Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains, Plant Materials, and Experimental Design
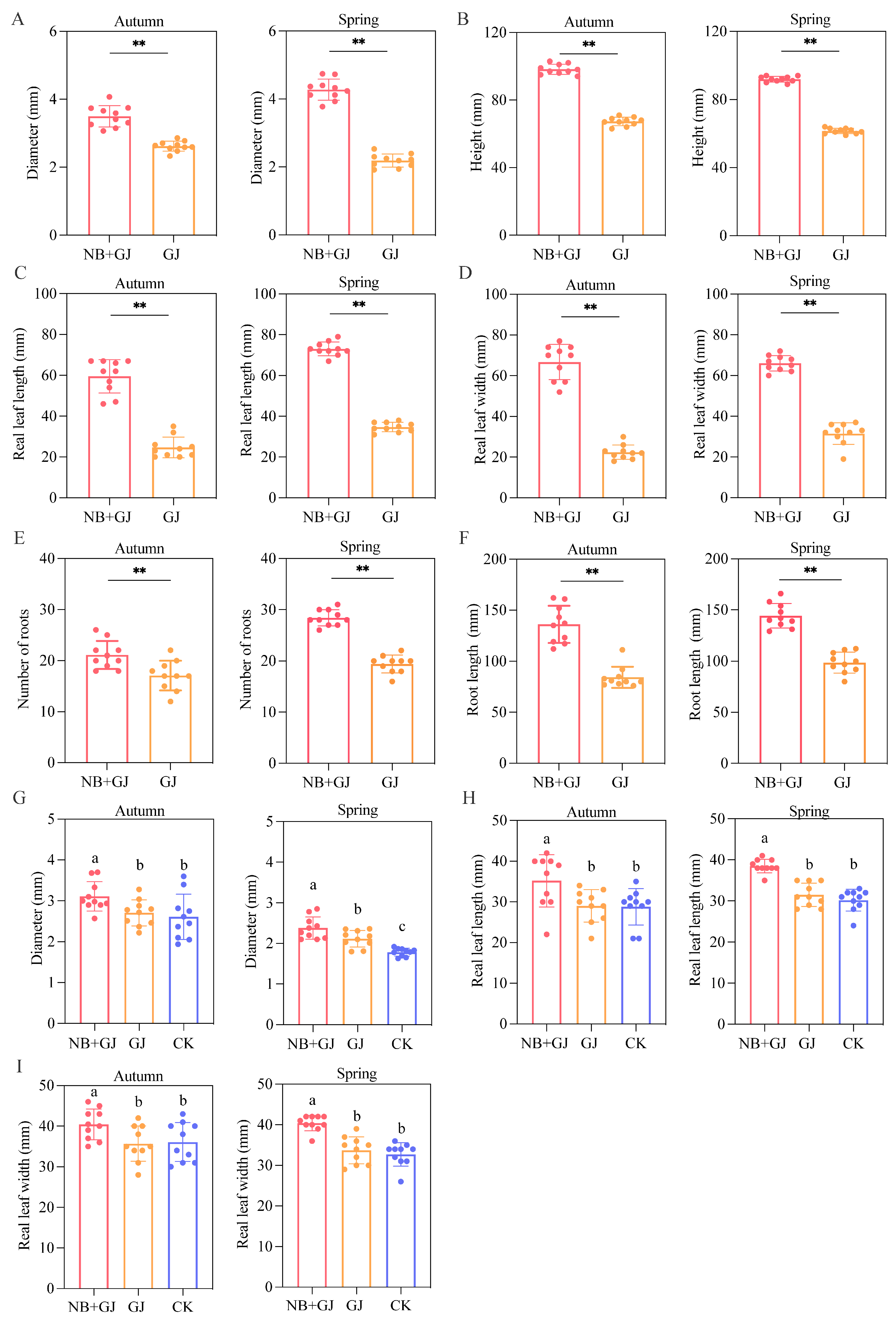
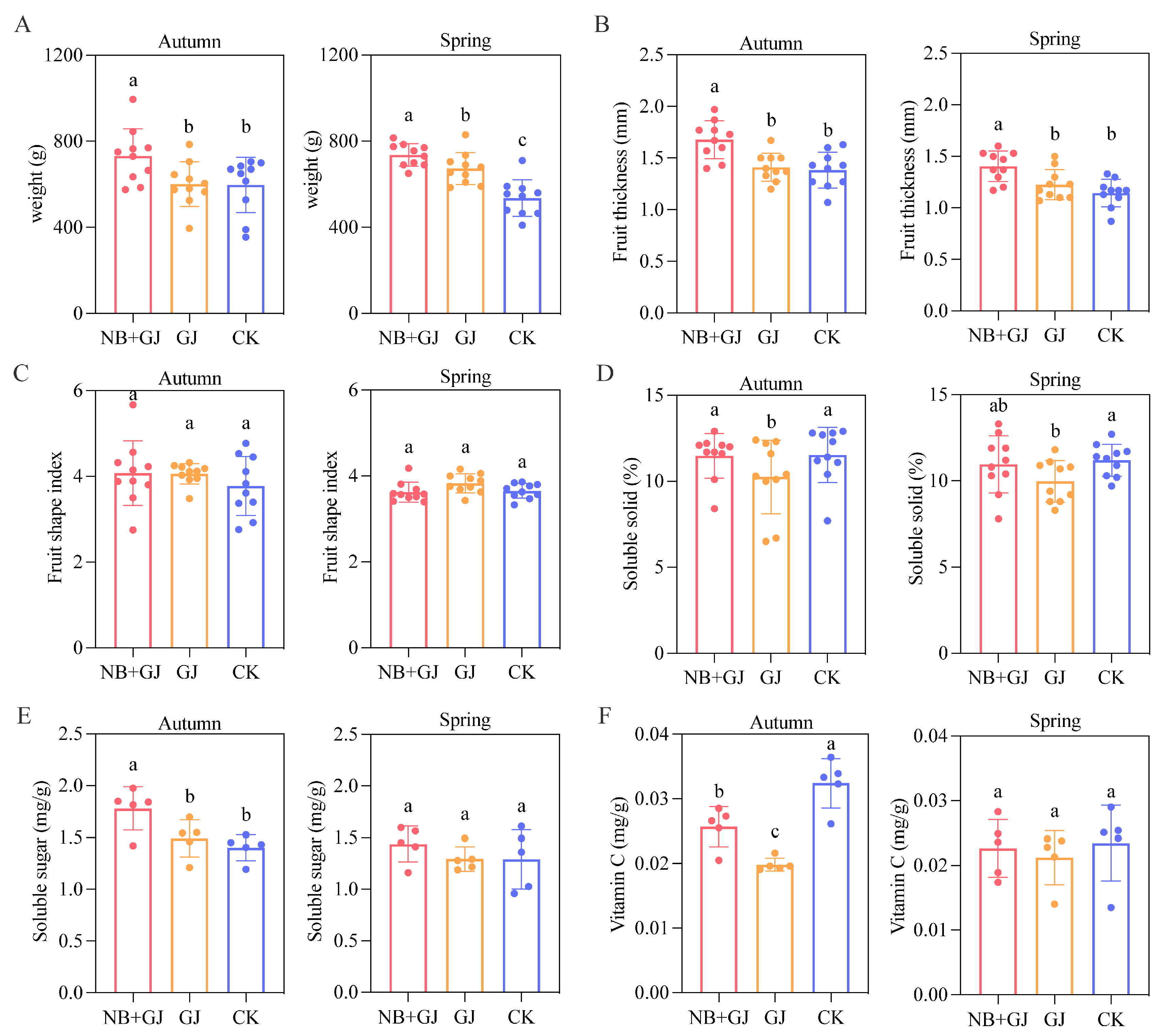
2.2. Phenotypic Trait Measurements
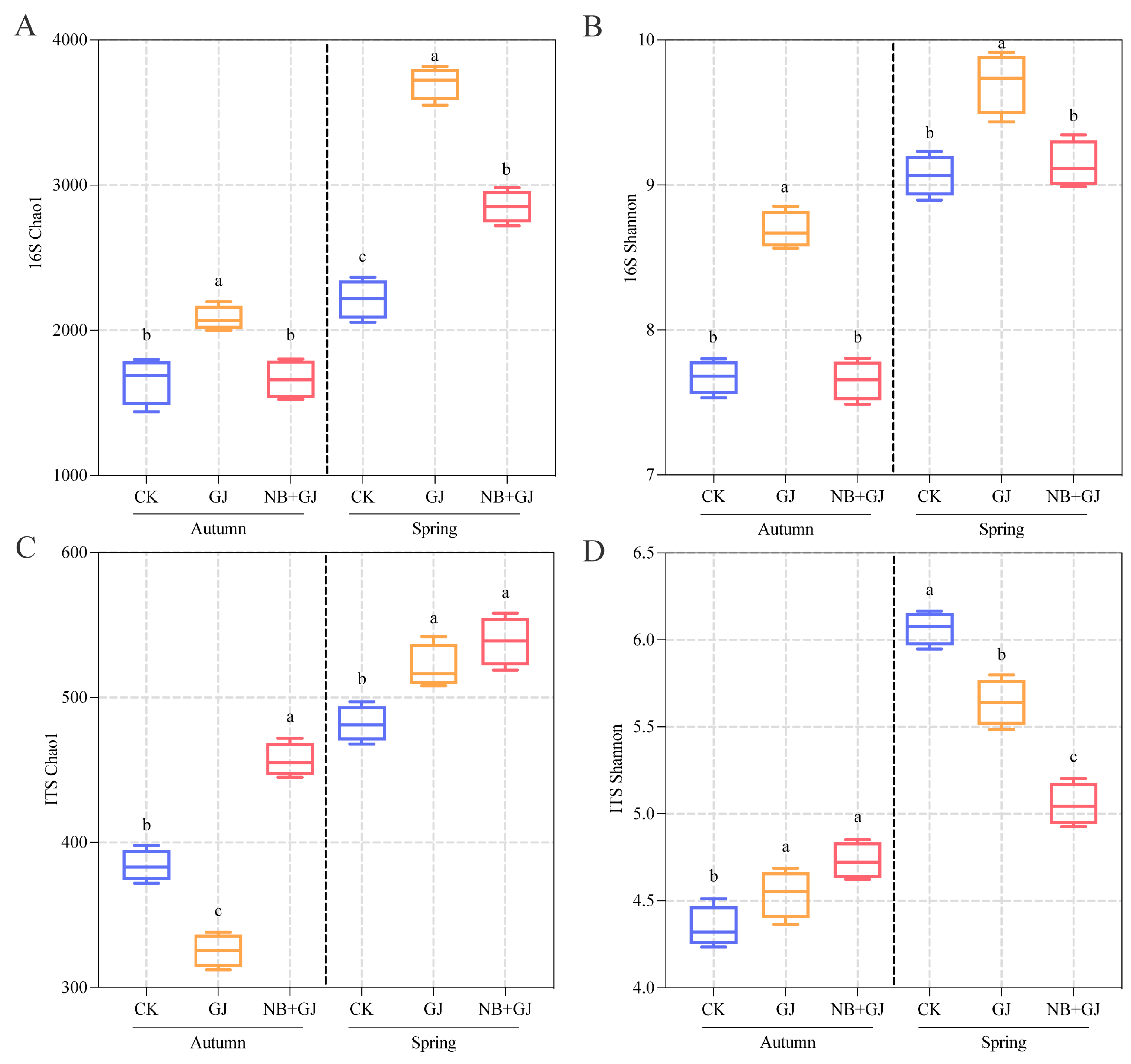
2.3. Rhizosphere Soil Sampling and DNA Sequencing
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Treatments on Growth of Melon Rootstock and Scion
3.2. Effects of Different Treatments on Fruit Quality and Yield
3.3. Effects of Different Treatments on Rhizosphere Microbial Diversity
3.4. Effects of Different Treatments on Rhizosphere Microbial Community Composition
3.5. Prediction of Rhizosphere Microbial Function of Melon Under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Autumn (%) | Spring (%) |
|---|---|---|
| NB+GJ | 96.0 | 98.5 |
| GJ | 85.0 | 82.5 |
| Treatment | Autumn (kg) | Spring (kg) |
|---|---|---|
| NB+GJ | 65.83 | 67.02 |
| GJ | 54.04 | 61.92 |
| CK | 50.71 | 49.08 |
References
- Li, J.P.; Ma, Y. Review and prospect of 70 years of development of watermelon and melon in China and 60 years of research and production cooperation. China Cucurbits Veg. 2019, 32, 1–8. [Google Scholar]
- Huang, X.Q.; Cai, Z.C. Soil microorganisms and soil-borne crop diseases control. Bull. Chin. Acad. Sci. 2017, 32, 593–600. [Google Scholar]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Anna, S.; Elvira, R. The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ. Exp. Botany 2010, 68, 283–291. [Google Scholar] [CrossRef]
- Xie, H.X.; Liu, R.J.; Sun, J.Q.; Li, M. Effects of AMF and grafting on soil physical, chemical and microbial characters in continuous cropping watermelon soil. Mycosystema 2018, 37, 830–836. [Google Scholar]
- Song, Y.; Ling, N.; Ma, J.H.; Wang, J.C.; Zhu, C.; Waseem, R.; Shen, Y.F.; Huang, Q.W.; Shen, Q.R. Grafting resulted in a distinct proteomic profile of watermelon root-exudates relative to the ungrafted watermelon and the rootstock plant. J. Plant Growth Regul. 2016, 35, 778–791. [Google Scholar] [CrossRef]
- Hai, R. Study on Grafting Sreeding Technology of Muskmelon. Master’s Thesis, Zhejiang University, Hangzhou, China, 2019. [Google Scholar]
- Wang, Y.H.; Li, L.Z.; Huang, Y.P.; Meng, Q.F.; Shao, B.F.; Wang, Z.L.; He, Z.L. Grafting experiment with different rootstocks in muskmelon. China Cucurbits Veg. 2005, 6, 30–32. [Google Scholar]
- Radhakrishnan, N.A.; Ravi, A.; Joseph, B.J.; Ashitha, J.; Jithesh, O.; Krishnankutty, R.E. Phenazine 1-carboxylic acid producing seed harbored endophytic bacteria from cultivated rice variety of kerala and its broad range antagonism to diverse plant pathogens. Probiot. Antimicrob. Prot. 2021, 15, 516–523. [Google Scholar] [CrossRef]
- Hao, F.M.; Zang, Q.Y.; Ma, E.L.; Ding, W.H.; Wang, Y.H.; Huang, Y.P. Identification, biocontrol and growth promoting effects of antagonistic bacteria NBmelon-1 of various fungal diseases in melon. China Cucurbits Veg. 2021, 34, 14–19. [Google Scholar]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS ONE 2014, 9, e100709. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; Oliveira, D.A.L.M. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl. Soil Ecol. 2021, 158, 103784. [Google Scholar] [CrossRef]
- Fang, S.Z.; Liu, D.; Tian, Y.; Deng, S.P.; Shang, X.L. Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: A rhizobox approach. PLoS ONE 2013, 8, e61461. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Zheng, L.X.; Feng, J.Y.; Zhou, J.X.; Wang, S.; Chen, F. Research progress on influencing factors of rhizosphere microbial assembly. Ind. Microbiol. 2024, 54, 7–9. [Google Scholar]
- Ling, N.; Zhang, W.W.; Wang, D.S.; Mao, J.G.; Huang, Q.W.; Guo, S.W.; Shen, Q.R. Root exudates from grafted-root watermelon showed a certain contribution in inhibiting Fusarium oxysporum f. sp. niveum. PLoS ONE 2013, 8, e63383. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J. Meta-analysis reveals the effects of microbial inoculants on the biomass and diversity of soil microbial communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpe a (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Wang, C.F.; Han, Y.; Wang, Y.B.; Wang, M.J.; Wang, X.J.; Ma, W.J. Determination and comparison of vitamin C content in fruits and vegetables. Agric. Technol. 2020, 40, 44–46. [Google Scholar]
- Zhang, S.B.; Jin, Y.X.; Zeng, Z.Y.; Liu, Z.Z.; Fu, Z.W. Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem. Res. Toxicol. 2015, 28, 2000–2009. [Google Scholar] [CrossRef]
- Pivato, B.; Semblat, A.; Guégan, T.; Jacquiod, S.; Martin, J.; Deau, F.; Moutier, N.; Lecomte, C.; Burstin, J.; Lemanceau, P. Rhizosphere bacterial networks, but not diversity, are impacted by pea-wheat intercropping. Front. Microbiol. 2021, 12, 674556. [Google Scholar] [CrossRef]
- Andre, P.M.; Andrea, K.B.; Jakub, M.T.; Daniel, G.B.; Josh, D.N. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Zhang, Z.R. Mechanism of Trichoderma viride on Alleviating Continuous Cropping Obstacle in Muskmelon. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Xu, S.L.; Li, X.M.; Chen, X.Q.; Duan, H.J. Effect of grafting and seedling cultivation on disease prevention and increase of muskmelon with thick skin. China Veg. 2000, 4, 19–21. [Google Scholar]
- Hao, T.Y.; Chen, S.F. Colonization of wheat, maize and cucumber by Paenibacillus polymyxa WLY78. PLoS ONE 2017, 12, e0169980. [Google Scholar] [CrossRef]
- Zaiter, H.Z.; Coyne, D.P.; Clark, R.B. Temperature, grafting methods and rootstock influence on iron deficiency chlorosis of bean. J. Am. Soc. Hortic. Sci. 1987, 112, 1023–1026. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Browning, J.A.; Frey, K.J. Multiline cultivars as a means of disease control. Annu. Rev. Phytopathol. 1969, 7, 355–382. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Peijnenburg, W.J.G.M.; Zhu, Y.C.; Zhang, M.; Sun, L.W.; Fu, Z.W.; Qian, H.F. Investigation of rhizospheric microbial communities in wheat, barley, and two rice varieties at the seedling stage. J. Agric. Food Chem. 2018, 66, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R.; Schmidt, T.M. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum acidobacteria. Appl. Environ. Microbiol. 2011, 77, 586–596. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Essarioui, A.; LeBlanc, N.; Kistler, H.; Kinkel, L. Plant community richness mediates inhibitory interactions and resource competition between Streptomyces and Fusarium populations in the rhizosphere. Microb. Ecol. 2017, 74, 157–167. [Google Scholar] [CrossRef]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, R.Y.; Liu, S.A.; Xing, W.; Yuan, Y.D. Seasonal changes in the soil microbial community structure in urban forests. Biology 2024, 13, 31. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Rfelt, H.D.; Gube, M.; Jackson, D.J.; Reitner, J.; Seyfullah, L.J. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenet. Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Voglmayr, H.; Rossman, A.Y.; Castlebury, L.A.; Jaklitsch, W.M. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Divers. 2012, 57, 1–44. [Google Scholar] [CrossRef]
- Takken, F.; Rep, M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chukwuneme, C.F.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic analyses of plant growth-promoting and carbon-cycling genes in maize rhizosphere soils with distinct land-use and management histories. Genes 2021, 12, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, P.N.; Liu, B.B.; Dai, W.C.; Cai, H.M.; Zheng, B.Q.; Li, J.C. Effects of late spring coldness during the anther differentiation period on bacterial community structure in wheat rhizosphere. Chin. J. Agrometeorol. 2024, 45, 756–765. [Google Scholar]
- Li, Y.Q.; Gao, X.Y.; Wang, L.Y. Microbial diversity and functions in the rhizosphere soil of different varieties of Pogostemon cablin. J. Northeast. For. Univ. 2025, 53, 137–144. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Zang, Q.; Wang, Y.; Ma, E.; Ding, W.; Yan, L.; Hao, F. Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks. Microorganisms 2025, 13, 1172. https://doi.org/10.3390/microorganisms13061172
Dong W, Zang Q, Wang Y, Ma E, Ding W, Yan L, Hao F. Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks. Microorganisms. 2025; 13(6):1172. https://doi.org/10.3390/microorganisms13061172
Chicago/Turabian StyleDong, Wenjie, Quanyu Zang, Yuhong Wang, Erlei Ma, Weihong Ding, Leiyan Yan, and Fangmin Hao. 2025. "Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks" Microorganisms 13, no. 6: 1172. https://doi.org/10.3390/microorganisms13061172
APA StyleDong, W., Zang, Q., Wang, Y., Ma, E., Ding, W., Yan, L., & Hao, F. (2025). Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks. Microorganisms, 13(6), 1172. https://doi.org/10.3390/microorganisms13061172




