPSC | Brain organoids * | NPCs | / | ZIKV strain FB-gweh-2016 and H/PF/2013 are able to cause the premature differentiation of neural precursor cells, the disruption of neurogenesis, and thinning of the cortex | II, III | [40] |
| ZIKV | Microcephaly | hiPSC | Brain organoids * | / | sfRNA | ZIKV sfRNA affects brain development through the Wnt signaling pathway and pro-apoptotic pathway | II, III | [41] |
| ZIKV | Microcephaly | hiPSC | Cerebral organoids * | / | Betulinic acid (BA) | BA inhibits neuronal cell death in ZIKV-infected brain organoids and protects the structural integrity of brain organoids. | I, II, III | [42] |
| JEV | Japanese encephalitis (JE) | hESC | Cortical organoids | NPCs oRGCs | Interferon (IFN) RIG-I | JEV infection can upregulate the expression of RIG-I and induce the expression of IFN-β. | I, II, III | [29] |
| LACV | La Crosse encephalitis (LCE) | hiPSC | Cerebral organoids * | Committed neurons | IFN | Weak interferon response in committed neurons is more sensitive to LACV | II, III | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Wang, Z.; Farid, A.; Wang, Z.; Wei, K.; Ren, N.; Yang, F.; Liu, H. The Application and Challenges of Brain Organoids in Exploring the Mechanism of Arbovirus Infection. Microorganisms 2025, 13, 1281. https://doi.org/10.3390/microorganisms13061281
Cui B, Wang Z, Farid A, Wang Z, Wei K, Ren N, Yang F, Liu H. The Application and Challenges of Brain Organoids in Exploring the Mechanism of Arbovirus Infection. Microorganisms. 2025; 13(6):1281. https://doi.org/10.3390/microorganisms13061281
Chicago/Turabian StyleCui, Baoqiu, Zhijie Wang, Anum Farid, Zeyu Wang, Kaiyue Wei, Naixia Ren, Fengtang Yang, and Hong Liu. 2025. "The Application and Challenges of Brain Organoids in Exploring the Mechanism of Arbovirus Infection" Microorganisms 13, no. 6: 1281. https://doi.org/10.3390/microorganisms13061281
APA StyleCui, B., Wang, Z., Farid, A., Wang, Z., Wei, K., Ren, N., Yang, F., & Liu, H. (2025). The Application and Challenges of Brain Organoids in Exploring the Mechanism of Arbovirus Infection. Microorganisms, 13(6), 1281. https://doi.org/10.3390/microorganisms13061281





