Exploring the Effect of Enzyme and Protein-Containing Toothpaste on Gum Health: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
- Population: humans of all ages;
- Intervention: toothpaste with enzymes and proteins tested in humans;
- Comparator: effect on gingivitis;
- Outcomes: possible effects of a toothpaste with enzymes and proteins on gingivitis and gum health.
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
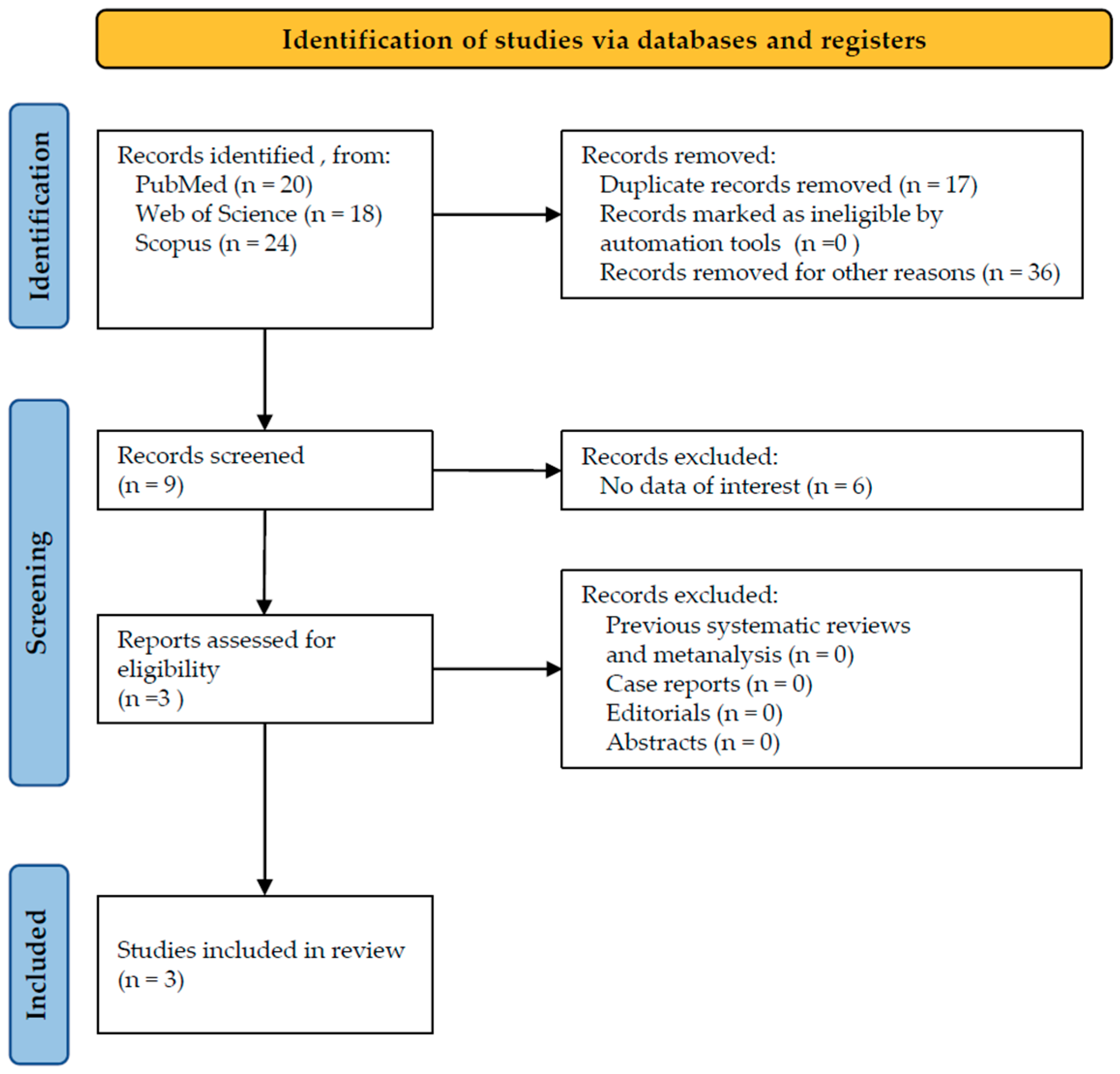
3.1. Study Selection
3.2. Detailed Results
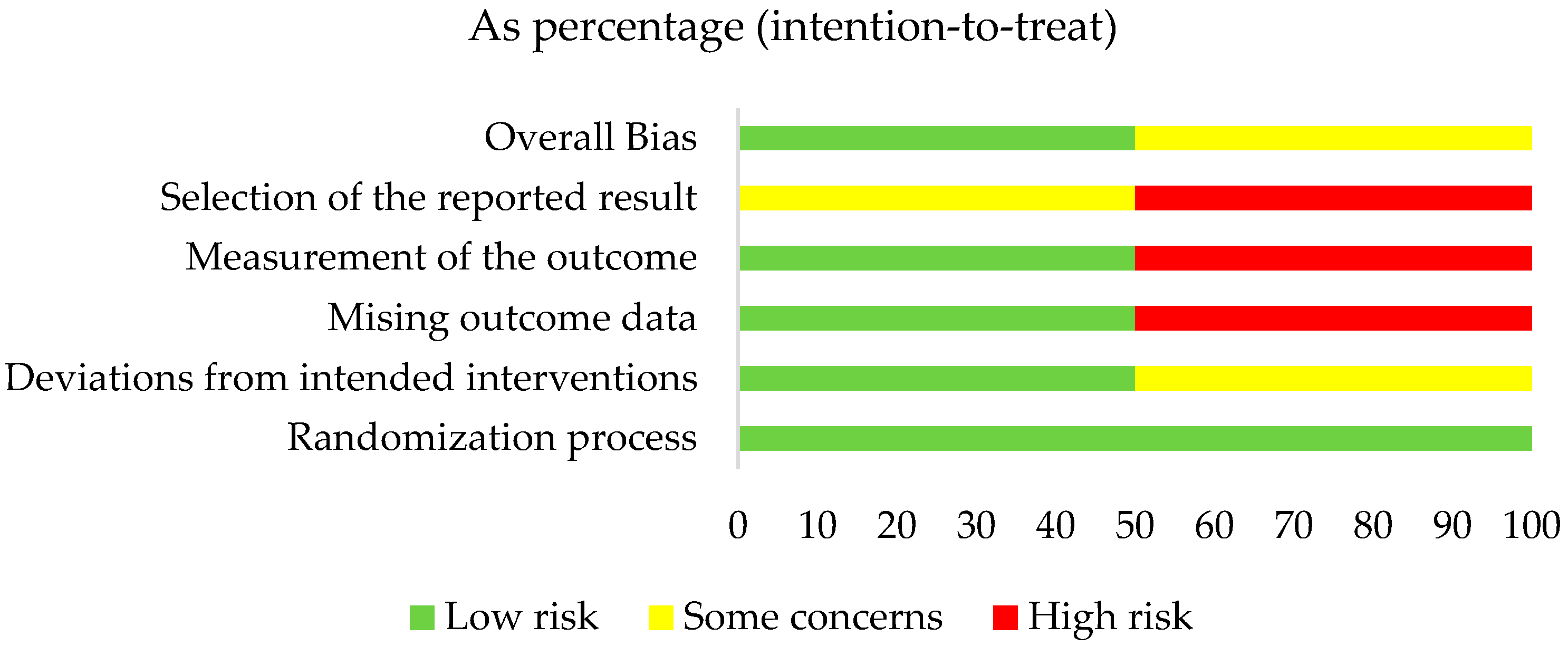
3.3. Quality Assessment Results
4. Discussion
4.1. Limitations
4.2. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Cawley, A.; Golding, S.; Goulsbra, A.; Hoptroff, M.; Kumaran, S.; Marriott, R. Microbiology insights into boosting salivary defences through the use of enzymes and proteins. J. Dent. 2019, 80 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Milligan, T.W.; Joyner, R.E.; Jefferson, M.M. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect. Immun. 1994, 62, 529–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wertz, P.W.; de Szalay, S. Innate Antimicrobial Defense of Skin and Oral Mucosa. Antibiotics 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ihalin, R.; Loimaranta, V.; Tenovuo, J. Origin, structure, and biological activities of peroxidases in human saliva. Arch. Biochem. Biophys. 2006, 445, 261–268. [Google Scholar] [CrossRef] [PubMed]
- van’t Hof, W.; Veerman, E.C.; Nieuw Amerongen, A.V.; Ligtenberg, A.J. Antimicrobial defense systems in saliva. Monogr. Oral Sci. 2014, 24, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.V.; Lin, T.; Siqueira, C.C.; Bruno, L.S.; Li, X.; Oppenheim, F.G.; Offner, G.; Troxler, R.F. Salivary micelles: Identification of complexes containing MG2, sIgA, lactoferrin, amylase, glycosylated proline-rich protein and lysozyme. Arch. Oral Biol. 2004, 49, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol 2000 2016, 70, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.E.; Arnold, D.; Murphy, B.; Carroll, P.; Green, A.K.; Smith, A.M.; Marsh, P.D.; Chen, T.; Marriott, R.E.; Brading, M.G. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci. Rep. 2017, 7, 43344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nandlal, B.; Anoop, N.K.; Ragavee, V.; Vanessa, L. In-vitro Evaluation of toothpaste containing enzymes and proteins on inhibiting plaque re-growth of the children with high caries experience. J. Clin. Exp. Dent. 2021, 13, e43–e47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, K.; Bosma, M.L.; John, G.; McGuire, J.A.; DelSasso, A.; Milleman, J.; Milleman, K.R. Quantitative analysis of the effects of brushing, flossing, and mouthrinsing on supragingival and subgingival plaque microbiota: 12-week clinical trial. BMC Oral Health 2024, 24, 575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 372. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carelli, M.; Zatochna, I.; Sandri, A.; Burlacchini, G.; Rosa, A.; Baccini, F.; Signoretto, C. Effect of A Fluoride Toothpaste Containing Enzymes and Salivary Proteins on Periodontal Pathogens in Subjects with Black Stain: A Pilot Study. Eur. J. Dent. 2024, 18, 109–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daly, S.; Seong, J.; Newcombe, R.; Davies, M.; Nicholson, J.; Edwards, M.; West, N. A randomised clinical trial to determine the effect of a toothpaste containing enzymes and proteins on gum health over 3 months. J. Dent. 2019, 80 (Suppl. S1), S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Darwish, M.; Nicholson, J.; Edwards, M.I.; Gupta, A.K.; Belstrøm, D. Gingival health status in individuals using different types of toothpaste. J. Dent. 2019, 80 (Suppl. S1), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Oppenheim, F.G. Saliva: A dynamic proteome. J. Dent. Res. 2007, 86, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haberska, K.; Svensson, O.; Shleev, S.; Lindh, L.; Arnebrant, T.; Ruzgas, T. Activity of lactoperoxidase when adsorbed on protein layers. Talanta 2008, 76, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.; Tenovuo, J.; Lenander-Lumikari, M.; Söderling, E.; Vilja, P. Lysozyme and lactoperoxidase inhibit the adherence of Streptococcus mutans NCTC 10449 (serotype c) to saliva-treated hydroxyapatite in vitro. Caries Res. 1994, 28, 421–428. [Google Scholar] [CrossRef]
- Korpela, A.; Yu, X.; Loimaranta, V.; Lenander-Lumikari, M.; Vacca-Smith, A.; Wunder, D.; Bowen, W.H.; Tenovuo, J. Lactoperoxidase inhibits glucosyltransferases from Streptococcus mutans in vitro. Caries Res. 2002, 36, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Sabir, S.; Jan, A. Physiology, Immune Response. 2024 Jul 27. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Ramenzoni, L.L.; Hofer, D.; Solderer, A.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Origin of MMP-8 and Lactoferrin levels from gingival crevicular fluid, salivary glands and whole saliva. BMC Oral Health 2021, 21, 385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jalil, R.A.; Ashley, F.P.; Wilson, R.F.; Wagaiyu, E.G. Concentrations of thiocyanate, hypothiocyanite, ‘free’ and ‘total’ lysozyme, lactoferrin and secretory IgA in resting and stimulated whole saliva of children aged 12–14 years and the relationship with plaque accumulation and gingivitis. J. Periodontal Res. 1993, 28, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Paqué, P.N.; Schmidlin, P.R.; Wiedemeier, D.B.; Wegehaupt, F.J.; Burrer, P.D.; Körner, P.; Deari, S.; Sciotti, M.A.; Attin, T. Toothpastes with Enzymes Support Gum Health and Reduce Plaque Formation. Int. J. Environ. Res. Public Health 2021, 18, 835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed] [PubMed Central]
- Belstrøm, D.; Holmstrup, P.; Nielsen, C.H.; Kirkby, N.; Twetman, S.; Heitmann, B.L.; Klepac-Ceraj, V.; Paster, B.J.; Fiehn, N.E. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J. Oral Microbiol. 2014, 6, 23609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, A.; Crichard, S.; Ling-Mountford, N.; Milward, M.; Hubber, N.; Platten, S.; Gupta, A.K.; Chapple, I.L.C. A randomised clinical study comparing the effect of Steareth 30 and SLS containing toothpastes on oral epithelial integrity (desquamation). J. Dent. 2019, 80 (Suppl. S1), S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 2011, 22, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Söderling, E.; Pienihäkkinen, K. Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: A systematic review. Acta Odontol. Scand. 2020, 78, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef] [PubMed]
- Lenander-Lumikari, M.; Tenovuo, J.; Mikola, H. Effects of a lactoperoxidase system-containing toothpaste on levels of hypothiocyanite and bacteria in saliva. Caries Res. 1993, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhou, H. Clinical evaluation of a toothpaste containing lysozyme for the treatment of recurrent aphthous stomatitis: A 3-month, double-blind, randomized study. Am. J. Dent. 2016, 29, 303–306. [Google Scholar] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar] [PubMed] [PubMed Central]
- Kirstilä, V.; Lenander-Lumikari, M.; Söderling, E.; Tenovuo, J. Effects of oral hygiene products containing lactoperoxidase, lysozyme, and lactoferrin on the composition of whole saliva and on subjective oral symptoms in patients with xerostomia. Acta Odontol. Scand. 1996, 54, 391–397. [Google Scholar] [CrossRef] [PubMed]

| Authors/Year | Study Design | Population /Age | Enzymes/ Proteins | Aim of Administration | Follow-Up | Systemic Conditions |
|---|---|---|---|---|---|---|
| Carelli M. et al., 2024 [17] | RCT | 26/22.5 | Amyloglucosidase, glucose oxidase, lactoperoxidase, lysozyme, lactoferrin, bovine colostrum (IgG) | Effects on DMFT, gingival indexes (GBI, plaque control) and black stains | 14 weeks | Healthy |
| Daly S. et al., 2019 [18] | RCT | 229/32.6 | Amyloglucosidase, glucose oxidase, lactoperoxidase, lysozyme, lactoferrin, bovine colostrum (IgG) | Effects on gingival indexes (MGI, BI, PI modified by Quigley and Hein) | 12 weeks | Healthy |
| Pedersen AML. et al., 2019 [19] | N-RCT | 305/18→56 | Amyloglucosidase, glucose oxidase, lactoperoxidase, lysozyme, lactoferrin, bovine colostrum (IgG) | Effects on gingival indexes (MGI, BI, PI modified by Quigley and Hein) | 12 months | Healthy |
| Authors/Year | Conclusions |
|---|---|
| Carelli M. et al., 2024 [17] | Brushing with an electric toothbrush seemed to be more effective in reducing black stains compared to a manual brush. This improvement was seen regardless of the toothpaste used. |
| Daly S. et al., 2019 [18] | Brushing with the enzyme and protein toothpaste resulted in lower plaque and bleeding indexes. |
| Pedersen AML. et al., 2019 [19] | Using a fluoride toothpaste containing enzymes and proteins for at least 1 year is associated with improved gum health compared to other fluoride toothpastes lacking these additional ingredients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, S.; Dolci, M. Exploring the Effect of Enzyme and Protein-Containing Toothpaste on Gum Health: A Systematic Review. Microorganisms 2025, 13, 1158. https://doi.org/10.3390/microorganisms13051158
D’Agostino S, Dolci M. Exploring the Effect of Enzyme and Protein-Containing Toothpaste on Gum Health: A Systematic Review. Microorganisms. 2025; 13(5):1158. https://doi.org/10.3390/microorganisms13051158
Chicago/Turabian StyleD’Agostino, Silvia, and Marco Dolci. 2025. "Exploring the Effect of Enzyme and Protein-Containing Toothpaste on Gum Health: A Systematic Review" Microorganisms 13, no. 5: 1158. https://doi.org/10.3390/microorganisms13051158
APA StyleD’Agostino, S., & Dolci, M. (2025). Exploring the Effect of Enzyme and Protein-Containing Toothpaste on Gum Health: A Systematic Review. Microorganisms, 13(5), 1158. https://doi.org/10.3390/microorganisms13051158








