Activated Carbon and Syntrophy Accelerate the Corrosion of Stainless Steel Under Strict Anaerobic Conditions by Methanosarcina barkeri
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Construction of the Culture System of Strains, Stainless Steel and GAC
2.3. Analytical and Detection Methods
2.3.1. Detection Method of Ions in Solution
2.3.2. Detection Method of Ions in Granular Activated Carbon
2.3.3. Determination of the Concentration of Substances in Solution
2.3.4. Testing Method for Gas Components and Contents
2.4. Surface Analysis Method
2.5. Detection Method for Electrochemical Indicators
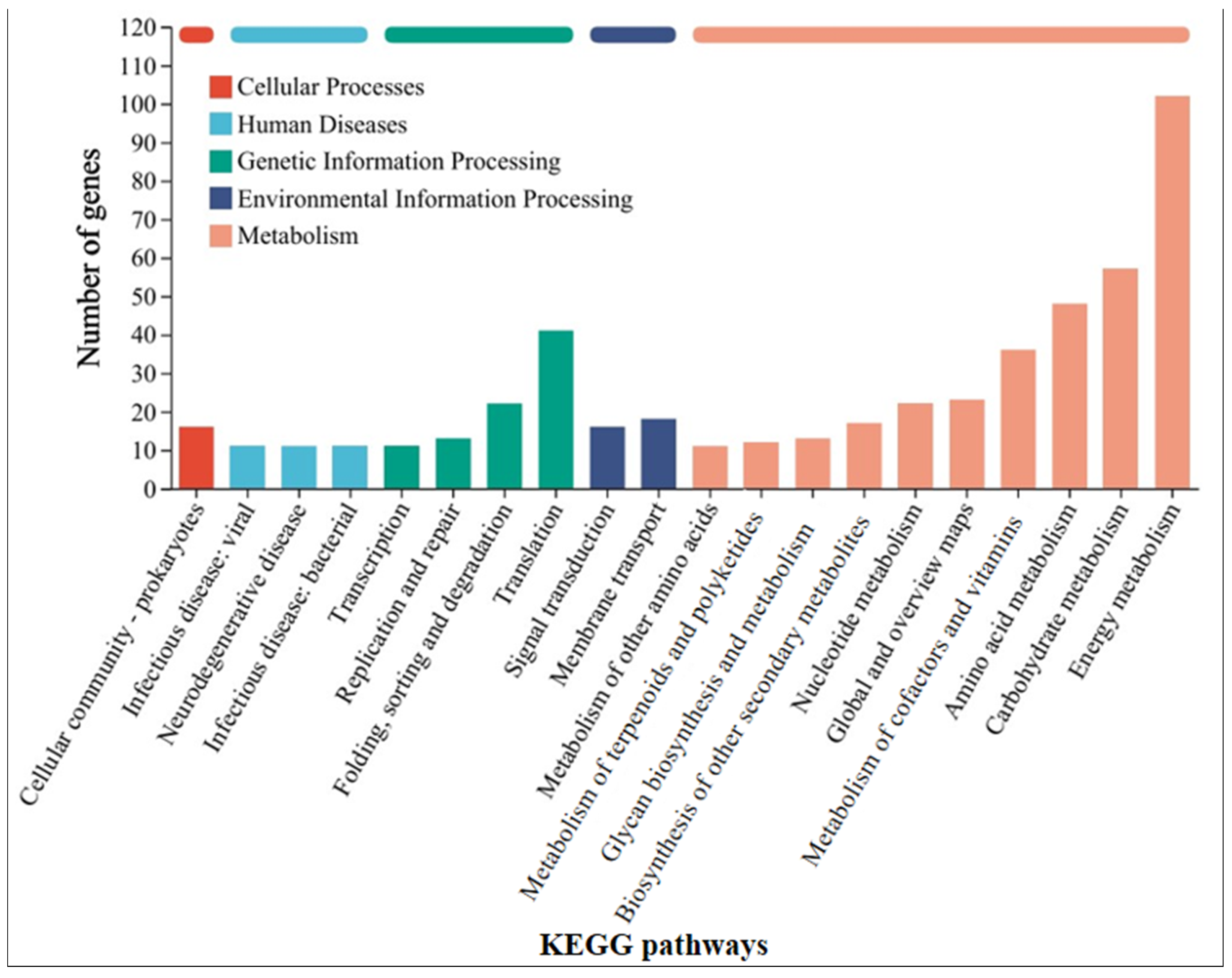
2.6. Transcriptomics
3. Results and Discussion
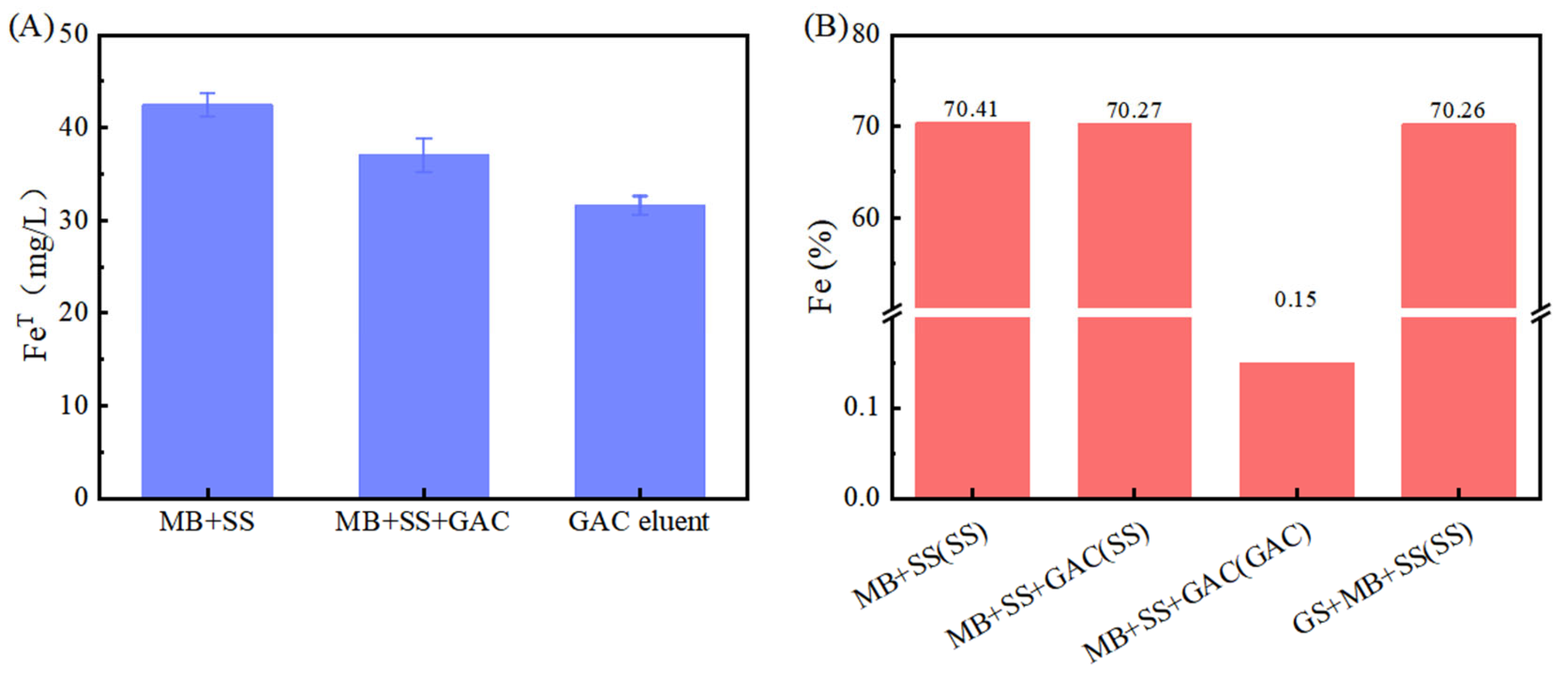
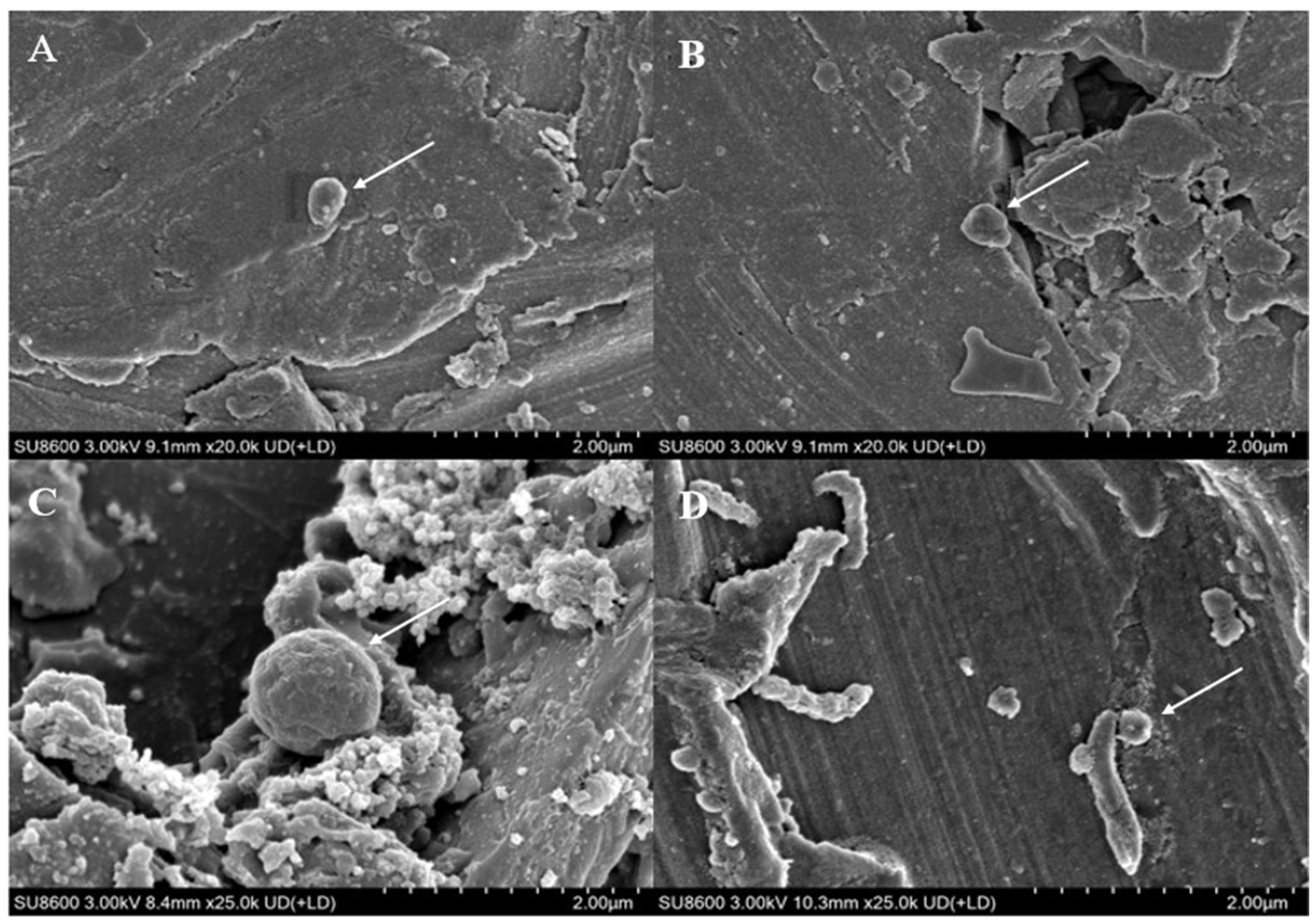
3.1. GAC Can Accelerate the Corrosion of Stainless Steel by Methanosarcina barkeri
3.2. Mechanism Analysis of GAC Accelerating the Corrosion of Stainless Steel by Methanosarcina barkeri
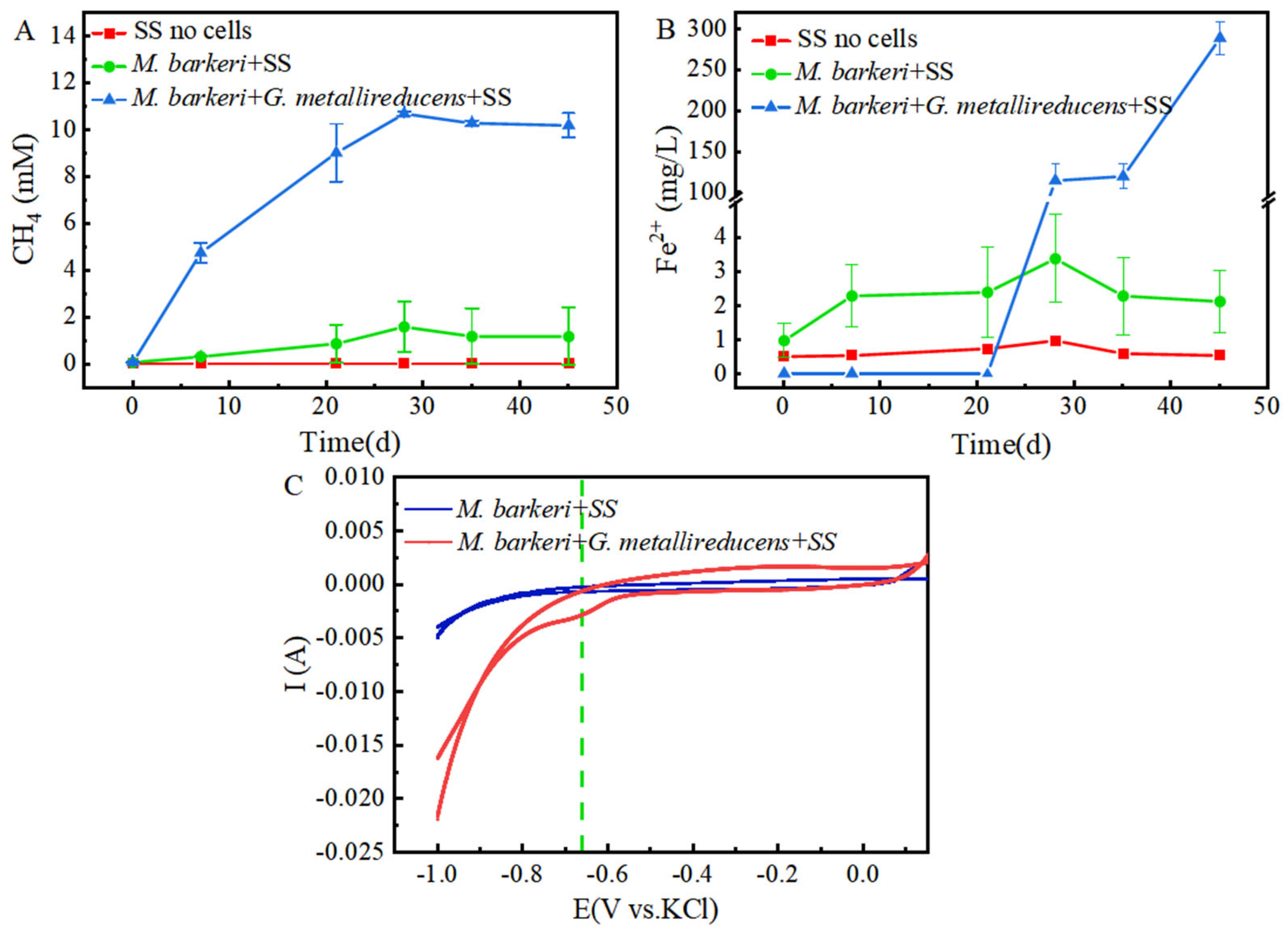
3.3. Syntrophy Can Accelerate the Corrosion of Stainless Steel by Methanosarcina barkeri
3.4. Mechanism Analysis of Syntrophy Accelerating the Corrosion of Stainless Steel by Methanosarcina barkeri
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Ionescu, G.C.; Ionescu, G.L.; Marin, L. Modern Theoretical and Practical Protection Methods of Metallic Structures Against the Effects of Corrosion. J. Appl. Eng. Sci. 2024, 14, 97–102. [Google Scholar] [CrossRef]
- Tang, H.Y.; Holmes, D.E.; Ueki, T.; Palacios, P.A.; Lovley, D.R. Iron Corrosion via Direct Metal-Microbe Electron Transfer. MBio 2019, 10, 1128. [Google Scholar] [CrossRef]
- Hernández Gayosso, M.J.; Zavala Olivares, G.; Ruiz Ordaz, N.; Juárez Ramirez, C.; García Esquivel, R.; Padilla Viveros, A. Microbial consortium influence upon steel corrosion rate, using polarisation resistance and electrochemical noise techniques. Electrochim. Acta 2004, 49, 4295–4301. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Liang, R.; Ji, S.; Li, J.; Liu, M. Chemical additives affect sulfate reducing bacteria biofilm properties adsorbed on stainless steel 316L surface in circulating cooling water system. Front. Environ. Sci. Eng. 2017, 11, 14. [Google Scholar] [CrossRef]
- Procópio, L. Microbially induced corrosion impacts on the oil industry. Arch. Microbiol. 2022, 204, 138. [Google Scholar] [CrossRef]
- Holmes, D.E.; Tang, H.; Woodard, T.; Liang, D.; Zhou, J.; Liu, X.; Lovley, D.R. Cytochrome-mediated direct electron uptake from metallic iron by Methanosarcina acetivorans. mLife 2022, 1, 443–447. [Google Scholar] [CrossRef]
- Paul, L.; Krzycki, J.A. Sequence and Transcript Analysis of a Novel Methanosarcina barkeri Methyltransferase II Homolog and Its Associated Corrinoid Protein Homologous to Methionine Synthase. J. Bacteriol. 1996, 178, 6599–6607. [Google Scholar] [CrossRef]
- Giangeri, G.; Tsapekos, P.; Gaspari, M.; Ghofrani Isfahani, P.; Treu, L.; Kougias, P.; Campanaro, S.; Angelidaki, I. A bioaugmentation strategy to recover methane production under sulfate-stressed conditions: Highlights on targeted sulfate-reducing bacteria and DIET-related species. Appl. Energy 2024, 362, 122940. [Google Scholar] [CrossRef]
- Wang, Z.; Gou, M.; Zheng, Q.; Xu, H.; Melhi, S.; El Bahy, Z.M.; Elsharkawy, E.R.; Dang, Y.; Qiu, B. Enhanced biogas upgrading by photocatalytic conversion of carbon dioxide to methane by Methanosarcina barkeri–cadmium sulfide biohybrid. Adv. Compos. Hybrid Mater. 2024, 7, 111. [Google Scholar] [CrossRef]
- Yu, L.; He, D.; Yang, L.; Rensing, C.; Zeng, R.J.; Zhou, S. Anaerobic methane oxidation coupled to ferrihydrite reduction by Methanosarcina barkeri. Sci. Total Environ. 2022, 844, 157235. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A.; Emmanuel, A.; Armand, F.; Sylvain, D.; Bernard, O. Iron corrosion induced by the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus at 70 °C. Int. Biodeterior. Biodegrad. 2020, 154, 105056. [Google Scholar] [CrossRef]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Chen, Y.; Ding, C.; Yan, Z.; Wang, S.; Song, C. Mechanistic investigation of ferric ion and ferriferous oxide on M. barkeri-mediated copper corrosion. J. Water Process Eng. 2024, 68, 106395. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Frkova, Z.; Lemaigre, S.; Goux, X.; Calusinska, M.; Roussel, J. Enhancement of anaerobic digestion of dairy wastewater by addition of conductive materials with or without the combination of external voltage application. J. Chem. Technol. Biotechnol. 2024, 99, 1837–1846. [Google Scholar] [CrossRef]
- Yahia, M.S.; Elzaref, A.S.; Awad, M.B.; Tony, A.M.; Elfeky, A.S. Efficient adsorption of chlorpyrifos onto modified activated carbon by gamma irradiation; a plausible adsorption mechanism. Z. Für Phys. Chem. 2022, 236, 1–25. [Google Scholar] [CrossRef]
- Voorhees, J.P.; Anderson, B.S.; Phillips, B.M.; Tjeerdema, R.S. Carbon Treatment as a Method to Remove Imidacloprid from Agriculture Runoff. Bull. Environ. Contam. Toxicol. 2017, 99, 200–202. [Google Scholar] [CrossRef]
- Yang, L.; Wen, Q.; Chen, Z.; Duan, R.; Yang, P. Impacts of advanced treatment processes on elimination of antibiotic resistance genes in a municipal wastewater treatment plant. Front. Environ. Sci. Eng. 2019, 13, 32. [Google Scholar] [CrossRef]
- Li, X.Y.; Xu, J.; Cheng, J.P.; Feng, L.; Shi, Y.F.; Ji, J. TiO2-SiO2/GAC particles for enhanced electrocatalytic removal of acid orange 7 (AO7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 2017, 187, 303–310. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Dong, H.; Cui, P.; Lu, W.; Xu, H.; Qiu, B.; Sun, D.; Ma, J.; Dang, Y. Enrichment of Methanothrix species via riboflavin-loaded granular activated carbon in anaerobic digestion of high-concentration brewery wastewater amidst continuous inoculation of Methanosarcina barkeri. Water Res. 2025, 268, 122739. [Google Scholar] [CrossRef]
- Roland, F.A.E.; Darchambeau, F.; Morana, C.; Bouillon, S.; Borges, A.V. Emission and oxidation of methane in a meromictic, eutrophic and temperate lake (Dendre, Belgium). Chemosphere 2017, 168, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, W.; Liu, D.; Xiang, J.; Lin, Y. Methane production potential and methanogenic archaea community dynamics along the Spartina alterniflora invasion chronosequence in a coastal salt marsh. Appl. Microbiol. Biotechnol. 2014, 98, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ni, B.J.; Ganigue, R.; Werner, U.; Sharma, K.R.; Yuan, Z. Sulfide and methane production in sewer sediments. Water Res. 2015, 70, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R.; Voordouw, G. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Tang, H.Y.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021, 15, 3084–3093. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Chan, C.S.; Emerson, D.; Luther, G.W., 3rd. The role of microaerophilic Fe-oxidizing micro-organisms in producing banded iron formations. Geobiology 2016, 14, 509–528. [Google Scholar] [CrossRef]
- Lovley, D.R.; Giovannoni, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.P.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef]
- Holmes, D.E.; Zhou, J.; Smith, J.A.; Wang, C.; Liu, X.; Lovley, D.R. Different outer membrane c-type cytochromes are involved in direct interspecies electron transfer to Geobacter or Methanosarcina species. mLife 2022, 1, 272–286. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, H.; Li, H.; Zhang, H.; Lu, W.; Sun, D.; Liu, X.; Dang, Y. Hybridization of photoanode and biocathode enables biogas upgrading via Methanosarcina barkeri. Renew. Energy 2025, 241, 122310. [Google Scholar] [CrossRef]
- Ueki, T.; Nevin, K.P.; Woodard, T.L.; Aklujkar, M.A.; Holmes, D.E.; Lovley, D.R. Construction of a Geobacter Strain With Exceptional Growth on Cathodes. Front. Microbiol. 2018, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, L.; Irfan, M.; Liang, T.T.; Cheng, L.; Liu, Y.F.; Liu, J.F.; Yang, S.Z.; Sand, W.; Gu, J.D.; et al. Bioelectrochemical methane production from CO2 by Methanosarcina barkeri via direct and H2-mediated indirect electron transfer. Energy 2020, 210, 118445. [Google Scholar] [CrossRef]
- Zhou, J.; Smith, J.A.; Li, M.; Holmes, D.E. Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens. mBio 2023, 14, e00360-23. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Woodard, T.L.; Smith, J.A.; Musat, F.; Lovley, D.R. Electrobiocorrosion by microbes without outer-surface cytochromes. mLife 2024, 3, 110–118. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, C.; Nnorom, M.A.; Avignone Rossa, C.; Yang, K.; Guo, B. Multi-faceted effects and mechanisms of granular activated carbon to enhance anaerobic ammonium oxidation (anammox) for nitrogen removal from wastewater. Bioresour. Technol. 2025, 418, 132001. [Google Scholar] [CrossRef]
- Burke, S.A.; Lo, S.L.; Krzycki, J.A. Clustered Genes Encoding the Methyltransferases of Methanogenesis from Monomethylamine. J. Bacteriol. 1998, 180, 3432–3440. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion: An update. Int. Mater. Rev. 2014, 59, 384–393. [Google Scholar] [CrossRef]
- Kip, N.; Veen, J.A.v. The dual role of microbes in corrosion. Int. Soc. Microb. Ecol. 2015, 9, 542–551. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, H.; Qi, X.; Yang, T.; Zhang, J.; Chen, W.; Li, M.; Xi, B. Direct interspecies electron transfer stimulated by coupling of modified anaerobic granular sludge with microbial electrolysis cell for biogas production enhancement. Appl. Energy 2023, 341, 121100. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.T.; Dinamarca, C. Electrochemically mediated CO2 reduction for bio-methane production: A review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 531–551. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Thauer, R.K. The active species of ‘CO2’ utilized by formylmethanofuran dehydrogenase from methanogenic Archaea. Eur. J. Biochem. 1997, 248, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, O.N.; Wagner, T. All-in-One CO2 Capture and Transformation: Lessons from Formylmethanofuran Dehydrogenases. Acc. Chem. Res. 2024, 57, 3512–3523. [Google Scholar] [CrossRef] [PubMed]
- Lekbach, Y.; Liu, T.; Li, Y.; Moradi, M.; Dou, W.; Xu, D.; Smith, J.A.; Lovley, D.R. Microbial corrosion of metals: The corrosion microbiome. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2021; Volume 78, pp. 317–390. [Google Scholar]
- Paul, L.; Donald, J.; Ferguson, J.; Krzycki, J.A. The Trimethylamine Methyltransferase Gene and Multiple Dimethylamine Methyltransferase Genes of Methanosarcina barkeri Contain In-Frame and Read-Through Amber Codons. J. Bacteriol. 2000, 182, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Tsola, S.L.; Zhu, Y.; Chen, Y.; Sanders, I.A.; Economou, C.K.; Bruchert, V.; Eyice, O. Methanolobus use unspecific methyltransferases to produce methane from dimethylsulphide in Baltic Sea sediments. Microbiome 2024, 12, 3. [Google Scholar] [CrossRef]

| Gene ID | KO Name | Gene Description | Pathway Definition | FC a (MB-GAC/MB) | Log2FC b (MB-GAC/MB) |
|---|---|---|---|---|---|
| MSBRM_3095 | ------ | DUF378 domain-containing protein | ------ | 23.949 | 4.582 |
| MSBRM_3339 | ------ | hypothetical protein | ------ | 24.252 | 4.600 |
| MSBRM_2922 | ------ | STARP antigen | ------ | 2.462 | 1.300 |
| MSBRM_2359 | ------ | hypothetical protein | ------ | 12.126 | 3.600 |
| MSBRM_1961 | ------ | hypothetical protein | ------ | 14.551 | 3.863 |
| MSBRM_2245 | ------ | putative membrane-associated protein | ------ | 13.338 | 3.738 |
| MSBRM_1851 | mtmC | Monomethylamine methyltransferase corrinoid protein | Methane metabolism | 7.276 | 2.863 |
| MSBRM_2105 | fabG | 3-oxoacyl-[acyl-carrier protein] reductase | Biotin metabolism Fatty acid biosynthesis | 3.638 | 1.863 |
| MSBRM_0533 | cofD | 2-phospho-L-lactate transferase | Methane metabolism | 2.122 | 1.085 |
| MSBRM_1439 | aor | Tungsten containing aldehyde:ferredoxin oxidoreductase | Pentose phosphate pathway | 1.819 | 0.863 |
| MSBRM_1541 | mtsB | Methylthiol:coenzyme M methyltransferase corrinoid protein | Sulfur metabolism | 1.819 | 0.863 |
| MSBRM_1197 | mfnE | delta 1-pyrroline-5-carboxylate synthetase | Methane metabolism | 1.819 | 0.863 |
| MSBRM_1079 | cbiQ | Transmembrane component CbiQ of energizing module of cobalt ECF transporter | ABC transporters | 1.819 | 0.863 |
| Gene ID | KO Name | Gene Description | Pathway Definition | FC(MB_GM/MB) | Log2FC(MB_GM/MB) |
|---|---|---|---|---|---|
| MSBRM_0413 | ------ | hypothetical protein | ------ | 44.909 | 5.489 |
| MSBRM_1961 | ------ | hypothetical protein | ------ | 35.886 | 5.165 |
| MSBRM_3095 | ------ | DUF378 domain-containing protein | ------ | 10.894 | 3.445 |
| MSBRM_0718 | ------ | collagen triple helix repeat domain protein | ------ | 5.554 | 2.473 |
| MSBRM_2952 | ------ | hypothetical protein | ------ | 19.968 | 4.320 |
| MSBRM_2105 | fabG | 3-oxoacyl-[acyl-carrier protein] reductase | Biotin metabolism Fatty acid biosynthesis | 6.408 | 2.680 |
| MSBRM_1954 | trpB | Tryptophan synthase beta chain | Phenylalanine, tyrosine, and tryptophan biosynthesis | 2.563 | 1.358 |
| MSBRM_1237 | puuE | 4-aminobutyrate aminotransferase | Alanine, aspartate, and glutamate metabolism | 2.563 | 1.358 |
| MSBRM_0462 | mtbC | Dimethylamine methyltransferase corrinoid protein | Methane metabolism | 2.563 | 1.358 |
| MSBRM_0475 | ipdC | Pyruvate decarboxylase | Tryptophan metabolism | 2.563 | 1.358 |
| MSBRM_0157 | pylC | Pyrrolysine synthetase | Lysine biosynthesis | 2.563 | 1.358 |
| MSBRM_2496 | ------ | 8-oxoguanine DNA glycosylase | Base excision repair | 2.563 | 1.358 |
| MSBRM_0641 | fwdC | Formylmethanofuran dehydrogenase | Methane metabolism | 12.816 | 3.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Huang, S.; Li, H.; Dong, H.; Zhang, H.; Chen, W.; Dang, Y. Activated Carbon and Syntrophy Accelerate the Corrosion of Stainless Steel Under Strict Anaerobic Conditions by Methanosarcina barkeri. Microorganisms 2025, 13, 1278. https://doi.org/10.3390/microorganisms13061278
Zhou C, Huang S, Li H, Dong H, Zhang H, Chen W, Dang Y. Activated Carbon and Syntrophy Accelerate the Corrosion of Stainless Steel Under Strict Anaerobic Conditions by Methanosarcina barkeri. Microorganisms. 2025; 13(6):1278. https://doi.org/10.3390/microorganisms13061278
Chicago/Turabian StyleZhou, Chunyu, Shiqi Huang, Haoyong Li, He Dong, Haowen Zhang, Wenwen Chen, and Yan Dang. 2025. "Activated Carbon and Syntrophy Accelerate the Corrosion of Stainless Steel Under Strict Anaerobic Conditions by Methanosarcina barkeri" Microorganisms 13, no. 6: 1278. https://doi.org/10.3390/microorganisms13061278
APA StyleZhou, C., Huang, S., Li, H., Dong, H., Zhang, H., Chen, W., & Dang, Y. (2025). Activated Carbon and Syntrophy Accelerate the Corrosion of Stainless Steel Under Strict Anaerobic Conditions by Methanosarcina barkeri. Microorganisms, 13(6), 1278. https://doi.org/10.3390/microorganisms13061278






