Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Material
2.2. Extract Preparation
2.3. Mycochemical Characterization
2.3.1. Atomic Absorption Spectrophotometry (AAS)
2.3.2. HPLC-FD Determination of Selected Polyamine Content
2.3.3. LC/MS-MS Quantification of Phenolic Compounds and Total Phenolic Content (TPC) Determination
2.3.4. Determination of Total Carbohydrate Content
2.4. Biological Activities
2.4.1. Determination of Antioxidant Activity
2.4.2. Determination of Anti-Acetylcholinesterase Activity (Anti-AChE)
2.4.3. Determination of Antiproliferative Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mycochemical Profile
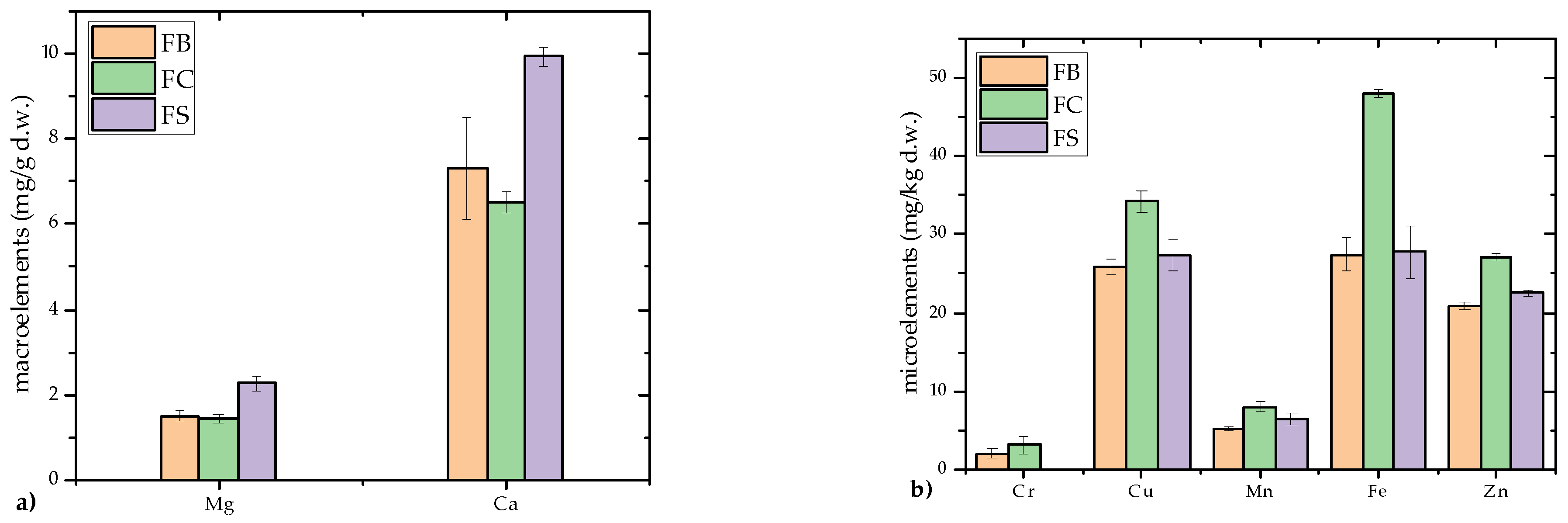
3.1.1. Macro- and Microelement Content and Biological Significance
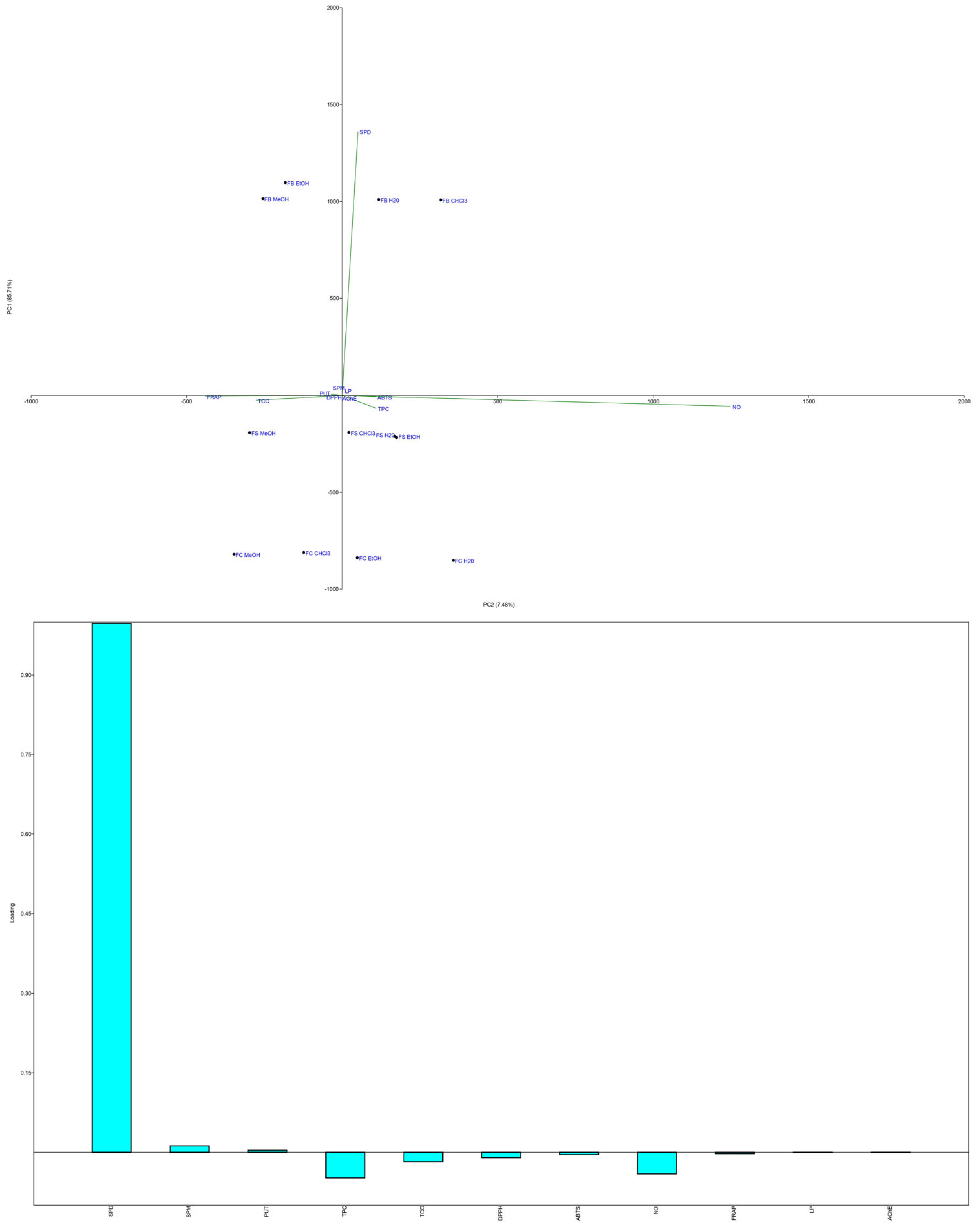
3.1.2. Content of Polyamines
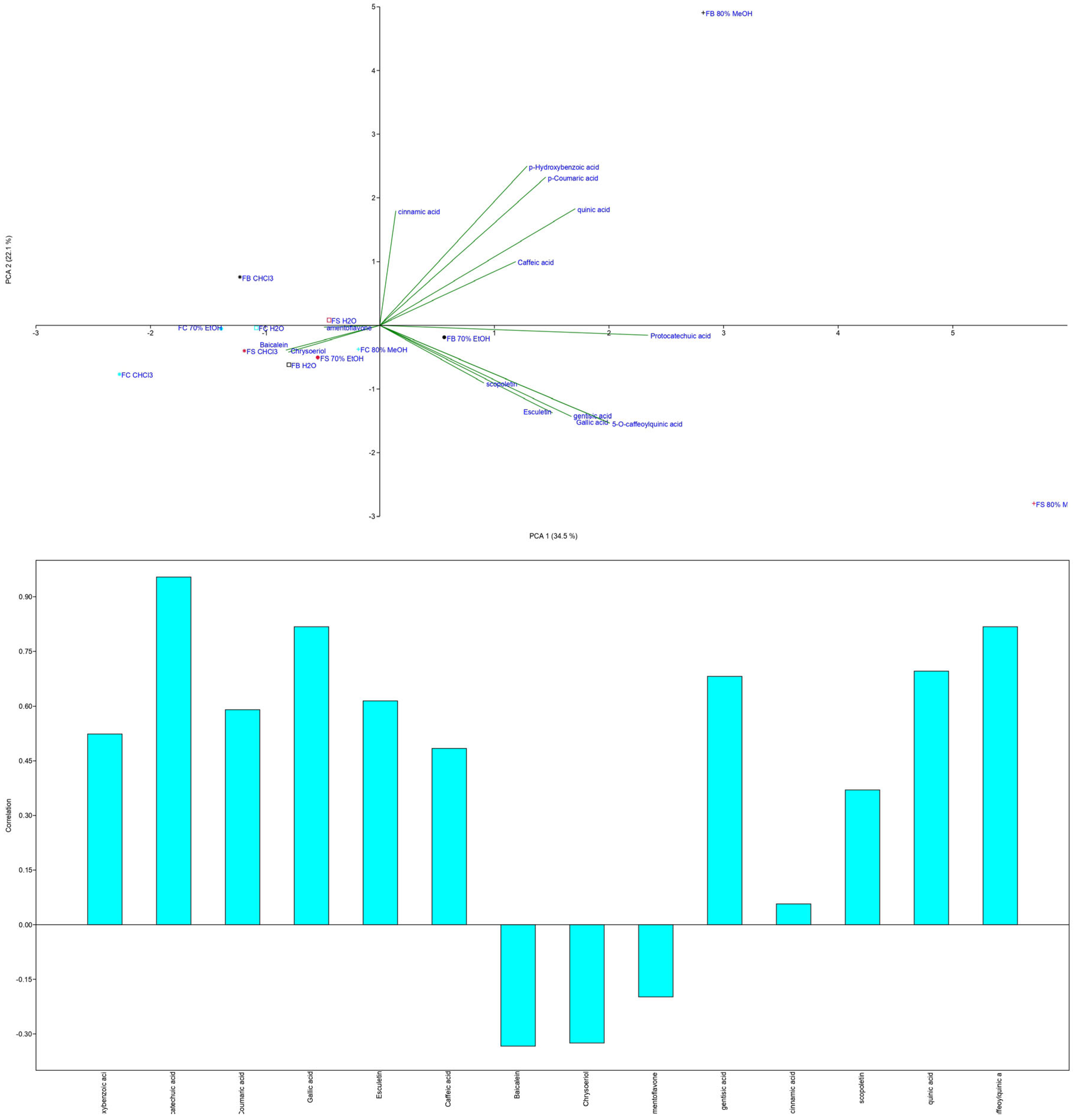
3.1.3. Phenolic Content Determined by LC-MS/MS
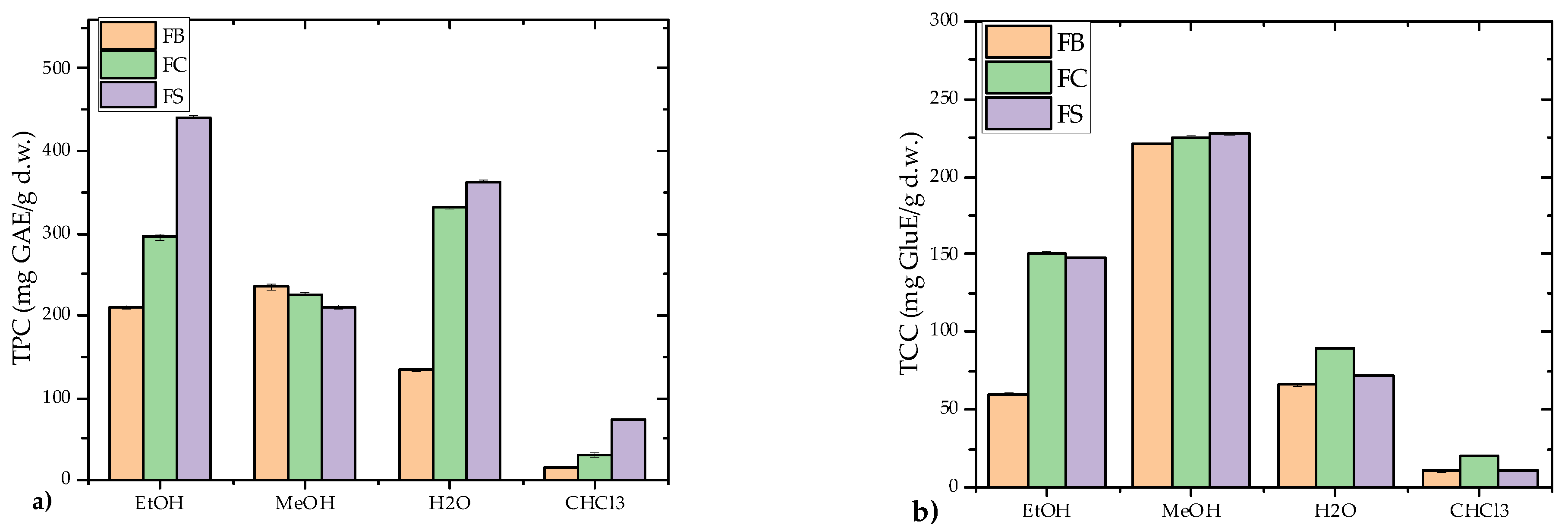
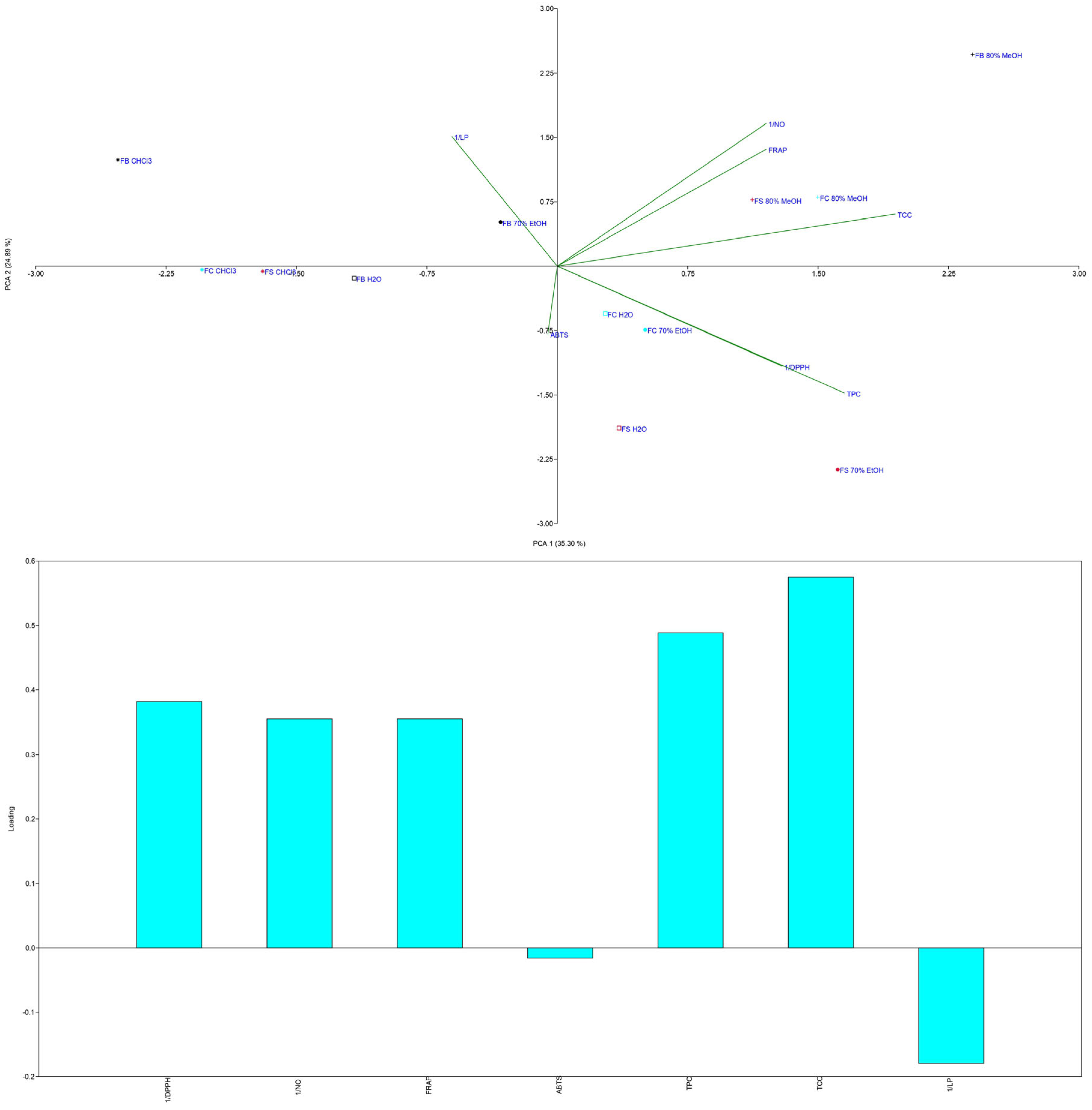
3.1.4. TPC and TCC Content
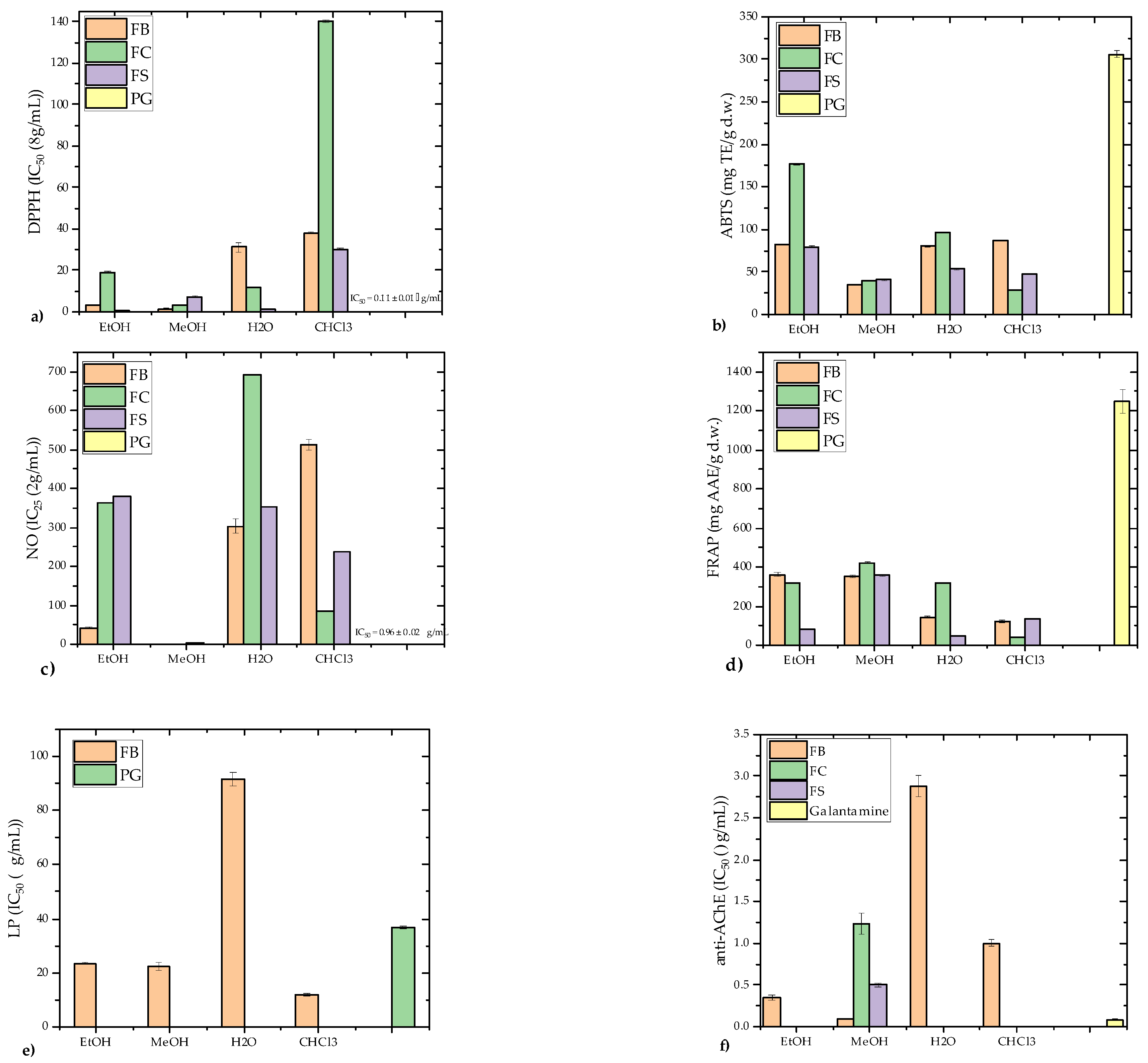
3.2. Antioxidant Activity
3.3. Anti-Acetylcholinesterase Activity
3.4. Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FC | Fomes fomentarius strain sampled from Croatia |
| FB | Fomes fomentarius strain sampled from Bosnia and Herzegovina |
| FS | Fomes fomentarius strain sampled from Serbia |
| H2O | Hot water extract |
| EtOH | Hydroethanolic extract prepared with 70% ethanol |
| MeOH | Hydromethanolic extract prepared with 80% methanol |
| CHCl3 | Chloroform extract |
| ROS | Reactive oxygen species |
| AChE | Acetylcholinesterase |
| AAS | Atomic absorption spectrophotometry |
| HPLC-FD | High-performance liquid chromatography coupled with fluorescence detector |
| LC-MS/MS | Liquid chromatography coupled with tandem mass-spectrometric detection |
| PA | Polyamine |
| PUT | Putrescine |
| SPD | Spermidine |
| SPM | Spermine |
| TPC | Total phenolic content |
| TCC | Total carbohydrate content |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radical, DPPH• |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), ABTS•+ |
| NO | Nitric oxide radical, NO• |
| FRAP | Ferric reducing antioxidant power |
| FC | Folin–Ciocalteu |
| AAE | Ascorbic acid equivalents |
| GAE | Gallic acid equivalents |
| GluE | Glucose equivalents |
| TE | Trolox equivalents |
| d.w. | Dry weight |
| dH2O | Distilled water |
| PG | Propyl gallate |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| PCA | Principal component analysis |
References
- Gafforov, Y.; Rašeta, M.; Rapior, S.; Yarasheva, M.; Wang, X.; Zhou, L.; Wan-Mohtar, W.A.A.Q.I.; Zafar, M.; Lim, Y.W.; Wang, M.; et al. Macrofungi as medicinal resources in Uzbekistan: Biodiversity, ethnomycology, and ethnomedicinal practices. J. Fungi 2023, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Morales, D. Fomes fomentarius: An underexplored mushroom as source of bioactive compounds. Food Biosci. 2024, 61, 104781. [Google Scholar] [CrossRef]
- Orhan, I.E.; Üstün, O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J. Food Compos. Anal. 2011, 24, 386–390. [Google Scholar] [CrossRef]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popović, M. Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat. Int. J. Food Sci. Technol. 2016, 51, 2583–2590. [Google Scholar] [CrossRef]
- Rašeta, J.; Vrbaški, S.; Bošković, V.E.; Popović, M.; Mimica-Dukić, N.M.; Karaman, A.M. Comparison of antioxidant capacities of two Ganoderma lucidum strains of different geographical origins. Matica Srp. J. Nat. Sci. 2017, 133, 209–219. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti-inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers. 2021, 18, e2000828. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Čapelja, E.; Zapora, E.; Petrović Fabijan, A.; Knežević, P.; Karaman, M. Do Ganoderma species represent novel sources of phenolic based antimicrobial agents? Molecules 2023, 28, 3264. [Google Scholar] [CrossRef]
- Rašeta, M.; Kebert, M.; Mišković, J.; Rakić, M.; Kostić, S.; Čapelja, E.; Karaman, M. Polyamines in edible and medicinal fungi from Serbia: A novel perspective on neuroprotective properties. J. Fungi 2024, 10, 21. [Google Scholar] [CrossRef]
- Khojimatov, O.K.; Gafforov, Y.; Bussmann, R.W. Ethnobiology of Uzbekistan: Ethnomedicinal Knowledge of Mountain Communities, 1st ed.; Springer Nature: Basel, Switzerland, 2023; 1564p. [Google Scholar]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores-a modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef]
- Kim, S.H.; Jakhar, R.; Kang, S.C. Apoptotic properties of polysaccharide isolated from fruiting bodies of medicinal mushroom Fomes fomentarius in human lung carcinoma cell line. Saudi J. Biol. Sci. 2015, 22, 484–490. [Google Scholar] [CrossRef]
- Gafforov, Y.; Kalitukha, L.; Tomšovský, M.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Yarasheva, M.; Wan-Mohtar, W.A.A.Q.I.; Rapior, S. Fomes fomentarius (L.) Fr.—POLYPORACEAE. In Ethnobiology of Uzbekistan: Ethnomedicinal Knowledge of Mountain Communities, 1st ed.; Khojimatov, O.K., Gafforov, Y., Bussmann, R.W., Eds.; Springer Nature: Basel, Switzerland, 2023; pp. 1045–1063. [Google Scholar]
- Tubić Vukajlović, J.; Djordjević, K.; Tosti, T.; Simić, I.; Grbović, F.; Milošević-Djordjević, O. In vitro effect of Lenzites betulinus mushroom against therapy-induced DNA damage in peripheral blood lymphocytes of patients with acute coronary syndrome. J. Ethnopharmacol. 2024, 335, 118640. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.O.; Wilson, R. Essential, deadly, enigmatic: Polyamine metabolism and roles in fungal cells. Fungal Biol. Rev. 2019, 33, 45–57. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Syatkin, S.P.; Poleshchuk, V.V.; Urazgildeeva, G.R.; Chigaleychik, L.A.; Sungrapova, C.Y.; Illarioshkin, S.N. Polyamines in Parkinson’s disease: Their role in oxidative stress induction and protein aggregation. J. Neurol. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Đorđievski, S.; Vukašinović, E.L.; Čelić, T.V.; Pihler, I.; Kebert, M.; Kojić, D.; Purać, J. Spermidine dietary supplementation and polyamines level in reference to survival and lifespan of honey bees. Sci. Rep. 2023, 13, 4329. [Google Scholar] [CrossRef]
- Vazirian, M.; Dianat, S.; Manayi, A.; Ziari, R.; Mousazadeh, A.R.; Habibi, E.; Saeidnia, S.; Amanzadeh, Y. Anti-inflammatory effect, total polysaccharide, total phenolics content and antioxidant activity of the aqueous extract of three basidiomycetes. Res. J. Pharmacogn. 2014, 1, 15–21. [Google Scholar]
- Kolundžić, M.; Grozdanić, N.Đ.; Dodevska, M.; Milenković, M.; Sisto, F.; Miani, A.; Farronato, G.; Kundaković, T. Antibacterial and cytotoxic activities of wild mushroom Fomes fomentarius (L.) Fr., Polyporaceae. Ind. Crops Prod. 2016, 79, 110–115. [Google Scholar] [CrossRef]
- Papp, N.; Rudolf, K.; Bencsik, T.; Czégényi, D. Ethnomycological use of Fomes fomentarius (L.) Fr. and Piptoporus betulinus (Bull.) P. Karst. in Transylvania, Romania. Genet. Resour. Crop Evol. 2017, 64, 101–111. [Google Scholar] [CrossRef]
- Pasailiuk, M.V. Total flavonoid content, lipid peroxidation and total antioxidant activity of Hericium coralloides, Fomes fomentarius and Schizophyllum commune cultivated by the method of direct confrontation. Ital. J. Mycol. 2020, 49, 25–37. [Google Scholar]
- Chen, W.; Zhao, Z.; Chen, S.; Li, Y. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008, 99, 3187–3194. [Google Scholar] [CrossRef]
- Dundar, A.; Okumuş, V.; Ozdemir, S.; Çelik, K.S.; Boğa, M.S.; Ozcagli, E. Determination of cytotoxic, anticholinesterase, antioxidant and antimicrobial activities of some wild mushroom species. Cogent Food Agric. 2016, 2, 1178060. [Google Scholar] [CrossRef]
- Gáper, J.; Gáperová, S.; Pristaš, P.; Náplavová, K. Medicinal value and taxonomy of the tinder polypore, Fomes fomentarius (Agaricomycetes): A review. Int. J. Med. Mushrooms 2016, 18, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Reinhardt, J.K.; Lindequist, U. European medicinal mushrooms: Do they have potential for modern medicine?—An update. Phytomedicine 2020, 66, 153131. [Google Scholar] [CrossRef] [PubMed]
- Tortić, M. Gljive kao lijek. Prir. Pop. Časopis Hrvat. Prirodosl. Društva 1961, 9, 272–275. [Google Scholar]
- Uzunov, B.A.; Stoyneva-Gärtner, M.P. Mushrooms and lichens in Bulgarian ethnomycology. J. Mycol. 2015, 2015, 1–7. [Google Scholar] [CrossRef][Green Version]
- Živković, J.; Ivanov, M.; Stojković, D.; Glamočlija, J. Ethnomycological investigation in Serbia: Astonishing realm of mycomedicines and mycofood. J. Fungi 2021, 75, 349. [Google Scholar] [CrossRef]
- Peintner, U.; Schwarz, S.; Mešić, A.; Moreau, P.; Moreno, G.; Saviuc, P. Mycophilic or mycophobic? Legislation and guidelines on wild mushroom commerce reveal different consumption behaviour in European countries. PLoS ONE 2013, 8, e63926. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Kebert, M.; Berežni, S.; Krstić, S.; Gojgić-Cvijović, G.; Pirker, T.; Bauer, R.; Karaman, M. Mycochemical profiles and bioactivities of Fistulina hepatica and Volvopluteus gloiocephalus from Serbia: Antioxidant, enzyme inhibition, and cytotoxic potentials. Food Biosci. 2025, 66, 106221. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Vuksanović, V.; Gavranović Markić, A.; Kiprovski, B.; Zorić, M.; Orlović, S. Metal- and organ-specific response to heavy metal-induced stress mediated by antioxidant enzymes’ activities, polyamines, and plant hormones levels in Populus deltoides. Plants 2022, 11, 3246. [Google Scholar] [CrossRef]
- Scaramagli, S.; Biondi, S.; Torrigiani, P. Methylglyoxal(bis-guanylhydrazone) inhibition of organogenesis is not due to S-adenosylmethionine decarboxylase inhibition/polyamine depletion in tobacco thin layers. Physiol. Plant. 1999, 107, 353–360. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass-spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Green, C.E.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal. Biochem. 1982, 243, 709–714. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Iron and free radical reactions: Two aspects of antioxidant protection. Trends Biochem. Sci. 1986, 11, 372–375. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid and concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andrés, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, S.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Mosmann, T.R. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis version 2.09. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Fouhy, L.E.; Mangano, K.M.; Zhang, X.; Hughes, B.D.; Tucker, K.L.; Noel, S.E. Association between a Calcium-to-magnesium ratio and osteoporosis among Puerto Rican adults. J. Nutr. 2023, 153, 2642–2650. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Karaman, M.; Matavulj, M. Macroelements and heavy metals in some lignicolous and tericolous fungi. Matica Srp. J. Nat. Sci. 2005, 108, 255–267. [Google Scholar] [CrossRef]
- Mallikarjuna, S.E.; Ranjini, A.; Haware, D.J.; Vijayalakshmi, M.R.; Shashirekha, M.; Rajarathnam, S. Mineral composition of four edible mushrooms. J. Chem. 2013, 2013, 805284. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Yabanlı, M.; Türkoğlu, A. Content of minerals and trace elements determined by ICP-MS in eleven mushroom species from Anatolia, Turkey. Chiang Mai J. Sci. 2017, 44, 939–945. [Google Scholar]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Stilinović, N.; Škrbić, B.; Živančev, J.; Mrmoš, N.; Pavlović, N.; Vukmirović, S. The level of elements and antioxidant activity of commercial dietary supplement formulations based on edible mushrooms. Food Funct. 2014, 5, 3170–3178. [Google Scholar] [CrossRef]
- Stilinović, N.; Čapo, I.; Vukmirović, S.; Rašković, A.; Tomas, A.; Popović, M.; Sabo, A. Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. R. Soc. Open Sci. 2020, 7, 200900. [Google Scholar] [CrossRef] [PubMed]
- Rašeta, M.; Rakić, M.; Čapelja, E.; Karaman, M. Chapter 2: Update on Research Data on the Nutrient Composition of Mushrooms and Their Potentials in Future Human Diets. In Food Chemistry, Function and Analysis, Edible Fungi: Chemical Composition, Nutrition and Health Effects, 1st ed.; Stojković, D., Barros, L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2022; pp. 27–67. [Google Scholar]
- Gałgowska, M.; Pietrzak-Fiećko, R. Mineral composition of three popular wild mushrooms from Poland. Molecules 2020, 25, 3588. [Google Scholar] [CrossRef] [PubMed]
- Gnusarev, S.; Mitrofanova, N.; Churakov, B.P. The effect of mixed rot from the present tinder (Fomes fomentarius (L.:Fr.) Gill.) on the accumulation of heavy metals in the hanging birch (Betula pendula Roth.). In Proceedings of the Materialy VIII Vserossijskoj Konferencii s Mezhdunarodnym Uchastiem «Mediko-Fiziologicheskie Problemy Jekologii Cheloveka», Ulyanovsk, Russia, 1–4 December 2021. [Google Scholar]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Content of biogenic amines and polyamines in some species of European wild-growing edible mushrooms. Eur. Food Res. Technol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Reis, G.L.; Custódio, F.B.; Botelho, B.G.; Guidi, L.R.; Glória, M.B. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compos. Anal. 2020, 86, 103375. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005-mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Bal, C.; Akgul, H.; Sevindik, M.; Akata, I.; Yumrutas, O. Determination of the anti-oxidative activities of six mushrooms. Fresenius Environ. Bull. 2017, 26, 6246–6252. [Google Scholar]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorović, N.; Vunduk, J.; Petrović, P.M.; Nikšić, M.P.; Vrvić, M.M.; Van Griensven, L.J. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Karaman, M.; Stahl, M.; Vulić, J.; Vesić, M.; Čanadanović-Brunet, J. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int. J. Food Sci. Nutr. 2014, 65, 311–319. [Google Scholar] [CrossRef]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: Fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Nicolescu, A.; Ielciu, I.; Păltinean, R.; Crişan, G.; Mocan, A. Chapter 4: Bioactive Phenolic Compounds from Mushrooms. In Edible Fungi: Chemical Composition, Nutrition and Health Effects, 1st ed.; Stojković, D., Barros, L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2022; pp. 139–160. [Google Scholar]
- Rašeta, M.; Popović, M.; Knežević, P.; Šibul, F.; Kaišarević, S.; Karaman, M. Bioactive phenolic compounds of two medicinal mushroom species Trametes versicolor and Stereum subtomentosum as antioxidant and antiproliferative agents. Chem. Biodivers. 2020, 17, e2000683. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 40, 3408–3437. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.S.; Florêncio, J.R.; Pinto, N.C.; Campos, L.M.; Silva, T.P.; Grazul, R.M.; Pinto, P.D.; Tavares, G.D.; Scio, E.; Apolônio, A.C.; et al. Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front. Microbiol. 2020, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Luo, J.; Xu, B. Insights into the chemical compositions and health promoting effects of wild edible mushroom Chroogomphus rutilus. Nutrients 2023, 15, 4030. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one Polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Karadeniz, M.; Bakır, T.K.; Ünal, S. Investigation of the antioxidant and total phenolic substance of Fomes fomentarius and Ganoderma applanatum mushrooms showing therapeutic properties. Bilge Int. J. Sci. Technol. Res. 2024, 8, 14–18. [Google Scholar] [CrossRef]
- Deveci, E.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. Structural characterization and determination of biological activities for different polysaccharides extracted from tree mushroom species. J. Food Biochem. 2019, 43, e12965. [Google Scholar] [CrossRef]
- Saltarelli, R.; Ceccaroli, P.; Iotti, M.; Zambonelli, A.; Buffalini, M.; Casadei, L.; Vallorani, L.; Stocchi, V. Biochemical characterisation and antioxidant activity of mycelium of Ganoderma lucidum from Central Italy. Food Chem. 2009, 116, 143–151. [Google Scholar] [CrossRef]
- On-nom, N.; Suttisansanee, U.; Chathiran, W.; Charoenkiatkul, S.; Thiyajai, P.; Srichamnong, W. Nutritional security: Carbohydrate profile and folk remedies of rare edible mushrooms to diversify food and diet: Thailand case study. Sustainability 2023, 15, 14034. [Google Scholar] [CrossRef]
- Ilyashenka, S. Biological activity of Fomes fomentarius. J. Pharm. Pharmacol. 2022, 10, 294–298. [Google Scholar] [CrossRef]
- Darkal, A.K.; Zuraik, M.M.; Ney, Y.; Nasim, M.J.; Jacob, C. Unleashing the biological potential of Fomes fomentarius via dry and wet milling. Antioxidants 2021, 10, 303. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Ho, T.P.; Mangelings, D.; Van Eeckhaut, A.; Vander Heyden, Y.; Tran, H.T. Antioxidant, neuroprotective, and neuroblastoma cells (SH-SY5Y) differentiation effects of melanins and arginine-modified melanins from Daedaleopsis tricolor and Fomes fomentarius. BMC Biotechnol. 2024, 24, 89. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant metabolism induced by quinic acid. increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 2009, 23, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Kang, H.K.; Hosseindoust, A.; Mun, J.Y.; Moturi, J.; Tajudeen, H.; Lee, H.; Cheong, E.J.; Kim, J.S. Effects of scopoletin supplementation and stocking density on growth performance, antioxidant activity, and meat quality of Korean native broiler chickens. Foods 2021, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Jiang, T.; Xu, J.; Xi, W.; Shang, E.; Xiao, P.; Duan, J. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer. 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Moss, D.E. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Are irreversible inhibitors the future? Int. J. Mol. Sci. 2020, 21, 3438. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.; Zargaham, M.K.; Khan, A.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with reference of acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Ghosh, I.; Sankhe, R.; Mudgal, J.; Arora, D.; Nampoothiri, M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides 2020, 83, 102083. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.; Jamwal, S.; Kumar, P. A review on polyamines as promising next-generation neuroprotective and anti-aging therapy. Eur. J. Pharmacol. 2024, 978, 176804. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Botić, T.; Gregori, A.; Pohleven, F.; Knez, Ž. The effects of different solvents on bioactive metabolites and “in vitro” antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and primordia extracts. Maced. J. Chem. Chem. Eng. 2017, 36, 129–141. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho Júnior, R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of learning and memory by natural polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.D. In vitro antioxidant activity of dietary polyamines. Food Res. Int. 2013, 51, 141–147. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Rikhireva, G.T.; Kirichenko, E.Y.; Trinitatsky, I.Y.; Vakulenko, M.Y.; Ermakov, A.M. The role of polyamines in the mechanisms of cognitive impairment. J. Neurochem. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, C.; Liu, J. Nature spermidine and spermine alkaloids: Occurrence and pharmacological effects. Arab. J. Chem. 2022, 15, 104367. [Google Scholar] [CrossRef]
- Shnyreva, A.V.; Shnyreva, A.A.; Espinoza, C.; Padrón, J.M.; Trigos, Á. Antiproliferative activity and cytotoxicity of some medicinal wood-destroying mushrooms from Russia. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernstrom, H. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor i receptor levels in human breast cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef] [PubMed]

| Class | Compound | Amount of Selected Compound (μg/g d.w. ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. fomentarius and Extract Type | |||||||||||||
| FB | FC | FS | |||||||||||
| CHCl3 | H2O | EtOH | MeOH | CHCl3 | H2O | EtOH | MeOH | CHCl3 | H2O | EtOH | MeOH | ||
| Flavones | Baicalein | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | 147.50 ± 29.50 b | 44.63 ± 8.93 c | <12.20 |
| Chrysoeriol | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | ||
| Biflavonoid | Amentoflavone | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 | 10.97 ± 0.33 a | <3.05 | <3.05 | <3.05 | <3.05 | <3.05 |
| Hydroxybenzoic acids | p-Hydroxybenzoic acid | <6.10 | <6.10 | 32.79 ± 1.97 c | 420.01 ± 25.20 a | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | 56.03 ± 3.36 b |
| Protocatechuic acid | <6.10 | <6.10 | 10.90 ± 0.87 c | 13.28 ± 1.06 b | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | 25.48 ± 2.04 a | |
| Gentisic acid | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | 22.46 ± 1.80 a | |
| Gallic acid | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | |
| Cinnamic acid | <97.50 | <97.50 | <97.50 | <97.5 | <97.50 | <97.50 | <97.50 | <97.50 | <97.50 | <97.50 | <97.50 | <97.50 | |
| Hydroxycinnamic acids | p-Coumaric acid | <12.20 | <12.20 | <12.20 | 83.12 ± 7.48 a | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | <12.20 | 19.23 ± 1.73 b |
| Caffeic acid | <6.10 | <6.10 | 22.18 ± 1.55 e | 83.36 ± 5.84 b | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | 105.95 ± 7.42 a | 63.32 ± 4.43 c | 49.23 ± 3.45 d | |
| Coumarins | Esculetin | <6.10 | 51.36 ± 3.08 b | 73.66 ± 4.42 a | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | 84.03 ± 5.04 a |
| Scopoletin | <6.10 | <6.10 | <6.10 | 88.02 ± 7.04 e | <6.10 | <6.10 | <6.10 | 23.63 ± 1.89 f | 209.10 ± 16.73 d | 511.70 ± 40.94 c | 853.07 ± 68.25 a | 580.80 ± 46.46 b | |
| Cyclohexanecarboxylic acid | Quinic acid | <48.85 | 58.99 ± 5.59 g | 414.20 ± 41.42 c | 996.80 ± 99.68 a | <48.85 | 207.50 ± 20.75 f | 211.70 ± 21.17 f | 379.30 ± 37.93 d | <48.85 | 247.80 ± 24.78 e | <48.85 | 454.20 ± 45.42 b |
| Chlorogenic acid | 5-O-caffeoylquinic acid | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | <6.10 | 6.74 ± 0.34 a |
| Cell Line | Extract Concentration (μg/mL) | Growth Inhibition (% ± SEM) | |||||
|---|---|---|---|---|---|---|---|
| FB | FC | FS | |||||
| H2O | EtOH | MeOH | CHCl3 | MeOH | MeOH | ||
| MDA-MB-231 | 50 | 12.77 ± 3.30 d | 29.15 ± 1.58 c | 74.17 ± 0.34 a | <10 * | 12.83 ± 0.59 d | 46.55 ± 1.79 b |
| 100 | 13.05 ± 2.57 d | 89.06 ± 0.87 b | 94.98 ± 0.78 a | <10 * | 51.39 ± 0.77 c | 91.39 ± 0.83 b | |
| MCF-7 | 50 | 29.17 ± 3.44 e | 65.84 ± 2.35 c | 85.23 ± 0.50 a | 38.14 ± 2.50 d | 60.99 ± 1.30 c | 75.88 ± 0.78 b |
| 100 | 70.97 ± 3.10 e | 91.34 ± 0.86 c | 95.96 ± 0.36 a | 49.22 ± 1.52 f | 80.84 ± 0.72 d | 93.42 ± 0.57 b | |
| T47D | 50 | 17.45 ± 2.08 e | 55.38 ± 0.72 b | 51.51 ± 1.50 cd | 49.19 ± 1.33 d | 53.06 ± 0.72 bc | 68.55 ± 0.94 a |
| 100 | 20.72 ± 1.80 e | 78.62 ± 0.83 b | 82.80 ± 1.58 a | 68.40 ± 1.19 c | 63.59 ± 1.12 d | 80.79 ± 0.90 ab | |
| A2780 | 50 | <10 | 91.12 ± 0.43 c | 100.30 ± 0.36 a | 25.07 ± 3.29 e | 69.20 ± 1.66 d | 96.35 ± 0.72 b |
| 100 | 36.95 ± 3.79 c | 100.50 ± 0.33 a | 99.94 ± 0.34 a | 52.43 ± 0.99 b | 100.20 ± 0.31 a | 99.21 ± 0.47 a | |
| SiHa | 50 | 16.44 ± 2.02 e | 66.97 ± 0.33 b | 93.13 ± 0.92 a | 44.56 ± 1.62 d | 51.43 ± 2.86 c | 68.77 ± 0.31 b |
| 100 | 20.09 ± 1.58 d | 95.42 ± 0.76 a | 95.75 ± 0.48 a | 43.25 ± 1.82 c | 73.51 ± 1.26 b | 93.13 ± 1.02 a | |
| HeLa | 50 | 15.46 ± 2.47 e | 50.25 ± 2.04 c | 60.86 ± 3.10 b | 36.87 ± 0.54 d | 62.75 ± 2.12 b | 68.69 ± 1.35 a |
| 100 | 27.81 ± 1.07 e | 92.80 ± 3.38 a | 96.31 ± 0.50 a | 50.79 ± 1.50 d | 70.31 ± 2.96 c | 82.82 ± 2.48 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rašeta, M.; Kebert, M.; Pintać Šarac, D.; Mišković, J.; Berežni, S.; Kulmány, Á.E.; Zupkó, I.; Karaman, M.; Jovanović-Šanta, S. Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities. Microorganisms 2025, 13, 1210. https://doi.org/10.3390/microorganisms13061210
Rašeta M, Kebert M, Pintać Šarac D, Mišković J, Berežni S, Kulmány ÁE, Zupkó I, Karaman M, Jovanović-Šanta S. Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities. Microorganisms. 2025; 13(6):1210. https://doi.org/10.3390/microorganisms13061210
Chicago/Turabian StyleRašeta, Milena, Marko Kebert, Diandra Pintać Šarac, Jovana Mišković, Sanja Berežni, Ágnes Erika Kulmány, István Zupkó, Maja Karaman, and Suzana Jovanović-Šanta. 2025. "Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities" Microorganisms 13, no. 6: 1210. https://doi.org/10.3390/microorganisms13061210
APA StyleRašeta, M., Kebert, M., Pintać Šarac, D., Mišković, J., Berežni, S., Kulmány, Á. E., Zupkó, I., Karaman, M., & Jovanović-Šanta, S. (2025). Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities. Microorganisms, 13(6), 1210. https://doi.org/10.3390/microorganisms13061210











