Protective Efficacy of an mRNA Vaccine Against HP-PRRSV Challenge in Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Cells and Reagents
2.3. Sample Source and Virus Isolation
2.4. Genome Cloning and Sequencing
2.5. mRNA-Based Vaccine
2.6. The Virulence of the JX021 Strain in Pigs
2.7. Vaccination and JX021 Challenge in Pigs
2.8. Immunofluorescence Assay (IFA)
2.9. Virus Neutralization Assay
2.10. Histopathological Examination
2.11. Statistical Analysis
3. Results
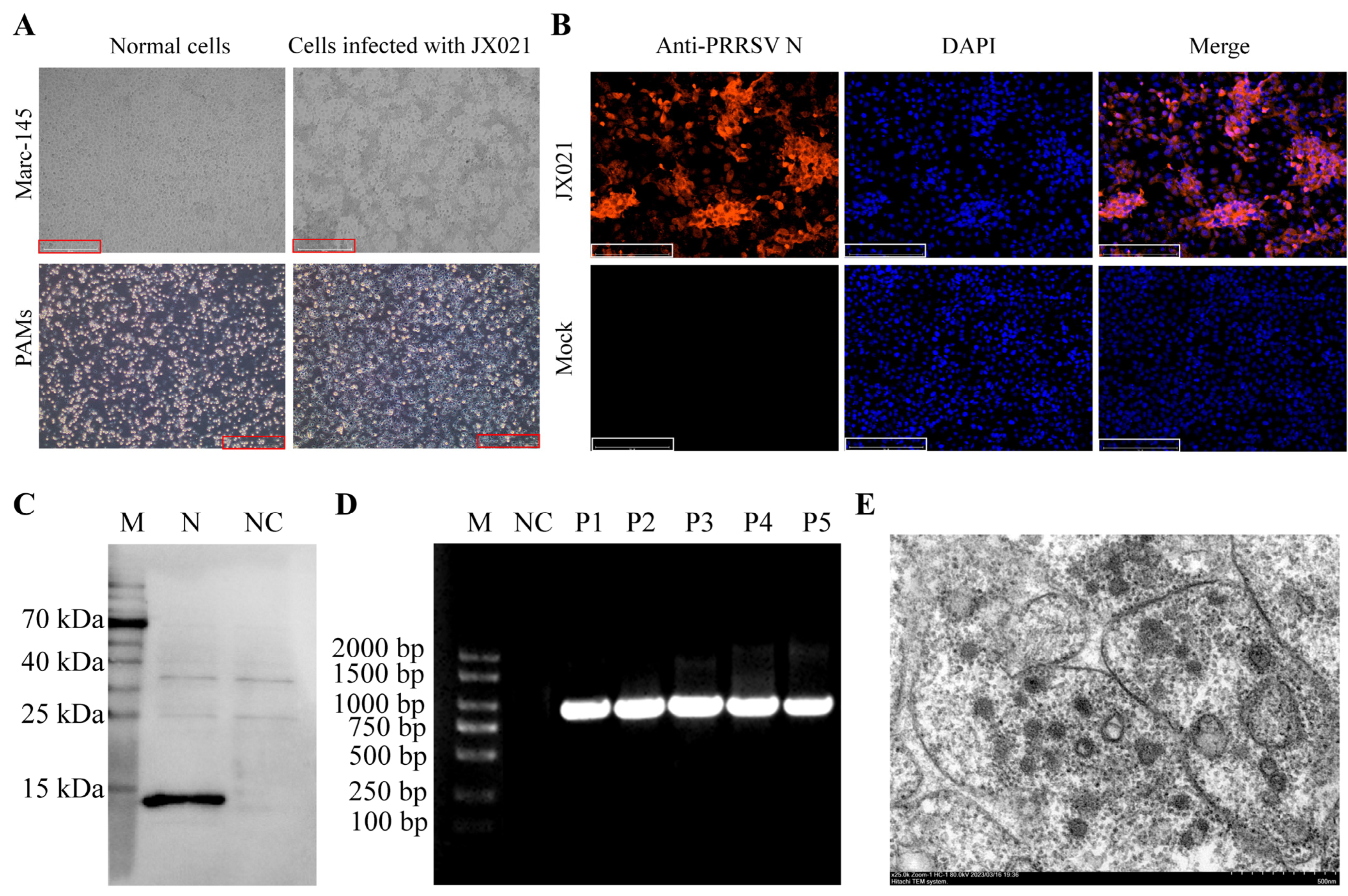
3.1. Isolation and Identification of JX021
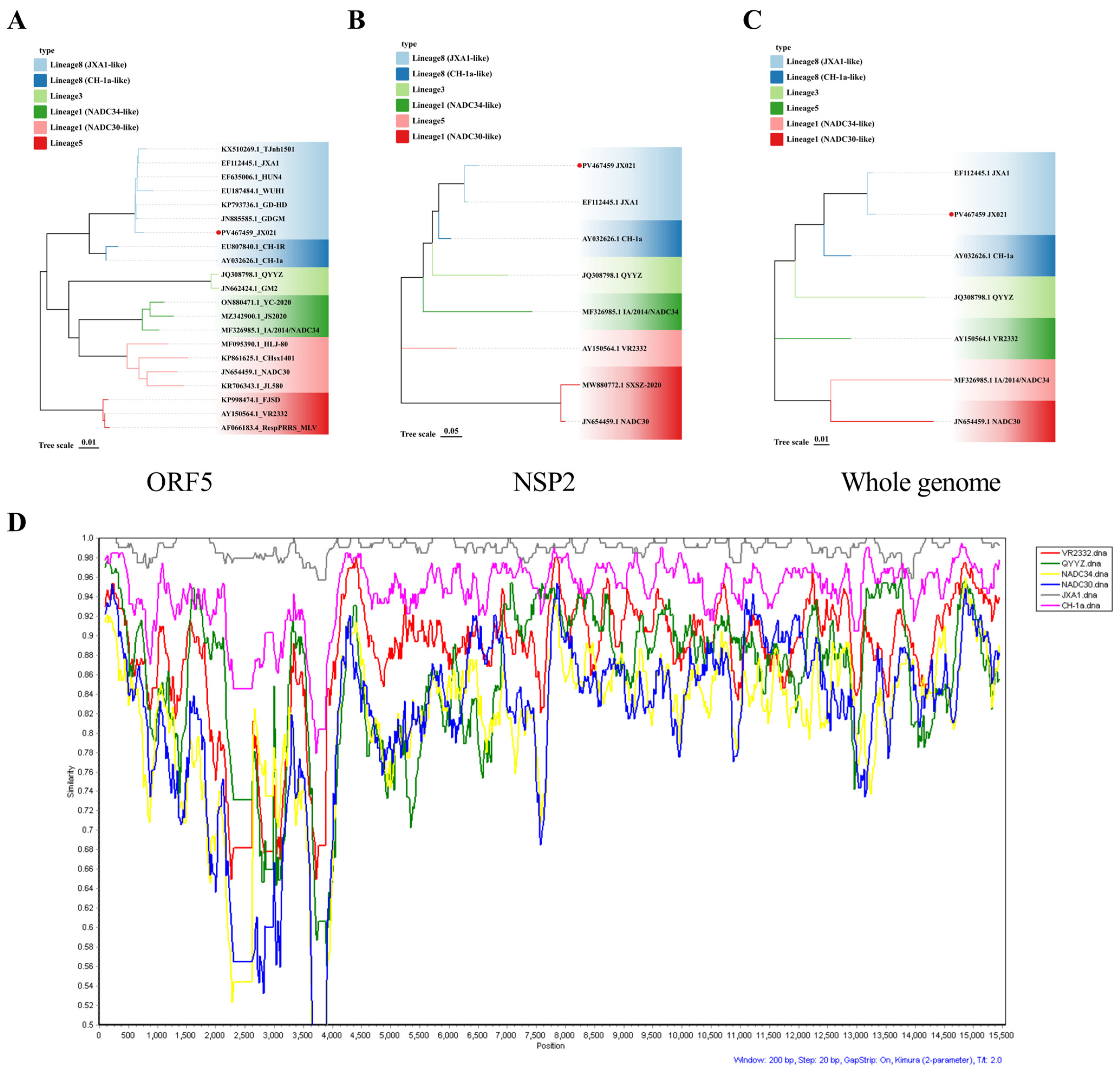
3.2. Genomic Characteristics of JX021 PRRSV
3.3. Pathogenicity Analysis of JX021 PRRSV
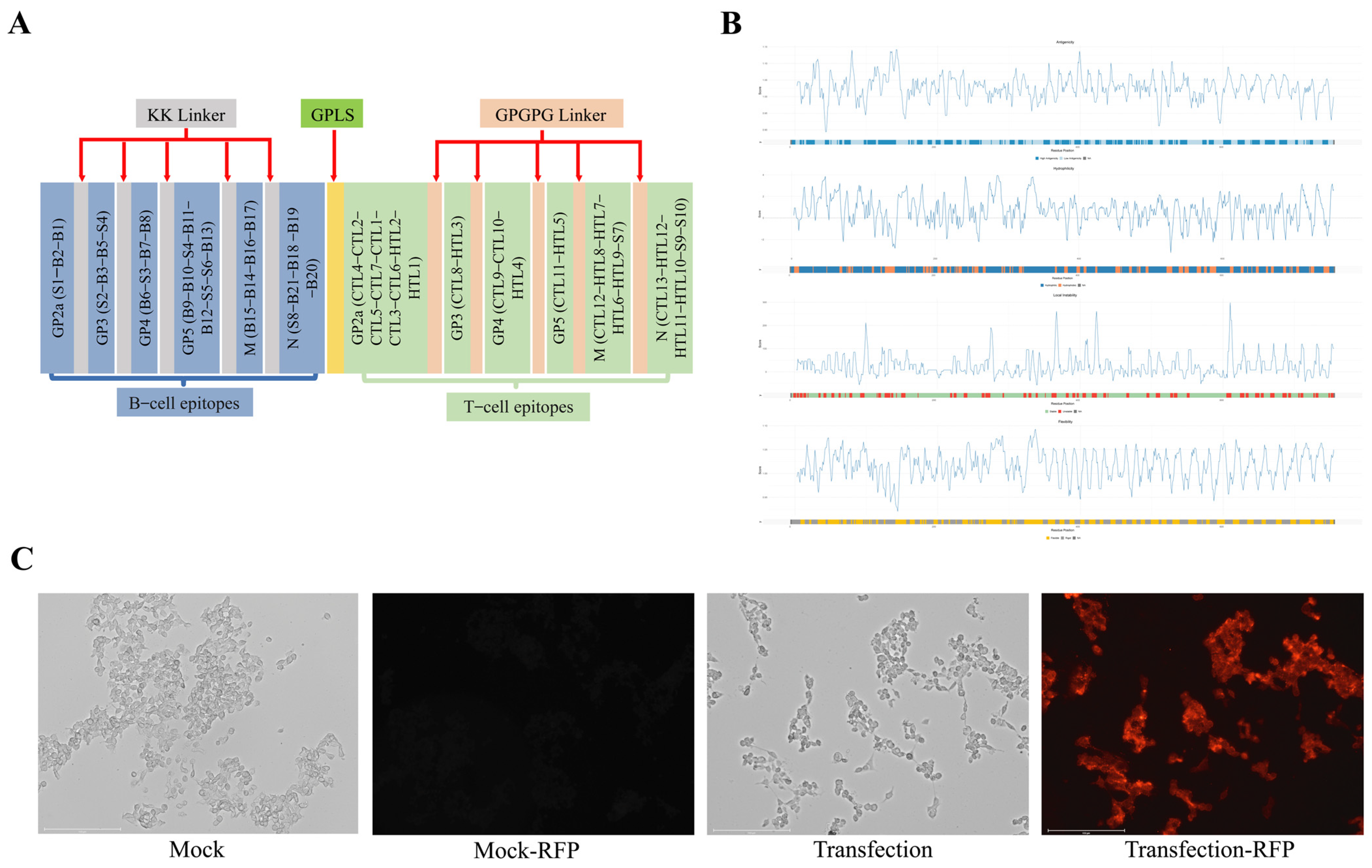
3.4. Design of PRRS mRNA Vaccine
3.5. Evaluation of the Immunoprotective Effect of the PRRS mRNA Vaccine in Piglets
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef]
- Allende, R.; Kutish, G.F.; Laegreid, W.; Lu, Z.; Lewis, T.L.; Rock, D.L.; Friesen, J.; Galeota, J.A.; Doster, A.R.; Osorio, F.A. Mutations in the genome of porcine reproductive and respiratory syndrome virus responsible for the attenuation phenotype. Arch. Virol. 2000, 145, 1149–1161. [Google Scholar] [CrossRef]
- Nelsen, C.J.; Murtaugh, M.P.; Faaberg, K.S. Porcine reproductive and respiratory syndrome virus comparison: Divergent evolution on two continents. J. Virol. 1999, 73, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.; Li, J.; Wong, L.T.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; You, S.; Li, Y.; Wang, H.; Bai, J.; Jiang, P. Emerging of two new subgenotypes of porcine reproductive and respiratory syndrome viruses in Southeast China. Microb. Pathog. 2016, 97, 27–33. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, C.; Chang, X.B.; Jiang, C.G.; Wang, S.J.; Cai, X.H.; Tong, G.Z.; Tian, Z.J.; Shi, M.; An, T.Q. Importation and Recombination Are Responsible for the Latest Emergence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhang, W.L.; Xiang, L.R.; Leng, C.L.; Tian, Z.J.; Tang, Y.D.; Cai, X.H. Emergence of novel porcine reproductive and respiratory syndrome viruses (ORF5 RFLP 1-7-4 viruses) in China. Vet. Microbiol. 2018, 222, 105–108. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, H.; An, T.; Cai, X.; Tian, Z.; Zhang, H. Recent Progress in Studies of Porcine Reproductive and Respiratory Syndrome Virus 1 in China. Viruses 2023, 15, 1528. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, K.K.; Visser, N.; Van Woensel, P.; Thiel, H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology 1993, 193, 329–339. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C. Porcine reproductive and respiratory syndrome virus vaccines: Current status and strategies to a universal vaccine. Transbound. Emerg. Dis. 2014, 61, 109–120. [Google Scholar] [CrossRef]
- Li, Y.; Shyu, D.L.; Shang, P.; Bai, J.; Ouyang, K.; Dhakal, S.; Hiremath, J.; Binjawadagi, B.; Renukaradhya, G.J.; Fang, Y. Mutations in a Highly Conserved Motif of nsp1β Protein Attenuate the Innate Immune Suppression Function of Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2016, 90, 3584–3599. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Q.; Hu, D.; Zhang, Q.; Han, T.; Ma, Y.; Gu, X.; Zhai, X.; Tian, K. Complete genomic characterization and genetic diversity of four European genotype porcine reproductive and respiratory syndrome virus isolates from China in 2011. Virus Genes 2015, 51, 375–384. [Google Scholar] [CrossRef]
- Liao, Y.C.; Lin, H.H.; Lin, C.H.; Chung, W.B. Identification of cytotoxic T lymphocyte epitopes on swine viruses: Multi-epitope design for universal T cell vaccine. PLoS ONE 2013, 8, e84443. [Google Scholar] [CrossRef]
- Ansari, I.H.; Kwon, B.; Osorio, F.A.; Pattnaik, A.K. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J. Virol. 2006, 80, 3994–4004. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Bollman, B.; Nunna, N.; Bahl, K.; Hsiao, C.J.; Bennett, H.; Butler, S.; Foreman, B.; Burgomaster, K.E.; Aleshnick, M.; Kong, W.P.; et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. NPJ Vaccines 2023, 8, 58. [Google Scholar] [CrossRef]
- Zhang, P.; Narayanan, E.; Liu, Q.; Tsybovsky, Y.; Boswell, K.; Ding, S.; Hu, Z.; Follmann, D.; Lin, Y.; Miao, H.; et al. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 2021, 27, 2234–2245. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e0295823. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, Y.G.; Tang, Y.D.; Liu, T.X.; Zheng, L.L.; Wang, T.Y.; Liu, S.G.; Wang, G.; Cai, X.H. A natural recombinant PRRSV between HP-PRRSV JXA1-like and NADC30-like strains. Transbound. Emerg. Dis. 2018, 65, 1078–1086. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Liu, X.; Bai, J.; Jiang, C.; Song, Z.; Zhao, Y.; Nauwynck, H.; Jiang, P. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2019, 238, 108431. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, Y.; Zhang, H.; Yang, Z.; Sha, H.; Kong, W.; Zhao, M.; Wang, N. Research Progress on Glycoprotein 5 of Porcine Reproductive and Respiratory Syndrome Virus. Animals 2023, 13, 813. [Google Scholar] [CrossRef]
- Fang, Y.; Kim, D.Y.; Ropp, S.; Steen, P.; Christopher-Hennings, J.; Nelson, E.A.; Rowland, R.R. Heterogeneity in Nsp2 of European-like porcine reproductive and respiratory syndrome viruses isolated in the United States. Virus Res. 2004, 100, 229–235. [Google Scholar] [CrossRef]
- Gonin, P.; Pirzadeh, B.; Gagnon, C.A.; Dea, S. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J. Vet. Diagn. Investig. 1999, 11, 20–26. [Google Scholar] [CrossRef]
- Piñeyro, P.E.; Kenney, S.P.; Giménez-Lirola, L.G.; Heffron, C.L.; Matzinger, S.R.; Opriessnig, T.; Meng, X.J. Expression of antigenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV) in a modified live-attenuated porcine circovirus type 2 (PCV2) vaccine virus (PCV1-2a) as a potential bivalent vaccine against both PCV2 and PRRSV. Virus Res. 2015, 210, 154–164. [Google Scholar] [CrossRef]
- Oleksiewicz, M.B.; Bøtner, A.; Normann, P. Porcine B-cells recognize epitopes that are conserved between the structural proteins of American- and European-type porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2002, 83, 1407–1418. [Google Scholar] [CrossRef]
- Kwon, T.; Yoo, S.J.; Sunwoo, S.Y.; Lee, D.U.; Je, S.H.; Park, J.W.; Park, C.K.; Lyoo, Y.S. Independent evolution of porcine reproductive and respiratory syndrome virus 2 with genetic heterogeneity in antigenic regions of structural proteins in Korea. Arch. Virol. 2019, 164, 213–224. [Google Scholar] [CrossRef]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002, 76, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhou, Y.J.; Li, G.X.; Zhang, S.R.; Jiang, Y.F.; Xu, A.T.; Yu, H.; Wang, M.M.; Yan, L.P.; Tong, G.Z. Identification of immunodominant T-cell epitopes in membrane protein of highly pathogenic porcine reproductive and respiratory syndrome virus. Virus Res. 2011, 158, 108–115. [Google Scholar] [CrossRef]
- Zhang, G.; Li, N.; Chen, Y.; Zhou, J.; Liu, H.; Qi, Y.; Liu, Y.; Peng, Y.; Sun, H.; Wang, A. Identification of the B-cell epitopes on N protein of type 2 porcine reproductive and respiratory syndrome virus, using monoclonal antibodies. Int. J. Biol. Macromol. 2019, 130, 300–306. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Bi, Y.; Yang, L.; Meng, S.; Zhou, Y.; Jia, X.; Meng, S.; Sun, L.; Liu, W. Synthetic B- and T-cell epitope peptides of porcine reproductive and respiratory syndrome virus with Gp96 as adjuvant induced humoral and cell-mediated immunity. Vaccine 2013, 31, 1838–1847. [Google Scholar] [CrossRef]
- Zhang, R.; Li, H.; Xie, H.; Hou, X.; Zhou, L.; Cao, A.; Zeshan, B.; Zhou, Y.; Wang, X. Comparing the molecular evolution and recombination patterns of predominant PRRSV-2 lineages co-circulating in China. Front. Microbiol. 2024, 15, 1398470. [Google Scholar] [CrossRef]
- Shabir, N.; Khatun, A.; Nazki, S.; Kim, B.; Choi, E.J.; Sun, D.; Yoon, K.J.; Kim, W.I. Evaluation of the Cross-Protective Efficacy of a Chimeric Porcine Reproductive and Respiratory Syndrome Virus Constructed Based on Two Field Strains. Viruses 2016, 8, 240. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, R.; Yu, J.; Xie, B.; Chen, C.; Li, X.; Xie, J.; Ye, Y.; Xiao, L.; Zhang, J.; et al. Genetic Characterization and Pathogenicity of a Novel Recombined Porcine Reproductive and Respiratory Syndrome Virus 2 among Nadc30-Like, Jxa1-Like, and Mlv-Like Strains. Viruses 2018, 10, 551. [Google Scholar] [CrossRef]
- Chen, P.; Tan, X.; Lao, M.; Wu, X.; Zhao, X.; Zhou, S.; Yu, J.; Zhu, J.; Yu, L.; Tong, W.; et al. The Novel PRRSV Strain HBap4-2018 with a Unique Recombinant Pattern Is Highly Pathogenic to Piglets. Virol. Sin. 2021, 36, 1611–1625. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Xu, X.; Leng, X.; Li, S.; Wen, Y.; Wang, F.; Xia, M.; Cheng, S.; Wu, H. Pathological and immunological characteristics of piglets infected experimentally with a HP-PRRSV TJ strain. BMC Vet. Res. 2016, 12, 230. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, J.; Sun, Y.; Liu, X.; Gao, Y.; Wang, X.; Yang, Y.; Jiang, P. Comparison of pathogenicity of different subgenotype porcine reproductive and respiratory syndrome viruses isolated in China. Microb. Pathog. 2022, 168, 105607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jiang, P.; Li, Y.; Tang, J.; Wang, X.; Ma, S. Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice. Vet. Immunol. Immunopathol. 2006, 113, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, J.; Sun, W.; Xie, C.; Zhang, H.; Gao, Y.; Wen, S.; Ha, Z.; Nan, F.; Zhu, X.; et al. Immunological evaluation of recombination PRRSV GP3 and GP5 DNA vaccines in vivo. Front. Cell. Infect. Microbiol. 2022, 12, 1016897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wubshet, A.K.; Zhang, J.; Hou, S.; Yao, K.; Zhao, Q.; Dai, J.; Liu, Y.; Ding, Y.; Zhang, J.; et al. The mRNA Vaccine Expressing Single and Fused Structural Proteins of Porcine Reproductive and Respiratory Syndrome Induces Strong Cellular and Humoral Immune Responses in BalB/C Mice. Viruses 2024, 16, 544. [Google Scholar] [CrossRef]
- Wissink, E.H.; Kroese, M.V.; van Wijk, H.A.; Rijsewijk, F.A.; Meulenberg, J.J.; Rottier, P.J. Envelope protein requirements for the assembly of infectious virions of porcine reproductive and respiratory syndrome virus. J. Virol. 2005, 79, 12495–12506. [Google Scholar] [CrossRef]
- Chai, H.; Wei, Y.; Chen, W.; Han, G.; Godspower, B.O.; Liu, Y.; Dong, C.; Zhang, Z.; Li, Y. Protection efficacy and the safety of the synergy between modified Bazhen powder and PRRSV modified-live virus vaccine against HP-PRRSV in piglets. Front. Vet. Sci. 2024, 11, 1436426. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Li, J.; Zhao, Z.; Zhang, H.; Hao, G.; Chen, H.; Qian, P. Immunization with a recombinant fusion of porcine reproductive and respiratory syndrome virus modified GP5 and ferritin elicits enhanced protective immunity in pigs. Virology 2021, 552, 112–120. [Google Scholar] [CrossRef]
- Mou, C.; Zhao, X.; Zhuo, C.; He, Q.; Xu, M.; Shi, K.; Han, T.; Xu, S.; Chen, Z. The mRNA vaccine expressing fused structural protein of PRRSV protects piglets against PRRSV challenge. Vet. Microbiol. 2025, 305, 110534. [Google Scholar] [CrossRef]
- Bi, C.; Shao, Z.; Li, J.; Weng, C. Identification of novel epitopes targeting non-structural protein 2 of PRRSV using monoclonal antibodies. Appl. Microbiol. Biotechnol. 2019, 103, 2689–2699. [Google Scholar] [CrossRef]
- Sha, H.; Zhang, H.; Chen, Y.; Huang, L.; Zhao, M.; Wang, N. Research Progress on the NSP9 Protein of Porcine Reproductive and Respiratory Syndrome Virus. Front. Vet. Sci. 2022, 9, 872205. [Google Scholar] [CrossRef]






| Virus Strain | Accession No. | Origin |
|---|---|---|
| GM2 | JN662424.1 | China, 2011 |
| JL580 | KR706343.1 | China, 2015 |
| R98 | DQ355796.1 | China, 2006 |
| SD16 | JX087437.1 | China, 2012 |
| WUH1 | EU187484.1 | China, 2016 |
| HuN4 | EF635006.1 | China, 2016 |
| JS2020 | MZ342900.1 | China, 2021 |
| JXA1 | EF112445.1 | China, 2016 |
| CH-1a | AY032626.1 | China, 1996 |
| CH-1R | EU807840.1 | China, 2008 |
| HUB1 | EF075945.1 | China, 2016 |
| QYYZ | JQ308798.1 | China, 2011 |
| CHsx1401 | KP861625.1 | China, 2014 |
| NADC30 | JN654459.1 | USA, 2008 |
| VR-2332 | AY150564.1 | USA, 1992 |
| Lelystad virus | M96262.2 | Netherlands, 1991 |
| RespPRRS MLV | AF066183.4 | USA, 1998 |
| IA/2014/NADC34 | MF326985.1 | USA, 2017 |
| IA/2015/NADC35 | MF326986.1 | USA, 2016 |
| IA/2015/NADC36 | MF326987.1 | USA, 2016 |
| Groups | Number of Piglets | Vaccination | Vaccine Type | Injection Method and Dosage | HP-PRRSV Challenge | |
|---|---|---|---|---|---|---|
| Strain | Method and Dosage | |||||
| mRNA-LNP group | 5 | Yes | mRNA-LNP | 150 μg, intramuscularly injected | JX021 | 2 × 105 TCID50, intranasally inoculated and intramuscularly injected |
| Inactivated vaccine group | 5 | Yes | Inactivated vaccine | 2 mL, intramuscularly injected | JX021 | 2 × 105 TCID50, intranasally inoculated and intramuscularly injected |
| PBS group | 5 | No | - | 2 mL PBS, intramuscularly injected | JX021 | 2 × 105 TCID50, intranasally inoculated and intramuscularly injected |
| Sentinel group | 5 | No | - | 2 mL PBS, intramuscularly injected | - | 2 mL DMEM, intranasally inoculated and intramuscularly injected |
| Protein | Name | Sequence | Start Position | End Position |
|---|---|---|---|---|
| GP2a | B1 | GQAAWKQVVSEATLSR | 123 | 138 |
| B2 | SYASDWFAPR | 49 | 58 | |
| GP3 | B3 | WCRIGHDRCSENDHDE | 74 | 89 |
| B4 | RVFRTSKPTPPQHQTS | 211 | 226 | |
| B5 | HPEIFGIGN | 123 | 131 | |
| GP4 | B6 | CKPCFSSSLSDIKTNT | 22 | 38 |
| B7 | HGDSSSPTIRKISQCR | 55 | 70 | |
| B8 | LLHFMTPETMRWATVL | 152 | 168 | |
| GP5 | B9 | TACCCSRLLFLWCIVP | 7 | 22 |
| B10 | NASNNNSSH | 30 | 38 | |
| B11 | TDWLAQKFDW | 53 | 62 | |
| B12 | VSTAGYYHGR | 96 | 105 | |
| B13 | VLDGSAATPLTRVSAEL | 180 | 196 | |
| M | B14 | GFHPIAANDNHAFVVR | 121 | 136 |
| B15 | LAPAHHVESAAGFHPIAAND | 110 | 129 | |
| B16 | RPGSTTV | 137 | 143 | |
| B17 | VPGLKSLVLGGRKAVKQGVVN | 148 | 168 | |
| N | B18 | QSRGKGPGKKNRKKNP | 35 | 50 |
| B19 | LSSIQTAFNQGAGTCA | 76 | 91 | |
| B20 | LPTQHTVRLIRATASP | 106 | 121 | |
| B21 | NGKQQKKKKGNGQPVN | 5 | 20 |
| Protein | Name | Sequence | Allele | Start Position |
|---|---|---|---|---|
| GP2a | CTL1 | ASDWFAPRY | SLA-1: 0201 | 51 |
| CTL2 | SQSPVGWWSY | SLA-1: 0501 | 41 | |
| CTL3 | HPLGVLWHH | SLA-1: 0701 | 93 | |
| CTL4 | SRNFWCPLL | SLA-3: 0101 | 21 | |
| CTL5 | SPVGWWSY | SLA-1: 0701 | 43 | |
| CTL6 | SQSPVGWWSY | SLA-2: 0301 | 151 | |
| CTL7 | SYASDWFAPRY | SLA-1-YC | 49 | |
| HTL1 | LDQVFAIFPTPGSRP | HLA-DRB1*04: 01 | 187 | |
| HTL2 | VVAHFQHLAAIEAET | HLA-DRB1*11: 01 | 144 | |
| GP3 | CTL8 | HQVDGGNWF | SLA-2: 0201 | 171 |
| HTL3 | GNVSQVYVDIKHQFI | HLA-DRB1*03: 01 | 130 | |
| GP4 | CTL9 | RTAIGTPVY | SLA-2: 0101 | 70 |
| CTL10 | ETMRWATVL | SLA-1-CHANGDA | 160 | |
| HTL4 | TPVYITITANVTDEN | HLA-DRB1*10: 01 | 75 | |
| GP5 | CTL11 | KFDWAVETF | SLA-1: 1301 | 59 |
| HTL5 | KGRLYRWRSPVIVEK | HLA-DRB1*15: 01 | 149 | |
| M | CTL12 | YSAIETWKF | SLA-2: 0101 | 86 |
| HTL6 | GRKYILAPAHHVESA | HLA-DRB1*01: 01 | 105 | |
| HTL7 | LGRKYILAPAHHVES | HLA-DRB1*01: 01 | 104 | |
| HTL8 | LLGRKYILAPAHHVE | HLA-DRB1*07: 01 | 103 | |
| HTL9 | RKYILAPAHHVESAA | HLA-DRB1*07: 01 | 106 | |
| N | CTL13 | ATEDDVRHHF | SLA-1: 0401 | 58 |
| HTL10 | GKIIAQQNQSRGKGP | HLA-DRB4*01: 01 | 27 | |
| HTL11 | LGKIIAQQNQSRGKG | HLA-DRB5*01: 01 | 26 | |
| HTL12 | MLGKIIAQQNQSRGK | HLA-DRB5*01: 01 | 25 |
| Protein | Name | Sequence | Start Position | End Position | Types |
|---|---|---|---|---|---|
| GP2a | S1 | RKAPPAHMARARGFKW [28] | 43 | 56 | B-cell epitope |
| GP3 | S2 | LEPGKSFWCRIGHDRCSEN [29] | 67 | 85 | B-cell epitope |
| GP4 | S3 | GDSSSPTIRK [30] | 51 | 65 | B-cell epitope |
| GP5-1 | S4 | SHIQLIYNL [31] | 37 | 45 | B-cell epitope |
| GP5-2 | S5 | LDTKGRLYRWR [31] | 146 | 156 | B-cell epitope |
| GP5-3 | S6 | GGKVEVEGHLIDLKRVV [31] | 164 | 180 | B-cell epitope |
| M | S7 | KFITSRCRLCLLGRK [32] | 93 | 107 | T-cell epitope |
| N-1 | S8 | MPNNNGKQQKKKKGN [33] | 1 | 15 | B-cell epitope |
| N-2 | S9 | IAQQNQSRGKGPGKKNRKKNPEKPHFPLA [34] | 30 | 58 | T-cell epitope |
| N-3 | S10 | VRHHFTPSE [34] | 63 | 71 | T-cell epitope |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ni, S.; Lv, Y.; Tong, Z.; Liu, P.; Zong, X.; Chen, G.; Zeng, Y.; Wang, C.; Tan, C. Protective Efficacy of an mRNA Vaccine Against HP-PRRSV Challenge in Piglets. Microorganisms 2025, 13, 1332. https://doi.org/10.3390/microorganisms13061332
Liu J, Ni S, Lv Y, Tong Z, Liu P, Zong X, Chen G, Zeng Y, Wang C, Tan C. Protective Efficacy of an mRNA Vaccine Against HP-PRRSV Challenge in Piglets. Microorganisms. 2025; 13(6):1332. https://doi.org/10.3390/microorganisms13061332
Chicago/Turabian StyleLiu, Jiaqi, Shiting Ni, Yaning Lv, Ze Tong, Pingxuan Liu, Xin Zong, Guosheng Chen, Yan Zeng, Chenchen Wang, and Chen Tan. 2025. "Protective Efficacy of an mRNA Vaccine Against HP-PRRSV Challenge in Piglets" Microorganisms 13, no. 6: 1332. https://doi.org/10.3390/microorganisms13061332
APA StyleLiu, J., Ni, S., Lv, Y., Tong, Z., Liu, P., Zong, X., Chen, G., Zeng, Y., Wang, C., & Tan, C. (2025). Protective Efficacy of an mRNA Vaccine Against HP-PRRSV Challenge in Piglets. Microorganisms, 13(6), 1332. https://doi.org/10.3390/microorganisms13061332





