The Impact of TRIM67 Knockout on Early Intestinal Antimicrobial Capacity in Mice Infected with Salmonella enterica serovar Typhimurium ATCC 14028
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacterial Strains
2.3. Construction of a S. Typhimurium Infection Mouse Model
2.4. Histological Analysis
2.5. qRT-PCR
2.6. Western Blot
2.7. Immunohistochemical Staining
2.8. Isolation and Culture of Primary Peritoneal Macrophages from Mice
2.9. In Vitro Infection
2.10. Bacterial Load Assay
2.11. Flow Cytometry
2.12. Data Analysis
3. Results
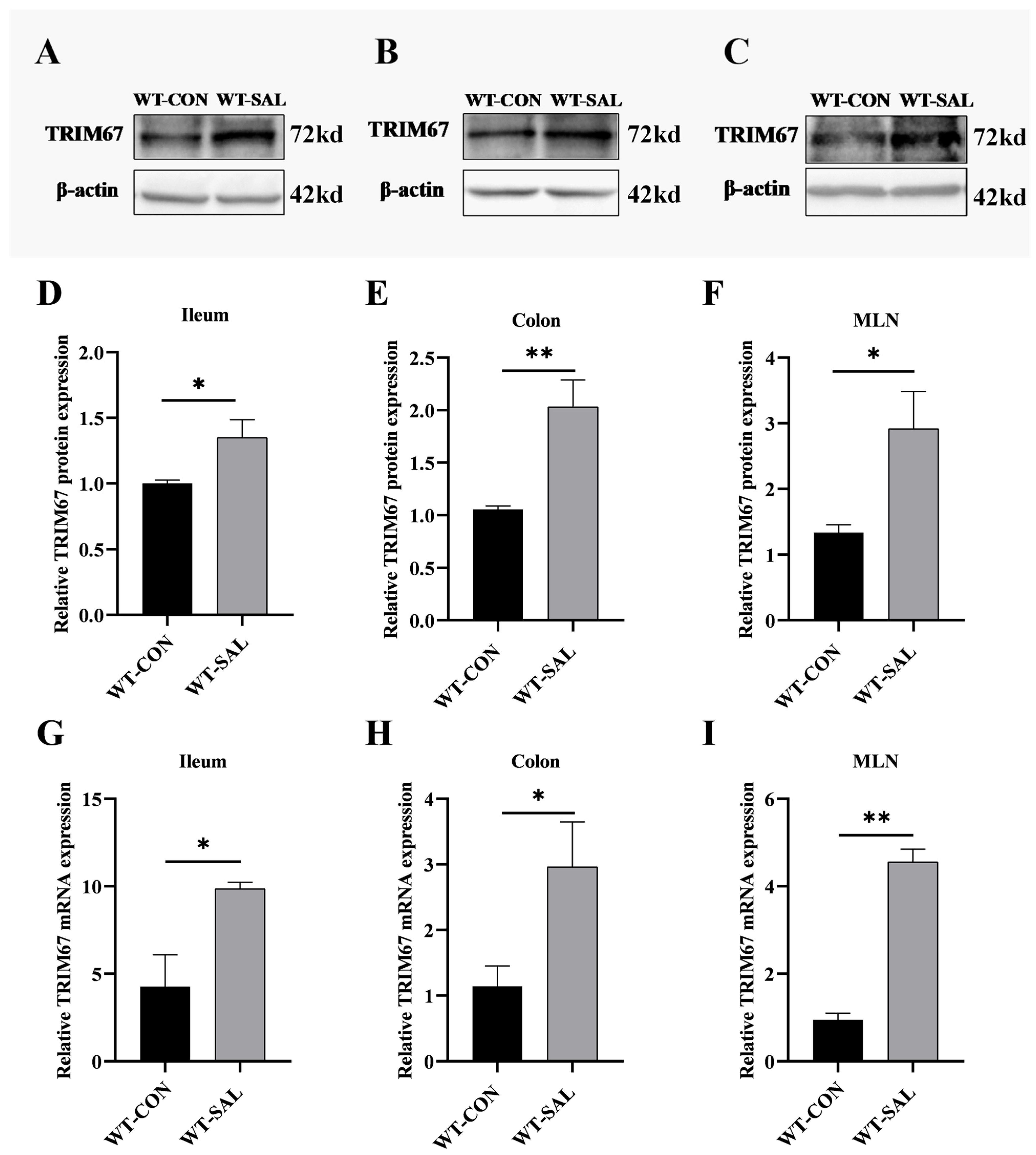
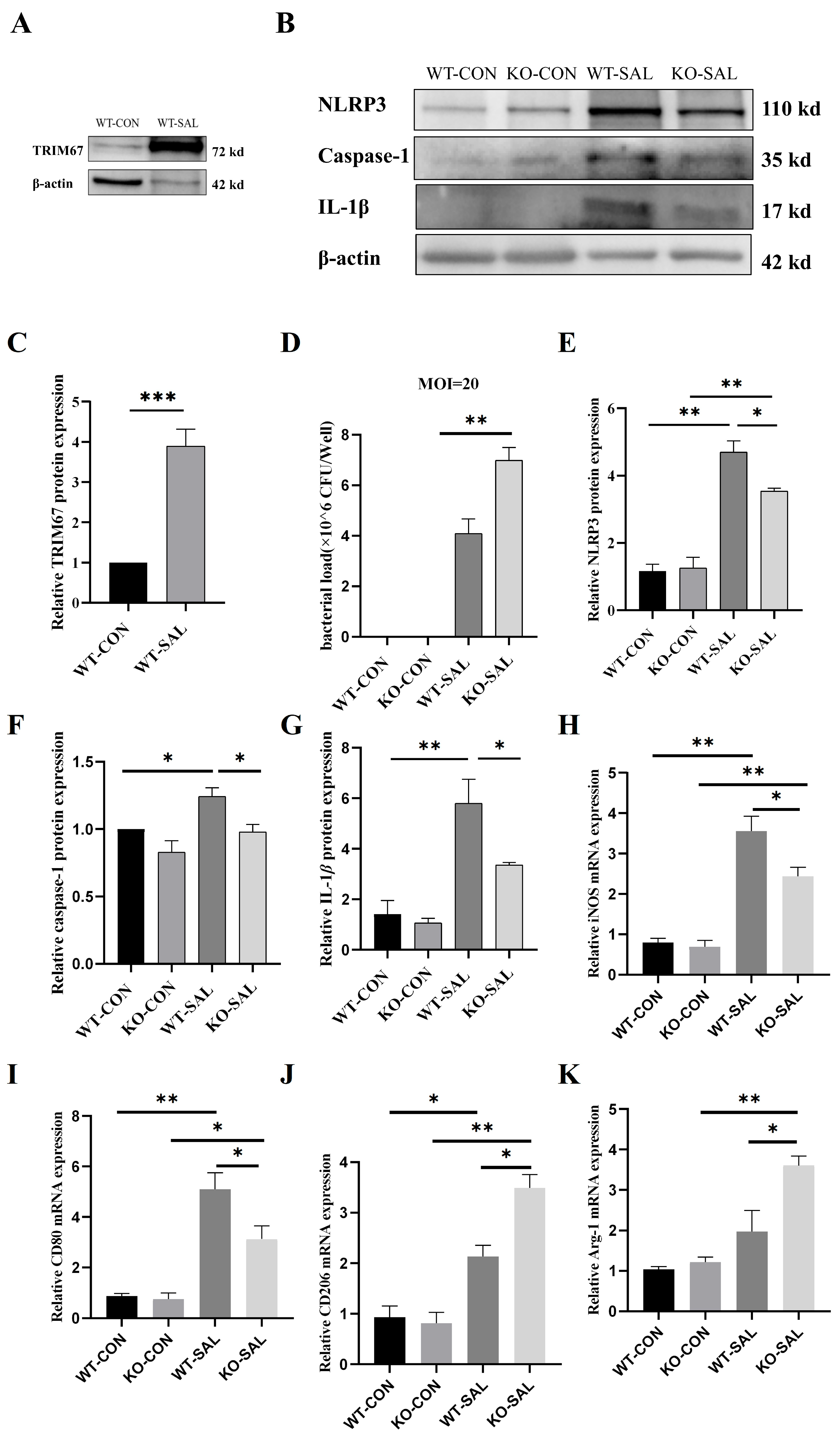
3.1. S. Typhimurium Infection Upregulates TRIM67 Expression in Mouse Ileum, Colon, and MLN
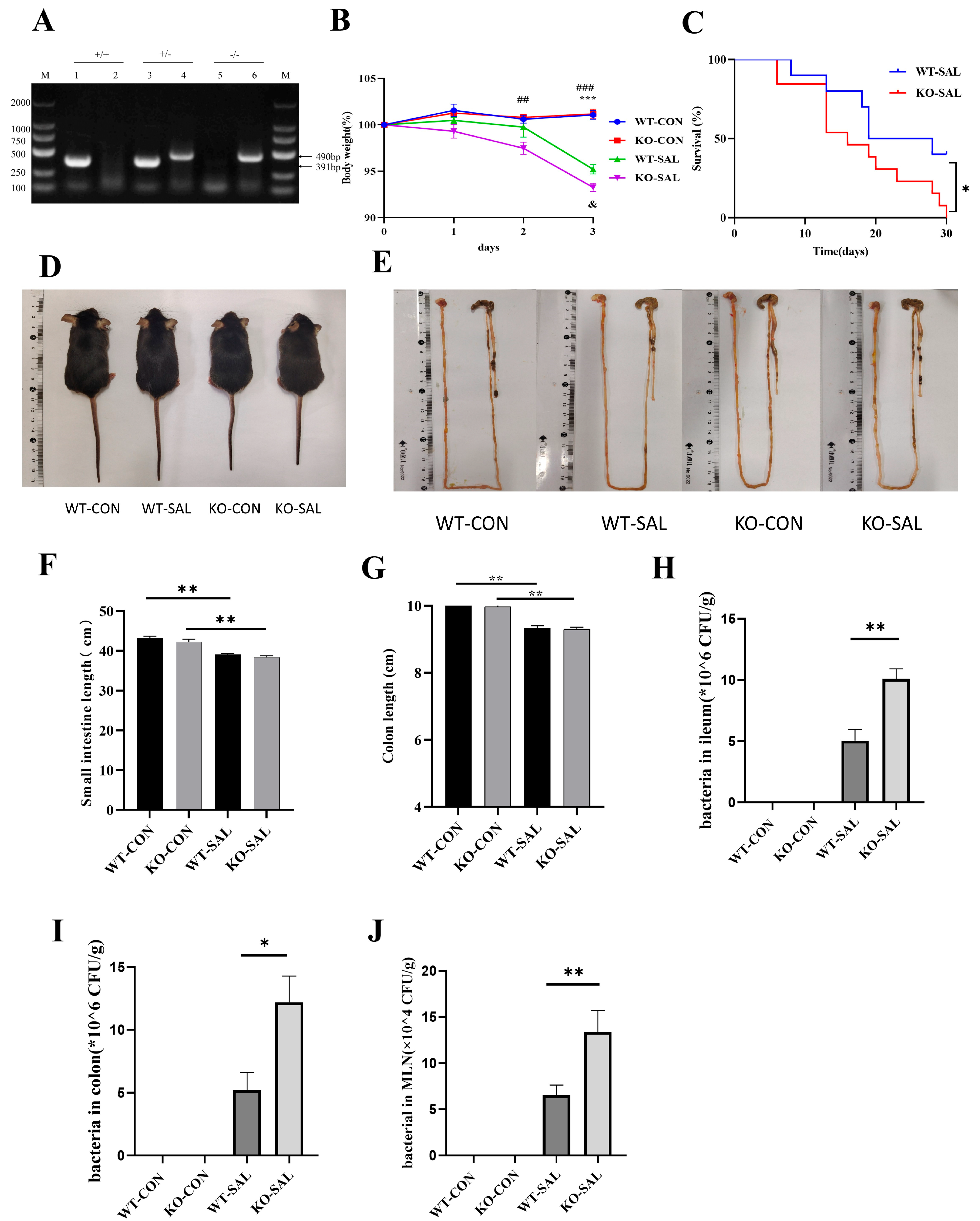
3.2. TRIM67 Knockout Exacerbates Weight Loss and Mortality in S. Typhimurium-Infected Mice
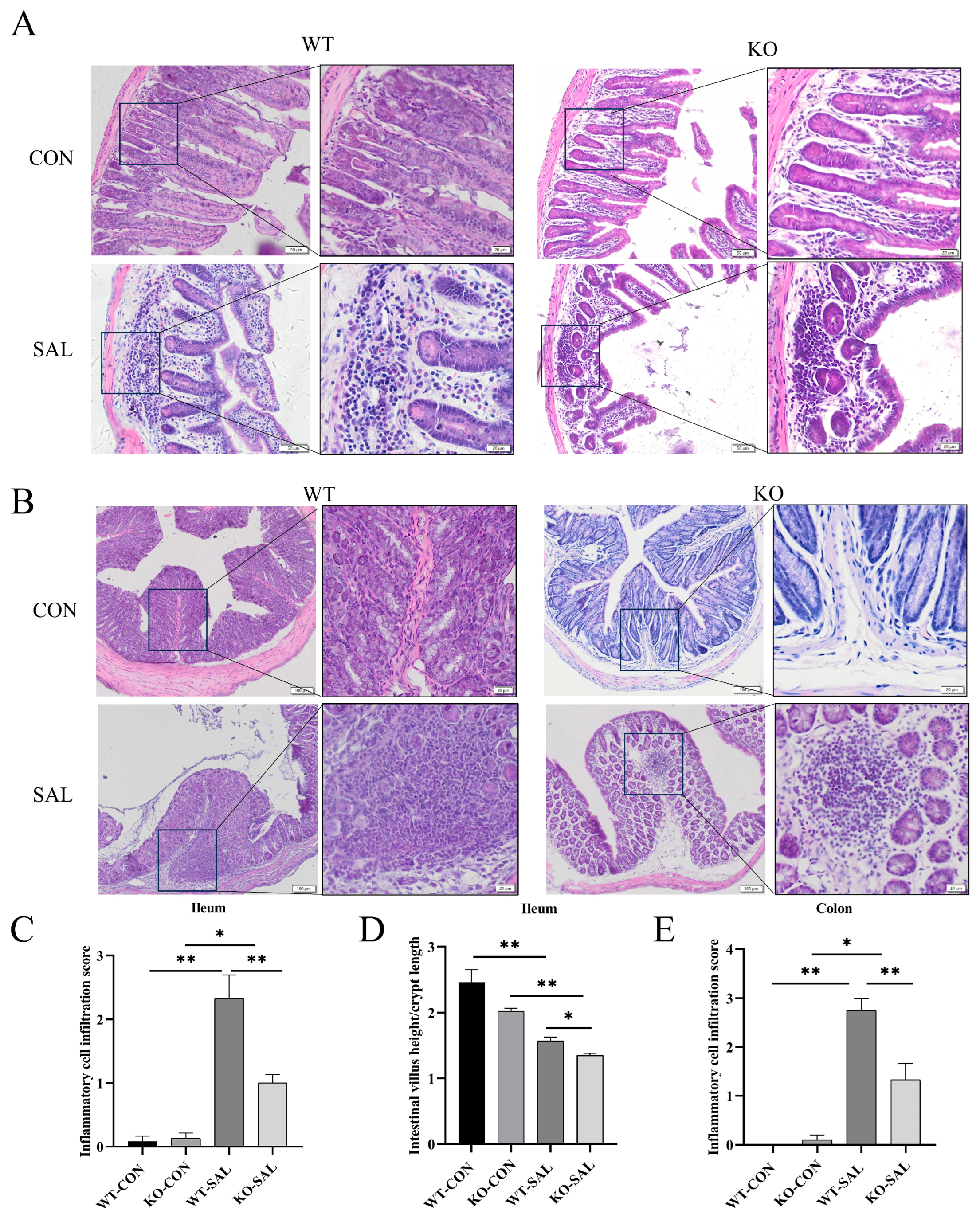
3.3. TRIM67 Knockout Inhibits Inflammatory Response and Exacerbates Intestinal Barrier Damage in the Gut of S. Typhimurium-Infected Mice
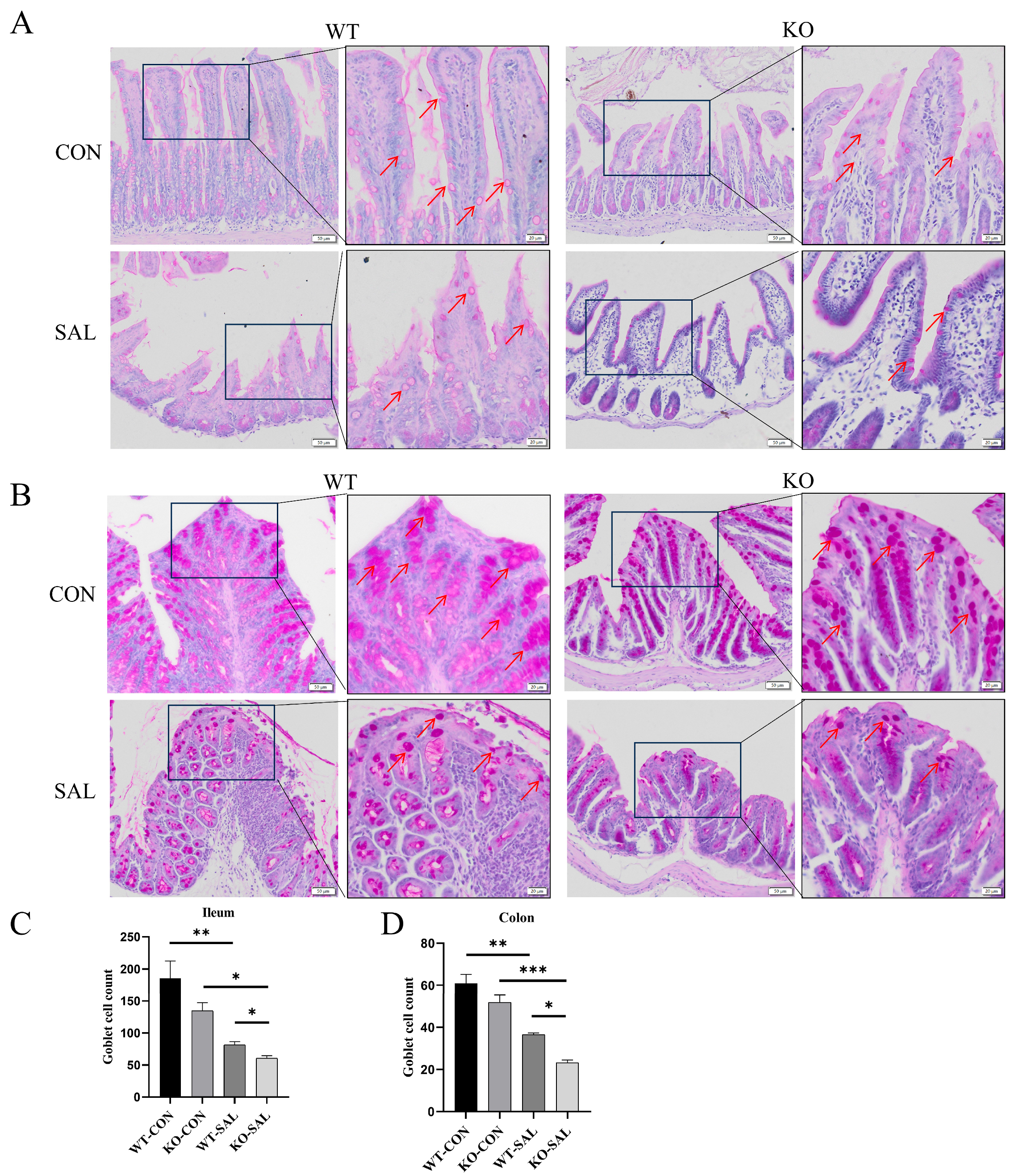
3.4. TRIM67 Regulates Intestinal Barrier Function by Modulating Goblet Cell Numbers in S. Typhimurium-Infected Mice
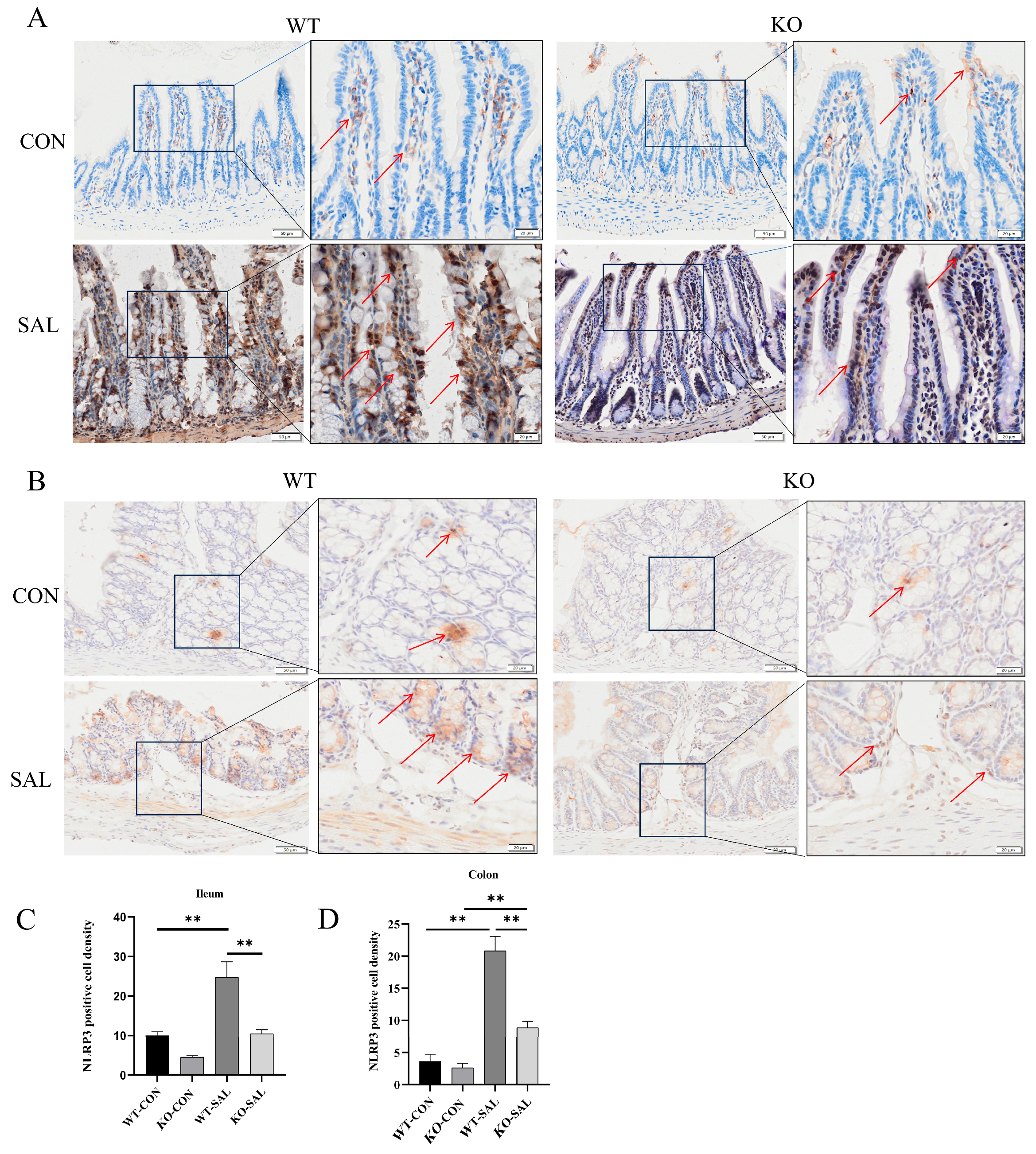
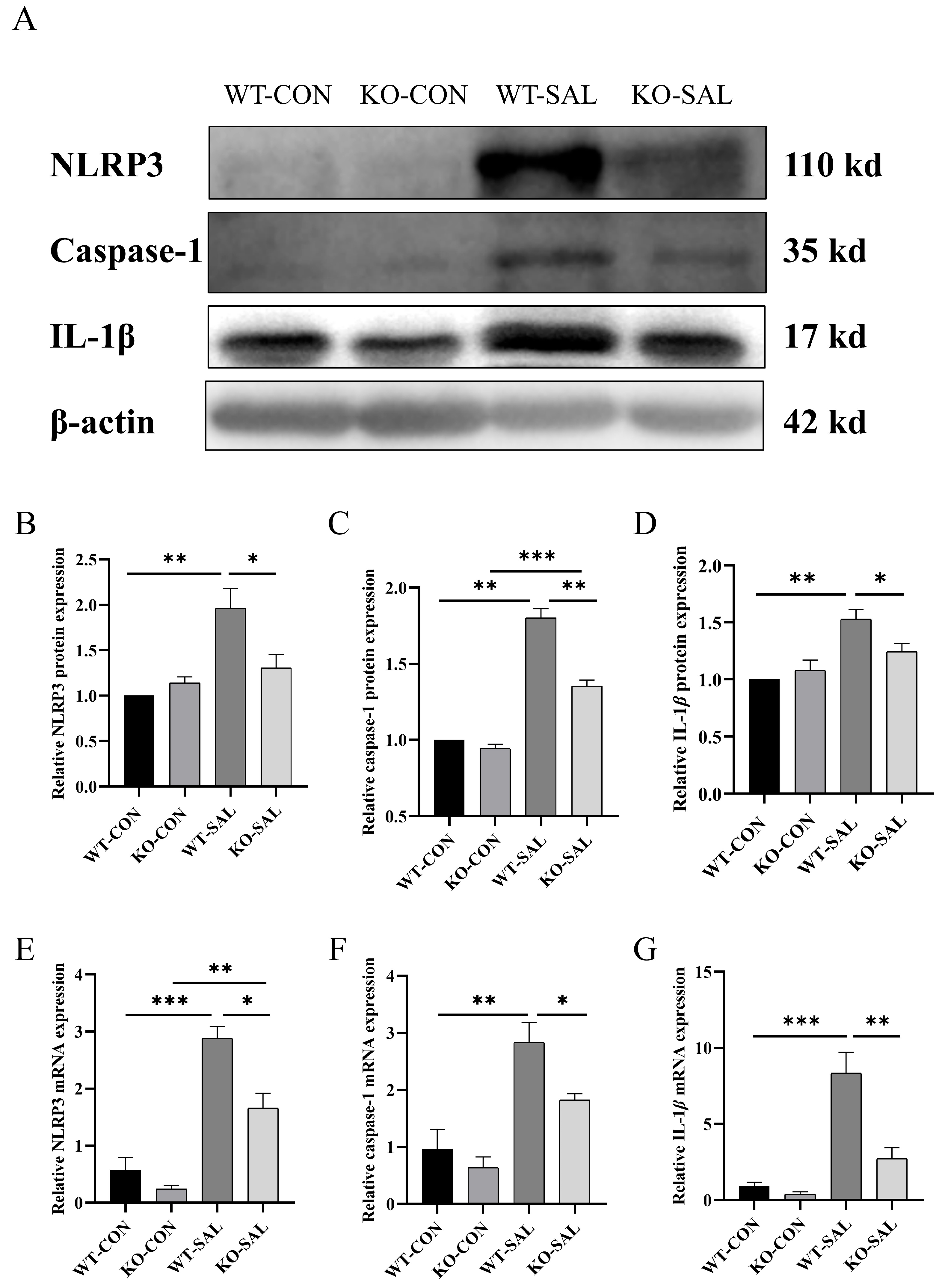
3.5. TRIM67 Knockout Reduces NLRP3 Inflammasome Activation in S. Typhimurium-Infected Mice
3.6. TRIM67 Knockout Reduces NLRP3 Inflammasome Activation in MLN of S. Typhimurium-Infected Mice
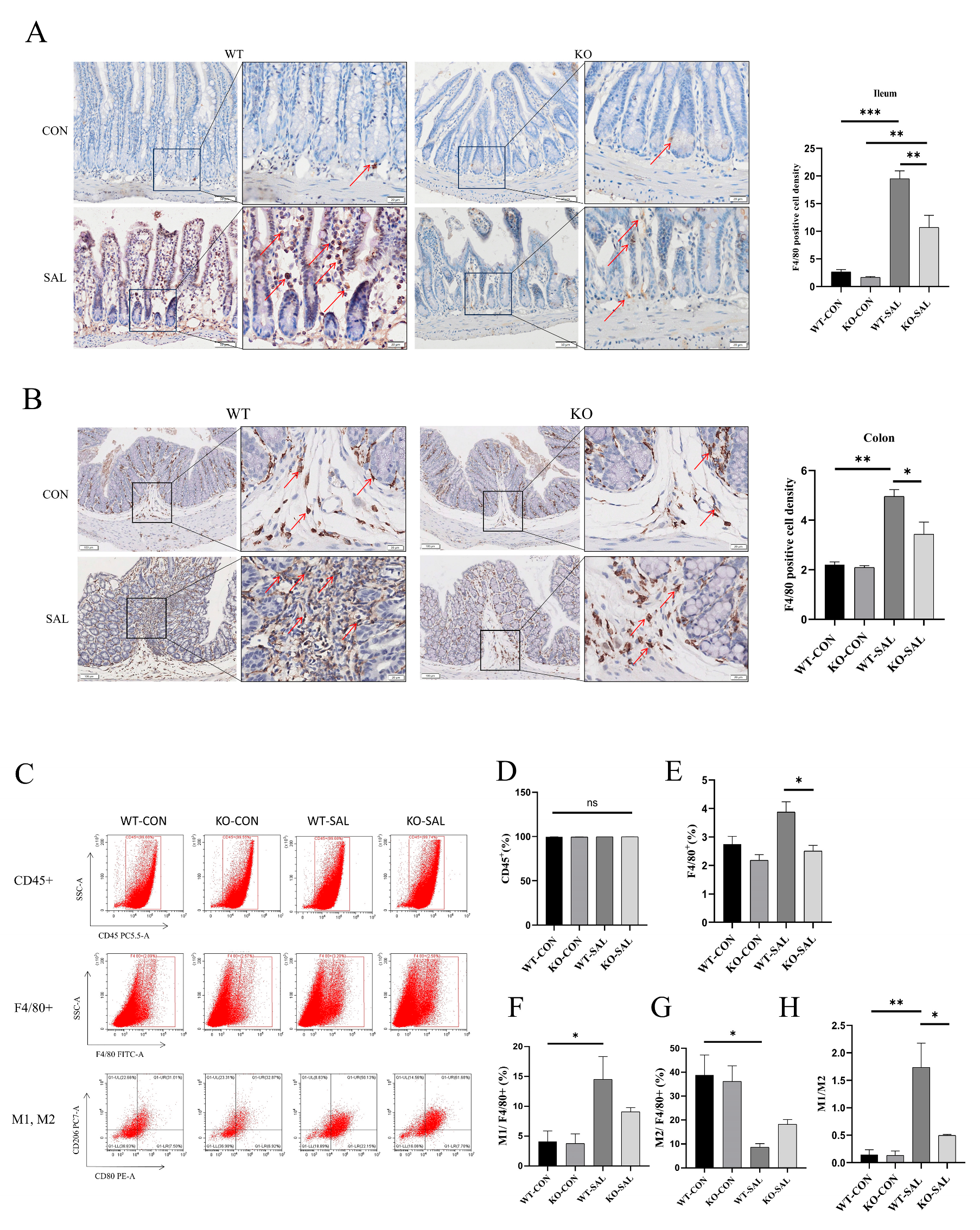
3.7. TRIM67 Knockout Inhibits Macrophage Recruitment and Polarization in the Intestine of S. Typhimurium-Infected Mice
3.8. TRIM67 Knockout Inhibits Polarization of PMs and NLRP3 Inflammasome Activation in S. Typhimurium Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.T.; Hur, S. Substrate recognition by TRIM and TRIM-like proteins in innate immunity. Semin. Cell Dev. Biol. 2021, 111, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Shariq, M.; Surolia, A.; Raj, R.; Khan, M.F.; Kumar, P. Multipronged regulation of autophagy and apoptosis: Emerging role of TRIM proteins. Cell. Mol. Biol. Lett. 2024, 29, 13. [Google Scholar] [CrossRef]
- Gushchina, L.V.; Kwiatkowski, T.A.; Bhattacharya, S.; Weisleder, N.L. Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol. Ther. 2018, 185, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, J.; Xiang, S.; Zhou, X.; Li, J. Intricate confrontation: Research progress and application potential of TRIM family proteins in tumor immune escape. J. Adv. Res. 2023, 54, 147–179. [Google Scholar] [CrossRef]
- Huang, N.; Sun, X.; Li, P.; Liu, X.; Zhang, X.; Chen, Q.; Xin, H. TRIM family contribute to tumorigenesis, cancer development, and drug resistance. Exp. Hematol. Oncol. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Y.; Xu, Q.; Xu, Y.; Guo, M.; Hu, Y.; Wang, Y.; Wang, Y. Britannilactone 1-O-acetate induced ubiquitination of NLRP3 inflammasome through TRIM31 as a protective mechanism against reflux esophagitis-induced esophageal injury. Chin. Med. 2024, 19, 118. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Q.; Liang, W.; Yan, R.; Tong, L.; Jia, M.; Zhao, C.; Zhao, W. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 2021, 12, 4794. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, H.; Ding, Z.; Wang, Z.; Yao, H.; Lin, R. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation in Helicobacter pylori-associated gastritis by regulating ROS and autophagy. Cell Commun. Signal. 2023, 21, 1. [Google Scholar] [CrossRef]
- Huang, C.; Wei, X.; Luo, Q.; Xia, Y.; Pan, T.; He, J.; Jahangir, A.; Jia, L.; Liu, W.; Zou, Y.; et al. Loss of TRIM67 Attenuates the Progress of Obesity-Induced Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7475. [Google Scholar] [CrossRef]
- Mandilara, G.; Sideroglou, T.; Chrysostomou, A.; Rentifis, I.; Papadopoulos, T.; Polemis, M.; Tzani, M.; Tryfinopoulou, K.; Mellou, K. The Rising Burden of Salmonellosis Caused by Monophasic Salmonella Typhimurium (1,4,[5],12:i:-) in Greece and New Food Vehicles. Antibiotics 2021, 10, 185. [Google Scholar] [CrossRef]
- Everest, P.; Ketley, J.; Hardy, S.; Douce, G.; Khan, S.; Shea, J.; Holden, D.; Maskell, D.; Dougan, G. Evaluation of Salmonella typhimurium mutants in a model of experimental gastroenteritis. Infect. Immun. 1999, 67, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Salmonella control in poultry flocks and its public health impact. EFSA J. Eur. Food Saf. Auth. 2019, 17, e05596. [Google Scholar]
- He, Y.; Wang, J.; Zhang, R.; Chen, L.; Zhang, H.; Qi, X.; Chen, J. Epidemiology of foodborne diseases caused by Salmonella in Zhejiang Province, China, between 2010 and 2021. Front. Public Health 2023, 11, 1127925. [Google Scholar] [CrossRef]
- Richter-Dahlfors, A.; Buchan, A.M.; Finlay, B.B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 1997, 186, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Reens, A.L.; Nagy, T.A.; Detweiler, C.S. Salmonella enterica Requires Lipid Metabolism Genes To Replicate in Proinflammatory Macrophages and Mice. Infect. Immun. 2019, 88. [Google Scholar] [CrossRef]
- Huang, K.; Fresno, A.H.; Skov, S.; Olsen, J.E. Dynamics and Outcome of Macrophage Interaction Between Salmonella Gallinarum, Salmonella Typhimurium, and Salmonella Dublin and Macrophages from Chicken and Cattle. Front. Cell. Infect. Microbiol. 2019, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Ma, S.; Yan, X.; Ma, S.; Sun, H.; Sun, Y.; Jiang, L. Lactate promotes Salmonella intracellular replication and systemic infection via driving macrophage M2 polarization. Microbiol. Spectr. 2023, 11, e0225323. [Google Scholar] [CrossRef]
- Voedisch, S.; Koenecke, C.; David, S.; Herbrand, H.; Förster, R.; Rhen, M.; Pabst, O. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect. Immun. 2009, 77, 3170–3780. [Google Scholar] [CrossRef]
- Flores-Langarica, A.; Marshall, J.L.; Bobat, S.; Mohr, E.; Hitchcock, J.; Ross, E.A.; Coughlan, R.E.; Khan, M.; Van Rooijen, N.; Henderson, I.R.; et al. T-zone localized monocyte-derived dendritic cells promote Th1 priming to Salmonella. Eur. J. Immunol. 2011, 41, 2654–2665. [Google Scholar] [CrossRef]
- Junt, T.; Moseman, E.A.; Iannacone, M.; Massberg, S.; Lang, P.A.; Boes, M.; Fink, K.; Henrickson, S.E.; Shayakhmetov, D.M.; Di Paolo, N.C.; et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007, 450, 110–114. [Google Scholar] [CrossRef]
- Lämmermann, T.; Sixt, M. The microanatomy of T-cell responses. Immunol. Rev. 2008, 221, 26–43. [Google Scholar] [CrossRef]
- Griffin, A.J.; Li, L.X.; Voedisch, S.; Pabst, O.; McSorley, S.J. Dissemination of persistent intestinal bacteria via the mesenteric lymph nodes causes typhoid relapse. Infect. Immun. 2011, 79, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, R.; Cao, Z.; Wu, Q.; Wang, J.; Lyu, W. NLRP3 Inflammasomes: Dual Function in Infectious Diseases. J. Immunol. 2024, 213, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Brickey, W.J.; Ting, J.P.; Sad, S. Isolates of Salmonella typhimurium circumvent NLRP3 inflammasome recognition in macrophages during the chronic phase of infection. J. Biol. Chem. 2022, 298, 101461. [Google Scholar]
- Diamond, C.E.; Leong, K.W.K.; Vacca, M.; Rivers-Auty, J.; Brough, D.; Mortellaro, A. Salmonella typhimurium-induced IL-1 release from primary human monocytes requires NLRP3 and can occur in the absence of pyroptosis. Sci. Rep. 2017, 7, 6861. [Google Scholar] [CrossRef]
- Gram, A.M.; Wright, J.A.; Pickering, R.J.; Lam, N.L.; Booty, L.M.; Webster, S.J.; Bryant, C.E. Salmonella Flagellin Activates NAIP/NLRC4 and Canonical NLRP3 Inflammasomes in Human Macrophages. J. Immunol. 2021, 206, 631–640. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, Q.; Wu, Z.; Huang, L.; Sun, T.; Ling, C.; Zhang, B.; Chen, C.; Wang, H. Berberine inhibits NLRP3 inflammasome activation and proinflammatory macrophage M1 polarization to accelerate peripheral nerve regeneration. Neurother. J. Am. Soc. Exp. Neurother. 2024, 21, e00347. [Google Scholar] [CrossRef]
- Nie, Z.; Fan, Q.; Jiang, W.; Wei, S.; Luo, R.; Hu, H.; Liu, G.; Lei, Y.; Xie, S. Placental mesenchymal stem cells suppress inflammation and promote M2-like macrophage polarization through the IL-10/STAT3/NLRP3 axis in acute lung injury. Front. Immunol. 2024, 15, 1422355. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Zhang, L.; Shi, C.; Zhou, J.; Wang, P.; Wang, H.; Shi, X.; Wei, S.; Wang, Q.; et al. TGR5 Regulates Macrophage Inflammation in Nonalcoholic Steatohepatitis by Modulating NLRP3 Inflammasome Activation. Front. Immunol. 2020, 11, 609060. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Liang, P.; Wang, X.; Liu, Y.; Cai, J.; She, Y.; Wang, D.; Wang, Z.; Guo, Z.; et al. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell. Mol. Immunol. 2021, 18, 2431–2442. [Google Scholar] [CrossRef]
- Luo, Q.; Jahangir, A.; He, J.; Huang, C.; Xia, Y.; Jia, L.; Wei, X.; Pan, T.; Du, Y.; Mu, B.; et al. Ameliorating Effects of TRIM67 against Intestinal Inflammation and Barrier Dysfunction Induced by High Fat Diet in Obese Mice. Int. J. Mol. Sci. 2022, 23, 7650. [Google Scholar] [CrossRef]
- Nilsson, O.R.; Kari, L.; Steele-Mortimer, O. Foodborne infection of mice with Salmonella Typhimurium. PLoS ONE 2019, 14, e0215190. [Google Scholar] [CrossRef]
- De Jesus, A.; Pusec, C.M.; Nguyen, T.; Keyhani-Nejad, F.; Gao, P.; Weinberg, S.E.; Ardehali, H. Optimized protocol to isolate primary mouse peritoneal macrophage metabolites. STAR Protoc. 2022, 3, 101668. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.Q.; Gordon, S. Isolation and Culture of Murine Macrophages. In Basic Cell Culture Protocols; Helgason, C.D., Miller, C.L., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 91–103. [Google Scholar]
- Liu, D.; Wang, Q.; He, W.; Ge, L.; Huang, K. Deoxynivalenol aggravates the immunosuppression in piglets and PAMs under the condition of PEDV infection through inhibiting TLR4/NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 231, 113209. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Xie, Q.M.; Zhao, C.C.; Xu, J.; Fan, X.Y.; Fei, G.H. Melatonin biosynthesis restored by CpG oligodeoxynucleotides attenuates allergic airway inflammation via regulating NLRP3 inflammasome. Life Sci. 2019, 239, 117067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zha, B.; Shen, Q.; Zou, H.; Cheng, C.; Wu, H.; Liu, R. Sevoflurane Inhibits the Th2 Response and NLRP3 Expression in Murine Allergic Airway Inflammation. J. Immunol. Res. 2018, 2018, 9021037. [Google Scholar] [CrossRef]
- Versteeg, G.A.; Benke, S.; García-Sastre, A.; Rajsbaum, R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014, 25, 563–576. [Google Scholar] [CrossRef]
- Kimura, T.; Jain, A.; Choi, S.W.; Mandell, M.A.; Johansen, T.; Deretic, V. TRIM-directed selective autophagy regulates immune activation. Autophagy 2017, 13, 989–990. [Google Scholar] [CrossRef]
- Jiang, M.X.; Hong, X.; Liao, B.B.; Shi, S.Z.; Lai, X.F.; Zheng, H.Y.; Xie, L.; Wang, Y.; Wang, X.L.; Xin, H.B.; et al. Expression profiling of TRIM protein family in THP1-derived macrophages following TLR stimulation. Sci. Rep. 2017, 7, 42781. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, T.; Li, Q.; Xiong, X.; Wang, J.; Zhu, X.; Zhou, X.; Zhang, L.; Zhu, Y.; Peng, Y.; et al. TRIM25 upregulation by Mycobacterium tuberculosis infection promotes intracellular survival of M.tb in RAW264.7 cells. Microb. Pathog. 2020, 148, 104456. [Google Scholar] [CrossRef] [PubMed]
- Hos, N.J.; Fischer, J.; Hos, D.; Hejazi, Z.; Calabrese, C.; Ganesan, R.; Murthy, A.M.V.; Rybniker, J.; Kumar, S.; Krönke, M.; et al. TRIM21 Is Targeted for Chaperone-Mediated Autophagy during Salmonella Typhimurium Infection. J. Immunol. 2020, 205, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lv, X.; Sun, C.; Sun, Y.; Yang, M.; Ma, D.; Jing, W.; Zhao, Y.; Cheng, Y.; Xuan, H.; et al. TRIM50 promotes NLRP3 inflammasome activation by directly inducing NLRP3 oligomerization. EMBO Rep. 2022, 23, e54569. [Google Scholar] [CrossRef]
- Deng, N.H.; Zhou, Z.X.; Liu, H.T.; Tian, Z.; Wu, Z.F.; Liu, X.Y.; Xiong, W.H.; Wang, Z.; Jiang, Z.S. TRIMs: Generalists Regulating the NLRP3 Inflammasome Signaling Pathway. DNA Cell Biol. 2022, 41, 262–275. [Google Scholar] [CrossRef]
- Tang, T.; Li, P.; Zhou, X.; Wang, R.; Fan, X.; Yang, M.; Qi, K. The E3 Ubiquitin Ligase TRIM65 Negatively Regulates Inflammasome Activation Through Promoting Ubiquitination of NLRP3. Front. Immunol. 2021, 12, 741839. [Google Scholar] [CrossRef]
- Naseer, N.; Egan, M.S.; Reyes Ruiz, V.M.; Scott, W.P.; Hunter, E.N.; Demissie, T.; Rauch, I.; Brodsky, I.E.; Shin, S. Human NAIP/NLRC4 and NLRP3 inflammasomes detect Salmonella type III secretion system activities to restrict intracellular bacterial replication. PLoS Pathog. 2022, 18, e1009718. [Google Scholar] [CrossRef]
- Wang, H.; Lou, J.; Liu, H.; Liu, Y.; Xie, B.; Zhang, W.; Xie, J.; Pan, H.; Han, W. TRIM59 deficiency promotes M1 macrophage activation and inhibits colorectal cancer through the STAT1 signaling pathway. Sci. Rep. 2024, 14, 16081. [Google Scholar] [CrossRef]
- Yu, T.; Gan, S.; Zhu, Q.; Dai, D.; Li, N.; Wang, H.; Chen, X.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef]
- Petit, V.; Parcelier, A.; Mathé, C.; Barroca, V.; Torres, C.; Lewandowski, D.; Ferri, F.; Gallouët, A.S.; Dalloz, M.; Dinet, O.; et al. TRIM33 deficiency in monocytes and macrophages impairs resolution of colonic inflammation. EBioMedicine 2019, 44, 60–70. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps-myth or reality? Clin. Sci. (Lond. Engl. 1979) 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
| Score | Description |
|---|---|
| 0 | No inflammatory cell infiltration or minimal infiltration confined to the mucosal layer. |
| 1 | Increased inflammatory cells, continuously distributed in the lamina propria. |
| 2 | Significantly increased inflammatory cells, but not fully penetrating the submucosal layer. |
| 3 | Transmural inflammatory cell infiltration, involving the lamina propria, submucosa, and muscularis mucosa. |
| Gene | Sequence (5′-3′) |
|---|---|
| β-actin | F: AGAGGGAAATCGTGCGTGAC |
| R: CAATAGTGATGACCTGGCCGT | |
| TRIM67 | F: ACTCGGCAGAAAGCCAAGC |
| R: CTGCTCTTGCGAGGTTTGC | |
| NLRP3 | F: TCCACAATTCTGACCCACAA |
| R: ACCTCACAGAGGGTCACCAC | |
| IL-1β | F: TCTTTGAAGTTGACGGACCC |
| R: TGAGTGATACTGCCTGCCTG | |
| Caspase-1 | F: AAACACCCACTCGTACACGTCTTG |
| R: AGGTCAACATCAGCTCCGACTCTC | |
| Arg-1 | F: CTCCAAGCCAAAGTCCTTAGAG |
| R: GGAGCTGTCATTAGGGACATCA | |
| iNOS | F: ACATCGACCCGTCCACAGTAT |
| R: CAGAGGGGTAGGCTTGTCTC | |
| CD206 | F: CTCTGTTCAGCTATTGGACGC |
| R: TGGCACTCCCAAACATAATTTGA | |
| CD80 | F: TCCAAGGCTCATTCT |
| R: TTGTAACGGCAAGG |
| Antibody | Company | Catalog/Purpose and Dilution Ratio |
|---|---|---|
| β-Actin Rabbit mAb | ABclonal | AC026/WB 1:100,000 |
| NLRP3 Rabbit mAb | ABclonal | A24294/WB 1:1000, IHC 1:200 |
| Caspase-1/Cleaved Caspase-1 | Wanleibio | WL03450/WB 1:1000 |
| IL-1β Rabbit pAb | ABclonal | A1112/WB 1:1000 |
| F4/80 Rabbit pAb | Bioss | bs11182R/IHC 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Q.; Zhang, T.; Jia, L.; Liu, W.; Huang, C.; Chen, Z.; Luo, Q. The Impact of TRIM67 Knockout on Early Intestinal Antimicrobial Capacity in Mice Infected with Salmonella enterica serovar Typhimurium ATCC 14028. Microorganisms 2025, 13, 1267. https://doi.org/10.3390/microorganisms13061267
Zhang X, Li Q, Zhang T, Jia L, Liu W, Huang C, Chen Z, Luo Q. The Impact of TRIM67 Knockout on Early Intestinal Antimicrobial Capacity in Mice Infected with Salmonella enterica serovar Typhimurium ATCC 14028. Microorganisms. 2025; 13(6):1267. https://doi.org/10.3390/microorganisms13061267
Chicago/Turabian StyleZhang, Xinyue, Qinyuan Li, Tingting Zhang, Lanlan Jia, Wentao Liu, Chao Huang, Zhengli Chen, and Qihui Luo. 2025. "The Impact of TRIM67 Knockout on Early Intestinal Antimicrobial Capacity in Mice Infected with Salmonella enterica serovar Typhimurium ATCC 14028" Microorganisms 13, no. 6: 1267. https://doi.org/10.3390/microorganisms13061267
APA StyleZhang, X., Li, Q., Zhang, T., Jia, L., Liu, W., Huang, C., Chen, Z., & Luo, Q. (2025). The Impact of TRIM67 Knockout on Early Intestinal Antimicrobial Capacity in Mice Infected with Salmonella enterica serovar Typhimurium ATCC 14028. Microorganisms, 13(6), 1267. https://doi.org/10.3390/microorganisms13061267






