Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study
Abstract
1. Introduction
1.1. Lactoferrin and Oral Disease
1.2. Oral Candidiasis
1.3. Photodynamic Therapy and Lactoferrin
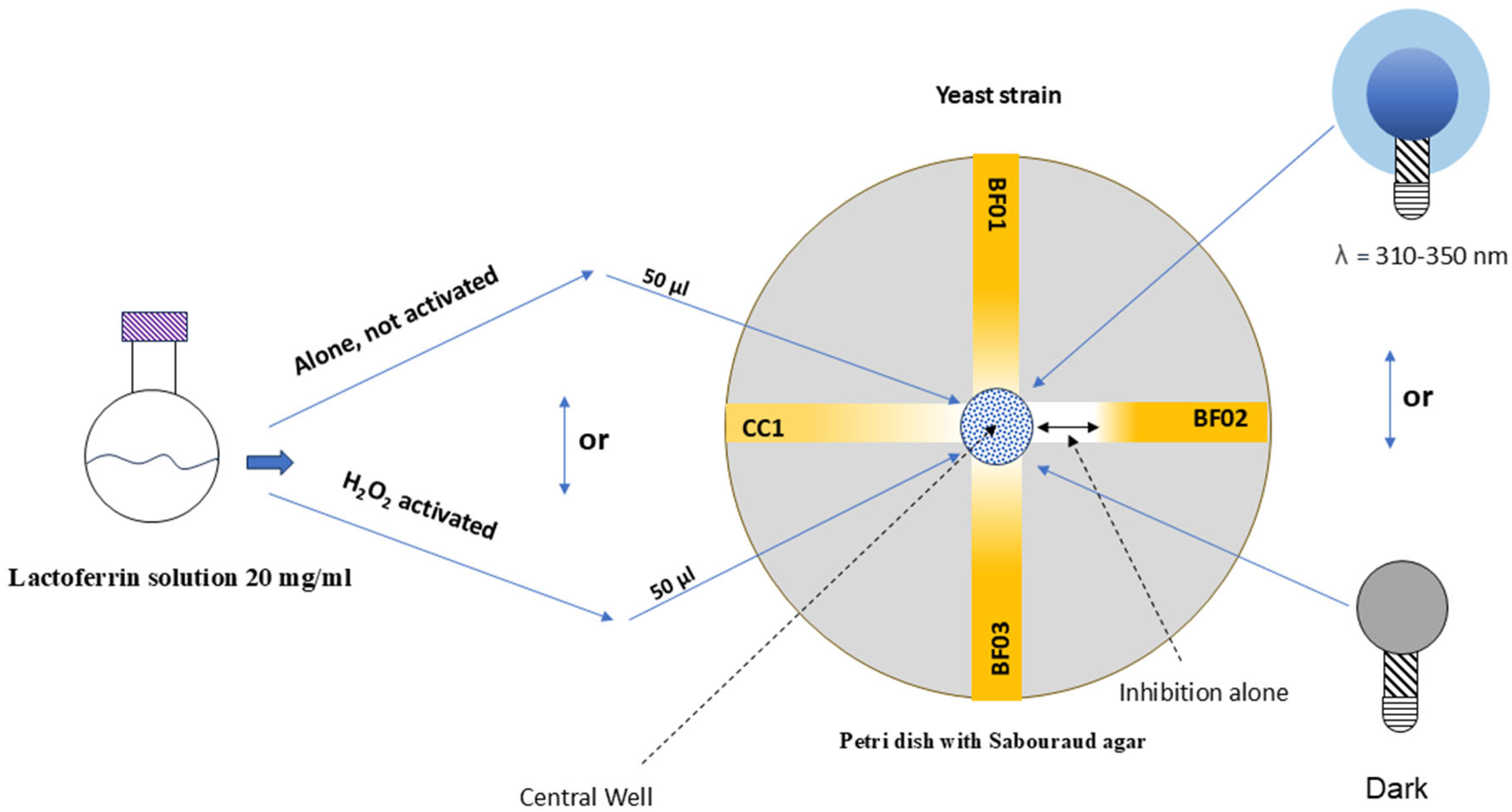
2. Materials and Methods
2.1. Candida spp. Tested
2.2. Lactoferrin Mixture Photosensitizer
2.3. Lights to Activate Lactoferrin Mixture
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrówka, M.; Duda-Madej, A.; Pietluch, F.; Mackiewicz, P.; Gagat, P. Testing Antimicrobial Properties of Human Lactoferrin-Derived Fragments. Int. J. Mol. Sci. 2023, 24, 10529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krupińska, A.M.; Bogucki, Z. Clinical aspects of the use of lactoferrin in dentistry. J. Oral Biosci. 2021, 63, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, K.E.; Payne, R.J.; Carter, D.A. Lactoferrin-Derived Peptide Lactofungin Is Potently Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e00842-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakano, M.; Suzuki, M.; Wakabayashi, H.; Hayama, K.; Yamauchi, K.; Abe, F.; Abe, S. Synergistic anti-candida activities of lactoferrin and the lactoperoxidase system. Drug Discov. Ther. 2019, 13, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Seo, M.; Park, T.E.; Lee, D.Y. A novel therapeutic strategy of multimodal nanoconjugates for state-of-the-art brain tumor phototherapy. J. Nanobiotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Telles, D.R.; Karki, N.; Marshall, M.W. Oral fungal infections: Diagnosis and management. Dent. Clin. N. Am. 2017, 61, 319–349. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D. Oral Fungal Infections: Past, Present, and Future. Front. Oral Health 2022, 3, 838639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, K.P.; Kuan, C.S.; Kaur, H.; Na, S.L.; Atiya, N.; Velayuthan, R.D. Candida species epidemiology 2000-2013: A laboratory-based report. Trop. Med. Int. Health 2015, 20, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, Q.; Zhu, F.; An, Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srivastava, N.; Ellepola, K.; Venkiteswaran, N.; Chai, L.Y.A.; Ohshima, T.; Seneviratne, C.J. Lactobacillus Plantarum 108 Inhibits Streptococcus mutans and Candida albicans Mixed-Species Biofilm Formation. Antibiotics 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens 2022, 11, 335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zomorodian, K.; Haghighi, N.N.; Rajaee, N.; Pakshir, K.; Tarazooie, B.; Vojdani, M.; Sedaghat, F.; Vosoghi, M. Assessment of Candida species colonization and denture-related stomatitis in complete denture wearers. Med. Mycol. 2011, 49, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.P.F.M.; Consani, R.L.X.; Sardi, J.C.O.; Mesquita, M.F.; Silva, M.C.V.S.; Sinhoreti, M.A.C. Biofilm formation in denture base acrylic resins and disinfection method using microwave. J. Res. Pract. Dent. 2014, 2014, 112424. [Google Scholar] [CrossRef][Green Version]
- Mothibe, J.V.; Patel, M. Pathogenic characteristics of Candida albicans isolated from oral cavities of denture wearers and cancer patients wearing oral prostheses. Microb. Pathog. 2017, 110, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Brizuela, M.; Raja, A. Oral Candidiasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; Lo Muzio, L.; Pérez-Sayáns, M. Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. J. Fungi 2022, 8, 1093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arya, C.P.; Jaiswal, R.; Tandon, A.; Jain, A. Isolation and identification of oral Candida species in potentially malignant disorder and oral squamous cell carcinoma. Natl. J. Maxillofac. Surg. 2021, 12, 387–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subramani, M.B.; Mahalakshmi, K.; Jaya, B.; Sankari, S.L.; Kumar, V.N. Candida Albicans Candidalysin ECE1 Gene—A Potent Virulence Factor for Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Indian. J. Dent. Res. 2024, 35, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Saraneva, O.; Furuholm, J.; Hagström, J.; Sorsa, T.; Rita, V.; Tervahartiala, T.; Välimaa, H.; Ruokonen, H. Oral Potentially Malignant Disorders and Candida in Oral Tongue Squamous Cell Carcinoma Patients. Dent. J. 2023, 11, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhodes, J.; Jacobs, J.; Dennis, E.K.; Manjari, S.R.; Banavali, N.K.; Marlow, R.; Rokebul, M.A.; Chaturvedi, S.; Chaturvedi, V. What makes Candida auris pan-drug resistant? Integrative insights from genomic, transcriptomic, and phenomic analysis of clinical strains resistant to all four major classes of antifungal drugs. Antimicrob. Agents Chemother. 2024, 68, e0091124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brassington, P.J.T.; Klefisch, F.R.; Graf, B.; Pfüller, R.; Kurzai, O.; Walther, G.; Barber, A.E. Genomic reconstruction of an azole-resistant Candida parapsilosis outbreak and the creation of a multi-locus sequence typing scheme: A retrospective observational and genomic epidemiology study. Lancet Microbe 2025, 6, 100949. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Jiang, L.; Huang, H. Photodynamic therapy with photodegradable photosensitizers. Chem. Commun. 2025, 61, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.C.D.S.; Calvi, G.S.; Rodrigues, A.B.F.; Costa, M.S. The inhibitory effect of photodynamic therapy on dual-species biofilms of Candida albicans and Candida krusei can be determined by Candida albicans/Candida krusei ratio. Photodiagn. Photodyn. Ther. 2023, 44, 103787. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Orrù, G. Potential of photodynamic therapy in the management of infectious oral diseases. World J. Exp. Med. 2024, 14, 84284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues, A.B.F.; Passos, J.C.D.S.; Costa, M.S. Effect of antimicrobial photodynamic therapy using toluidine blue on dual-species biofilms of Candida albicans and Candida krusei. Photodiagn. Photodyn. Ther. 2023, 42, 103600. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.B.; Ziental, D.; Dlugaszewska, J.; Sobotta, L.; Torres, T.; Rodríguez-Morgade, M.S. Multicationic ruthenium phthalocyanines as photosensitizers for photodynamic inactivation of multiresistant microbes. Eur. J. Med. Chem. 2025, 285, 117214. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Orrù, G.; Scano, A. Curcumin/H2O2 photodynamically activated: An antimicrobial time-response assessment against an MDR strain of Candida albicans. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8841–8851. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Piras, V.; Ciusa, M.L.; Taccori, F.; Pisano, M.B.; Montaldo, C.; Cosentino, S.; Fadda, M.E. Azole resistance and ERG11 464 polymorphism in oral Candida albicans clinical strains isolated in Sardinia. Open Mycol. 2008, 2, 82–85. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, H.G.; Li, P.; Wang, Y.Z.; Huang, G.X.; Xing, L.; Wang, J.Q.; Zheng, N. Effect of Heat Treatment on the Antitumor Activity of Lactoferrin in Human Colon Tumor (HT29) Model. J. Agric. Food Chem. 2019, 67, 140–147. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Short communication: Antimicrobial effect of lactoferrin and its amidated and pepsin-digested derivatives against Salmonella Enteritidis and Pseudomonas fluorescens. J. Dairy Sci. 2010, 93, 3965–3969. [Google Scholar] [CrossRef] [PubMed]
- Soldà, A.; Cantelli, A.; Di Giosia, M.; Montalti, M.; Zerbetto, F.; Rapino, S.; Calvaresi, M. C60@lysozyme: A new photosensitizing agent for photodynamic therapy. J. Mater. Chem. B 2017, 5, 6608–6615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust. Dent. J. 2000, 45, 257–269; quiz 289. [Google Scholar] [CrossRef] [PubMed]
- Casu, C. Spray of hydrogen peroxide for infection prevention and control of SARS COV 2 infection: Could this be possible? Pan Afr. Med. J. 2020, 35 (Suppl. 2), 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cervantes Trejo, A.; Castañeda, I.D.; Rodríguez, A.C.; Andrade Carmona, V.R.; Mercado, M.D.P.C.; Vale, L.S.; Cruz, M.; Barrero Castillero, S.; Consuelo, L.C.; Di Silvio, M. Hydrogen Peroxide as an Adjuvant Therapy for COVID-19: A Case Series of Patients and Caregivers in the Mexico City Metropolitan Area. Evid. Based Complement. Alternat Med. 2021, 2021, 5592042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mailart, M.C.; Carvalho Dos Santos, K.; Torres, C.R.G.; Borges, A.B. Efficacy and safety of peroxide-based mouthrinse on whitening treatment: A randomized controlled clinical trial. J. Dent. 2025, 154, 105584. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.T.; Majoie, I.M.; van Dongen, J.W.; van Weelden, H.; van Vloten, W.A. Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.A.; Baioni, J.C.; de Oliveira, J.; Sendyk, W.R.; Kimura, J.S.; Tanaka, M.H.; Scatolin, R.S. Evaluation of the efficacy of the use of violet LED light in the bleaching of damaged primary incisors darkened by trauma. Photodiagn. Photodyn. Ther. 2023, 41, 103239. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, E.V.; Costa, J.L.S.G.; Silva, C.L.A.; Barros, A.P.O.; Machado, B.L.; Casarin, H.H.; Besegato, J.F.; Kuga, M.C.; Silva, C.M. Influence of blue and violet LED and infrared laser on the temperature of bleaching protocols in different concentrations of hydrogen peroxide. Photodiagn. Photodyn. Ther. 2024, 45, 104006. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.R.; Kim, J.H.; Hong, J.Y.; Seok, J.; Kim, J.M.; Bak, D.H.; Choi, M.J.; Mun, S.K.; Kim, C.W.; Kim, B.J. Irradiation with 310 nm and 340 nm ultraviolet light-emitting-diodes can improve atopic dermatitis-like skin lesions in NC/Nga mice. Photochem. Photobiol. Sci. 2018, 17, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Chiam, T.L.; Choo, J.; Ashar, A.; Hussaini, H.M.; Rajandram, R.K.; Nordin, R. Efficacy of natural enzymes mouthwash: A randomised controlled trial. Clin. Oral Investig. 2024, 28, 259. [Google Scholar] [CrossRef] [PubMed]
- Sindhusha, V.B.; Rajasekar, A. Efficacy of Oxygen-Enriched Mouthwash as a Pre-procedural Mouth Rinse Against Oral Microbes Produced During Ultrasonic Scaling. Cureus 2023, 15, e49164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, T.A.; Ibrahim, H.M.; Ibrahim, F.; Samy, A.M.; Fetoh, E.; Nutan, M.T. In vitro release, rheological, and stability studies of mefenamic acid coprecipitates in topical formulations. Pharm. Dev. Technol. 2011, 16, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Clares, B.; Mallandrich, M.; Suñer-Carbó, J.; Montes, M.J.; Calpena, A.C. Development of a mucoadhesive delivery system for control release of doxepin with application in vaginal pain relief associated with gynecological surgery. Int. J. Pharm. 2018, 535, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Tonguc-Altin, K.; Selvi-Kuvvetli, S.; Topcuoglu, N.; Kulekci, G. Antibacterial effects of dentifrices against Streptococcus mutans in children: A comparative in vitro study. J. Clin. Pediatr. Dent. 2024, 48, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Nardi, M.G.; Ogliari, C.; Chiesa, A.; Preda, C.; Perego, G.; Scribante, A. Clinical Use of Paraprobiotics for Pregnant Women with Periodontitis: Randomized Clinical Trial. Dent. J. 2024, 12, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, N.; Liu, Q.; Dong, Q.; Gu, J.; Yang, Y. Effects of probiotics on preventing caries in preschool children: A systematic review and meta-analysis. J. Clin. Pediatr. Dent. 2023, 47, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2024, 30, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Henrique Soares, K.; Firoozi, P.; Maria de Souza, G.; Beatriz Lopes Martins, O.; Gabriel Moreira Falci, S.; Rocha Dos Santos, C.R. Efficacy of Probiotics Compared to Chlorhexidine Mouthwash in Improving Periodontal Status: A Systematic Review and Meta-Analysis. Int. J. Dent. 2023, 2023, 4013004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, E.; Kobayashi, T.; Wakabayashi, H.; Yamauchi, K.; Iwatsuki, K.; Yoshie, H. Effects of orally administered lactoferrin and lactoperoxidase-containing tablets on clinical and bacteriological profiles in chronic periodontitis patients. Int. J. Dent. 2011, 2011, 405139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, L.; Azizi, A.; Vatanpour, M.; Ramezani, G. Antibacterial Efficacy of Cold Atmospheric Plasma, Photodynamic Therapy with Two Photosensitizers, and Diode Laser on Primary Mandibular Second Molar Root Canals Infected with Enterococcus faecalis: An In Vitro Study. Int. J. Dent. 2023, 2023, 5514829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
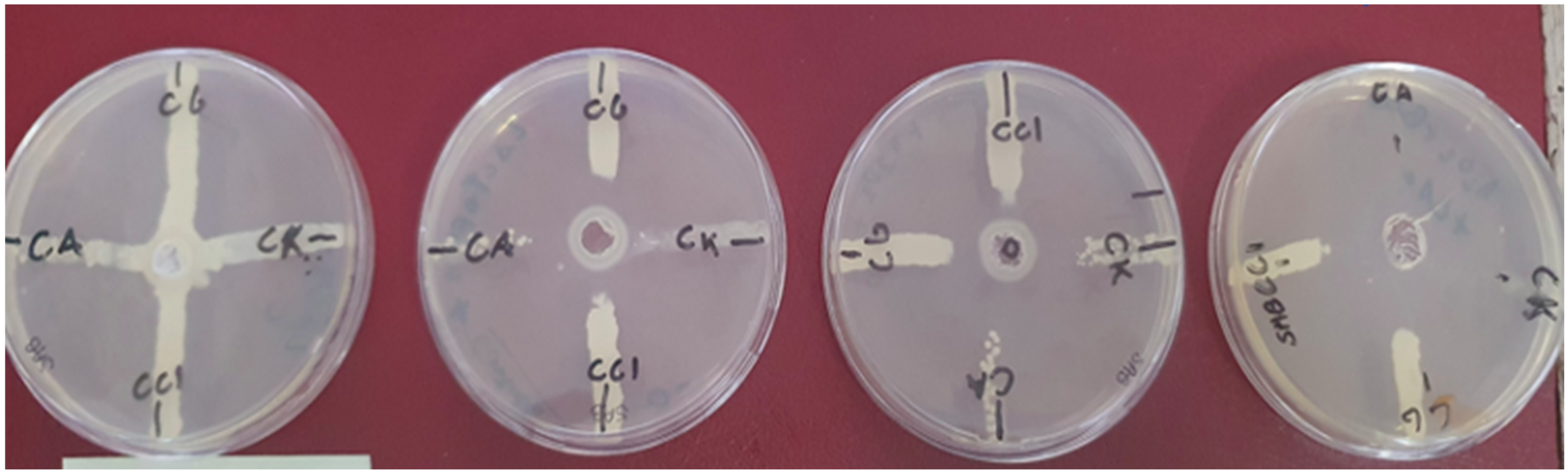
| Strain | Negative Control | Lactoferrin | H2O2 | Lactoferrin + Light | H2O2 + Light | Lactoferrin H2O2 + Light |
|---|---|---|---|---|---|---|
| Inhibition halos ø ± 1–2 mm | ||||||
| C. albicans | 0 | 10 | 23 | 22 | 30 | 40 |
| C. glabrata | 0 | 20 | 28 | 30 | 30 | 35 |
| C. krusei | 0 | 10 | 12 | 10 | 30 | 30 |
| CC1 | 0 | 10 | 18 | 20 | 18 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casu, C.; Butera, A.; Piga, A.; Scribante, A.; Fais, S.; Orrù, G. Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study. Microorganisms 2025, 13, 1255. https://doi.org/10.3390/microorganisms13061255
Casu C, Butera A, Piga A, Scribante A, Fais S, Orrù G. Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study. Microorganisms. 2025; 13(6):1255. https://doi.org/10.3390/microorganisms13061255
Chicago/Turabian StyleCasu, Cinzia, Andrea Butera, Alice Piga, Andrea Scribante, Sara Fais, and Germano Orrù. 2025. "Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study" Microorganisms 13, no. 6: 1255. https://doi.org/10.3390/microorganisms13061255
APA StyleCasu, C., Butera, A., Piga, A., Scribante, A., Fais, S., & Orrù, G. (2025). Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study. Microorganisms, 13(6), 1255. https://doi.org/10.3390/microorganisms13061255








