RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Bacterial Strains, and Culture Conditions
2.2. Sample Collection
2.3. RNA Extraction and Quality Control
2.4. cDNA Library Construction and Sequencing
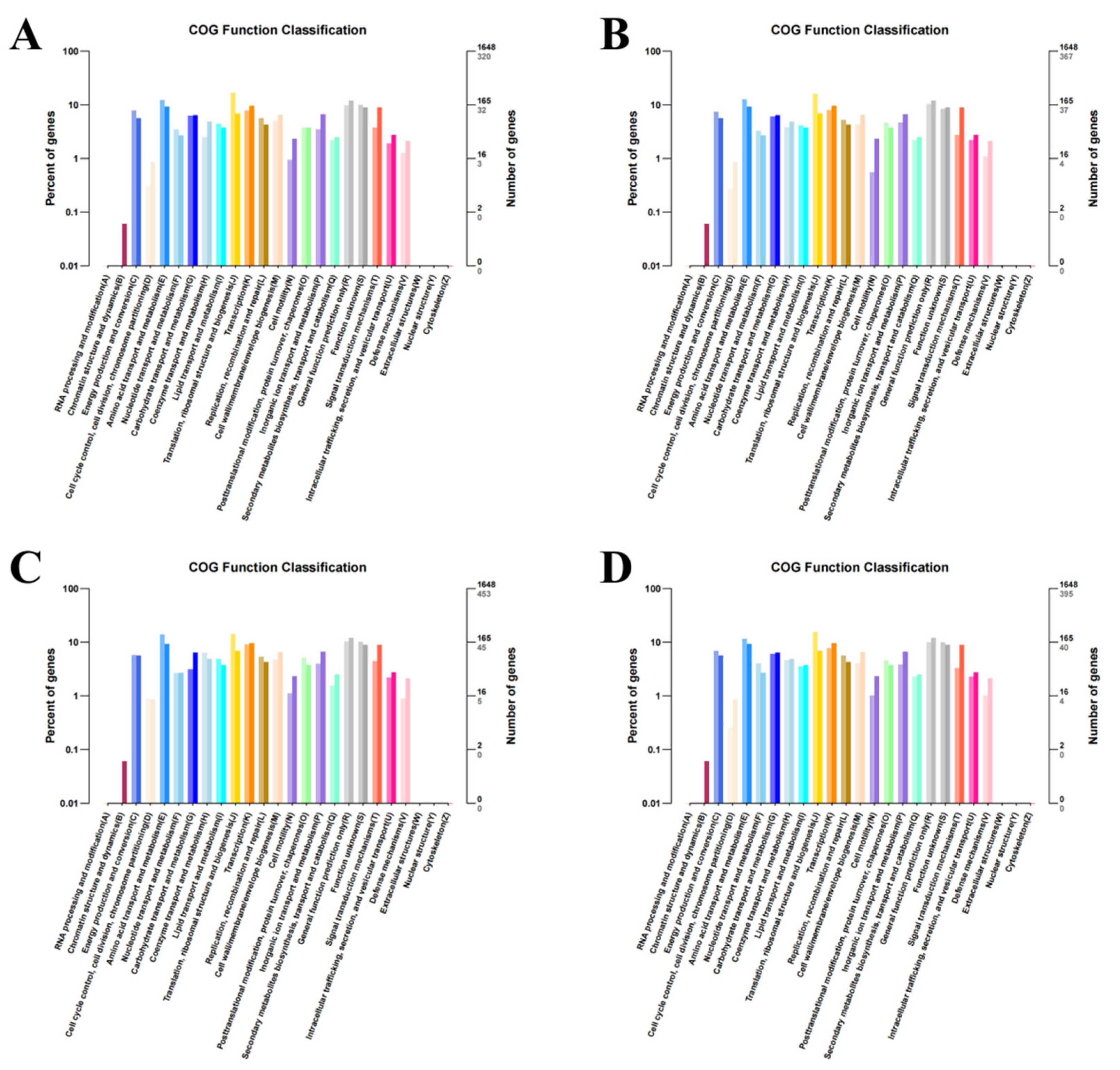
2.5. Sequence Data Analysis and Functional Annotation
2.6. Gene Expression Difference Analysis
2.7. RNA Isolation and qRT-PCR
2.8. H2O2 Stress, Bacterial Interspecies Competition, and Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
3.1. Library Sequencing and De Novo Transcriptome Assembly
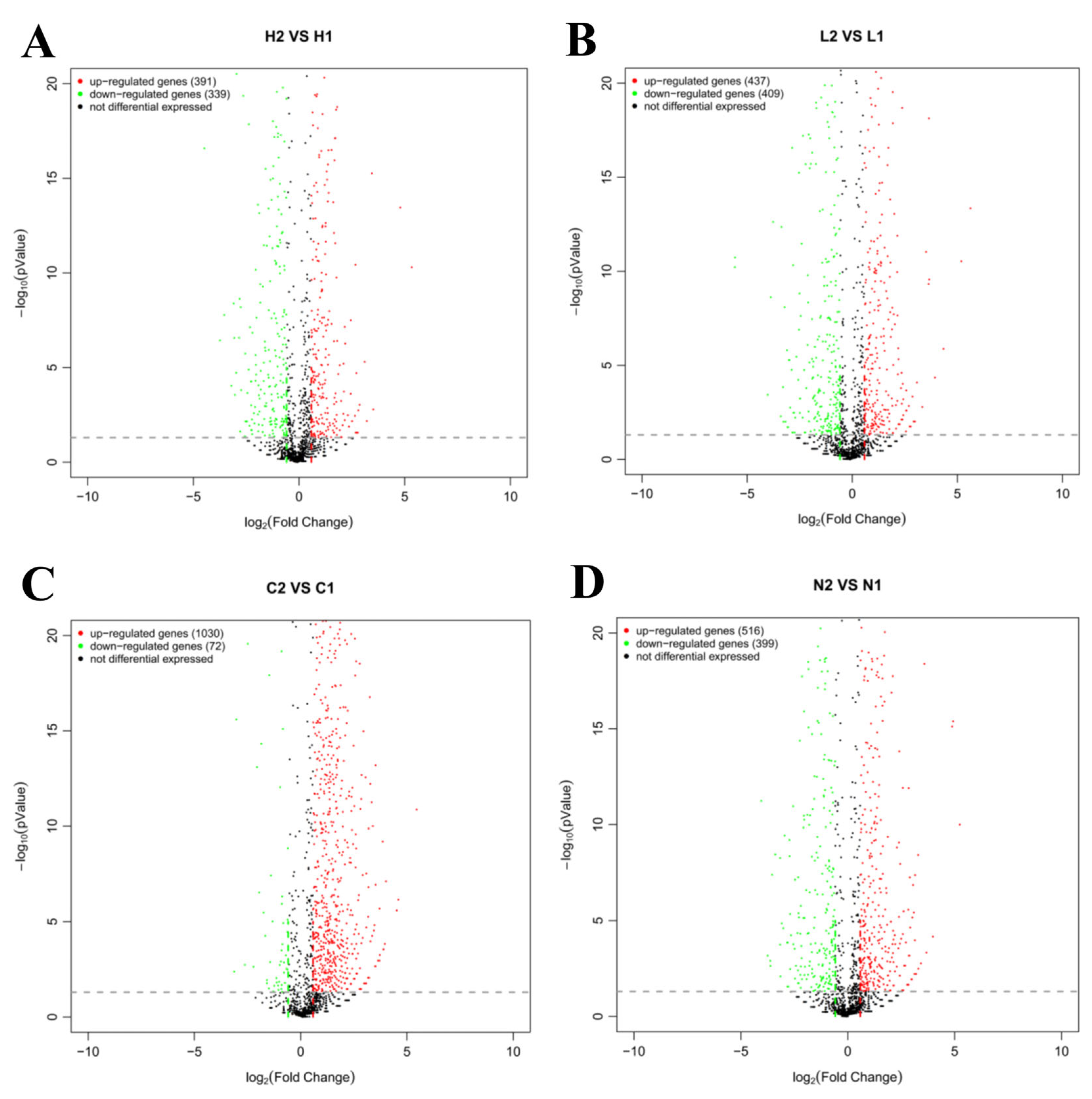
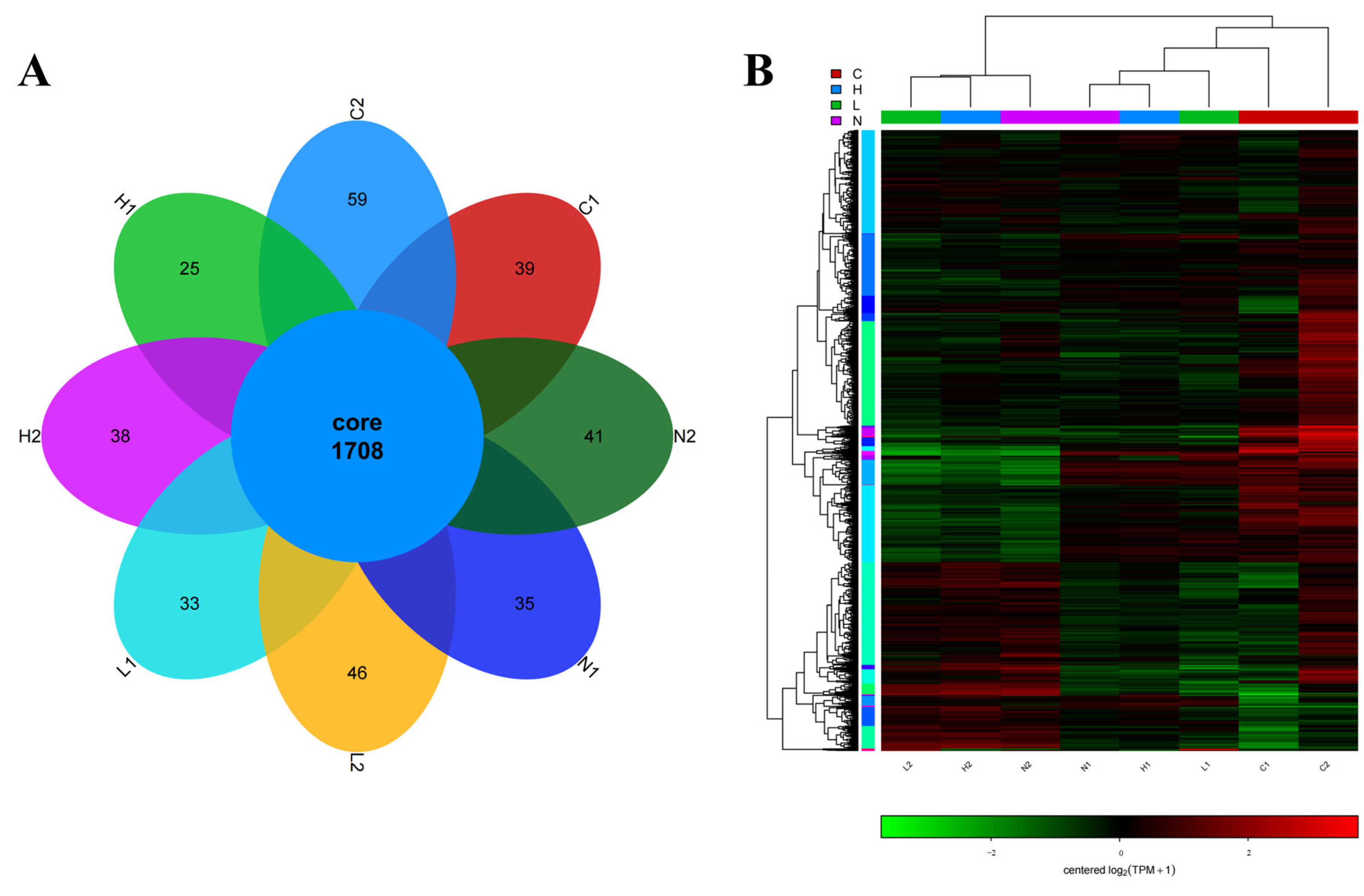
3.2. Identification of Differentially Expressed Transcripts in Different Iron Environments
3.3. Identification of Differentially Expressed Transcripts Regulated by Vsfur
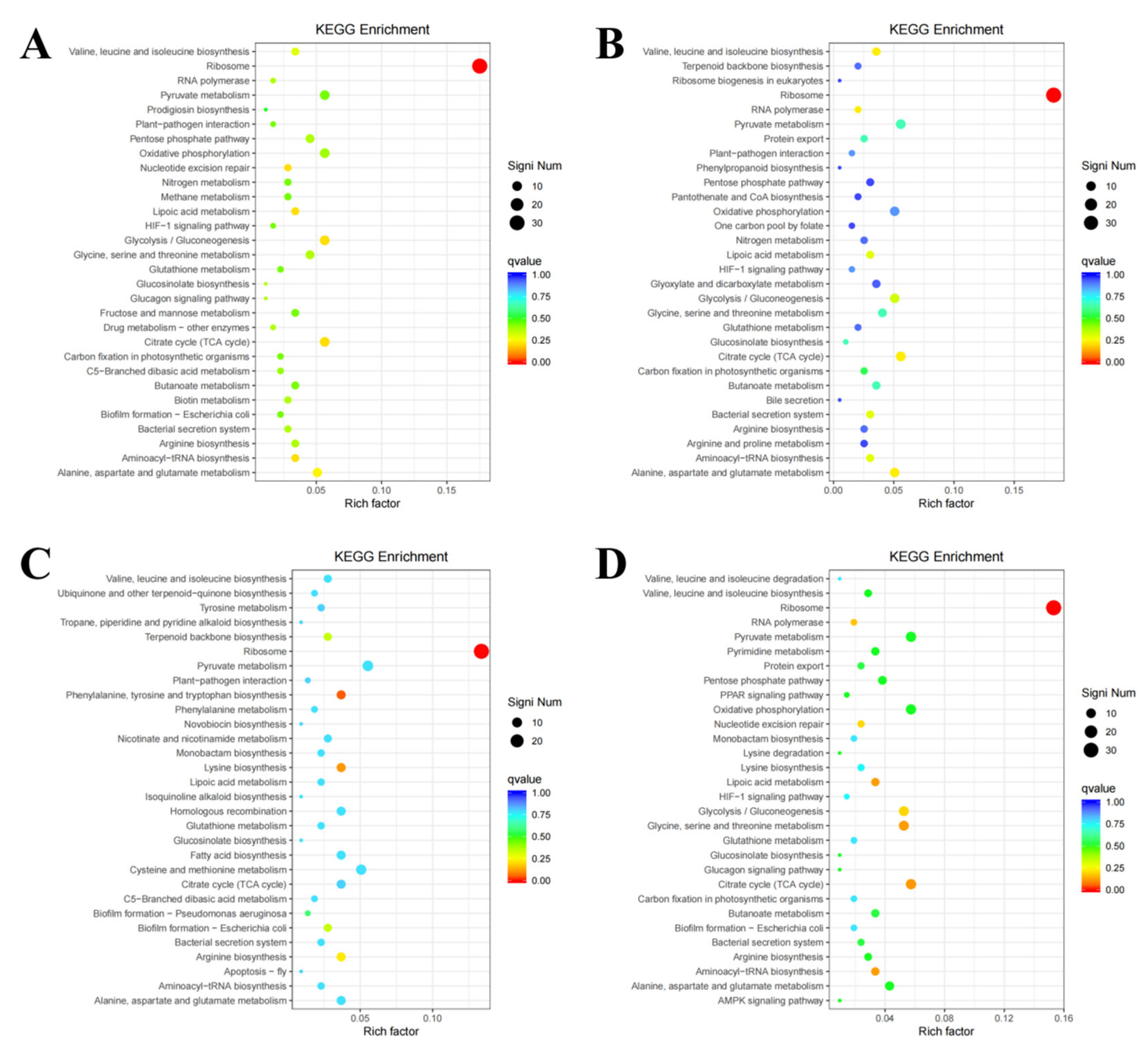
3.4. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
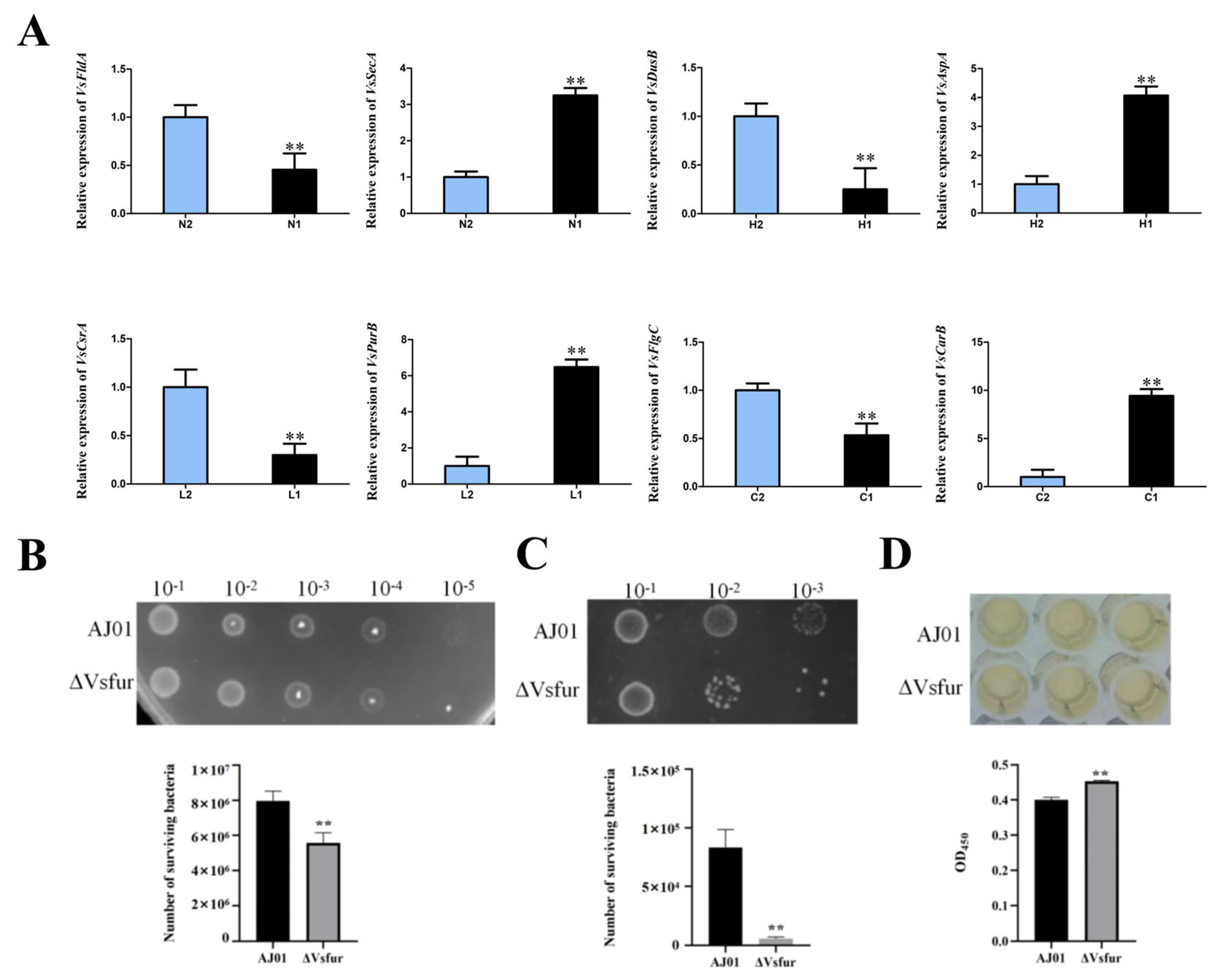
3.5. Validation of Differentially Expressed Genes by qRT-PCR
3.6. Vsfur Gene Deletion Affects Antioxidant Capacity, Bacterial Competitiveness, and Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksson, H.; Robinson, G.; Slater, M.J.; Troell, M. Sea cucumber aquaculture in the Western Indian ocean: Challenges for sustainable livelihood and stock improvement. Ambio 2012, 41, 109–121. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, Z.C.; Wang, N.B.; Liu, C. The syndrome of sea cucumber (Apostichopus japonicus) infected by virus and bacteria. Virol. Sin. 2008, 23, 63–67. [Google Scholar] [CrossRef]
- Liu, H.Z.; Zheng, F.R.; Sun, X.Q.; Hong, X.G.; Dong, S.L.; Wang, B.; Tang, X.X.; Wang, Y.Q. Identification of the pathogens associated with skin ulceration and peristome tumescence in cultured sea cucumbers Apostichopus japonicus (Selenka). J. Invertebr. Pathol. 2010, 105, 236–242. [Google Scholar] [CrossRef]
- Deng, H.; He, C.B.; Zhou, Z.C.; Liu, C.; Tan, K.F.; Wang, N.B.; Jiang, B.; Gao, X.G.; Liu, W.D. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture 2009, 287, 18–27. [Google Scholar] [CrossRef]
- Yang, A.F.; Zhou, Z.C.; Pan, Y.J.; Jiang, J.W.; Dong, Y.; Guan, X.Y.; Sun, H.J.; Gao, S.; Chen, Z. RNA sequencing analysis to capture the transcriptome landscape during skin ulceration syndrome progression in sea cucumber Apostichopus japonicus. BMC Genom. 2016, 17, 459. [Google Scholar] [CrossRef]
- Liang, W.K.; Zhang, C.; Liu, N.N.; Zhang, W.W.; Han, Q.X.; Li, C.H. Cloning and characterization of Vshppd, a gene inducing haemolysis and immune response of Apostichopus japonicus. Aquaculture 2016, 464, 246–252. [Google Scholar] [CrossRef]
- Liang, W.K.; Zhang, W.W.; Shao, Y.N.; Zhao, X.L.; Li, C.H. Dual functions of a 4-hydroxyphenylpyruvate dioxygenase for Vibrio splendidus survival and infection. Microb. Pathog. 2018, 120, 47–54. [Google Scholar] [CrossRef]
- Dai, F.; Guo, M.; Shao, Y.N.; Li, C.H. Novel secreted STPKLRR from Vibrio splendidus AJ01 promotes pathogen internalization via mediating tropomodulin phosphorylationdependent cytoskeleton rearrangement. PLoS. Pathog. 2023, 19, e1011419. [Google Scholar] [CrossRef]
- Dai, F.; Liang, W.K.; Liu, J.Q.; Guo, M.; Li, C.H. Eeukaryotic-like Sppsk1 from Vibrio splendidus AJ01 mediates phagosome escape via inhibiting phagosome acidification and maturation. Cell. Mol. Life Sci. 2025, 82, 88. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, Q.; Eggermont, M.; Defoirdt, T. Quorum-sensing interference in vibrios. Rev. Aquac. 2018, 10, 670–682. [Google Scholar] [CrossRef]
- Pajuelo, D.; Hernandez-Cabanyero, C.; Sanjuan, E.; Lee, C.T.; Silva-Hernandez, F.X.; Hor, L.I.; Mackenzie, S.; Amaro, C. Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ. Microbiol. 2016, 18, 4005–4022. [Google Scholar] [CrossRef] [PubMed]
- Troxell, B.; Hassan, H.M. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 59. [Google Scholar] [CrossRef]
- Yu, C.X.; McClure, R.; Nudel, K.; Daou, N.; Genco, C.A. Characterization of the Neisseria gonorrhoeae iron and Fur regulatory network. J. Bacteriol. 2016, 198, 2180–2191. [Google Scholar] [CrossRef]
- Shi, Y.; Liao, C.Y.; Dai, F.; Zhang, Y.W.; Li, C.H.; Liang, W.K. Vibrio splendidus Fur regulates virulence gene expression, swarming motility, and biofilm formation, affecting its pathogenicity in Apostichopus japonicus. Front. Vet. Sci. 2023, 10, 1207831. [Google Scholar] [CrossRef]
- Liang, W.K.; Zhang, W.W.; Li, C.H. Vibrio splendidus virulence to Apostichopus japonicus is mediated by hppD through glutamate metabolism and flagellum assembly. Virulence 2022, 13, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Vasta, G.R. Introduction to special issue: Pattern recognition receptors and their roles in immunity in invertebrates. Dev. Comp. Immunol. 2020, 109, 103712. [Google Scholar] [CrossRef]
- Kloc, M.; Halasa, M.; Kubiak, J.Z.; Ghobrial, R.M. Invertebrate immunity, natural transplantation immunity, somatic and germ cell parasitism, and transposon defense. Int. J. Mol. Sci. 2024, 25, 1072. [Google Scholar] [CrossRef] [PubMed]
- Eliseikina, M.G.; Magarlamov, T.Y. Coelomocyte morphology in the holothurians Apostichopus japonicus, (Aspidochirota: Stichopodidae) and Cucumaria japonica (Dendrochirota: Cucumariidae). Russ. J. Mar. Biol. 2002, 28, 197–202. [Google Scholar] [CrossRef]
- Chen, P.; Meulenaere, E.D.; Deheyn, D.D.; Bandaru, P.R. Iron redox pathway revealed in ferritin via electron transfer analysis. Sci. Rep. 2020, 10, 4033. [Google Scholar] [CrossRef]
- Porcheron, G.; Dozois, C.M. Interplay between iron homeostasis and virulence: Fur and RyhB as major regulators of bacterial pathogenicity. Vet. Microbiol. 2015, 179, 2–14. [Google Scholar] [CrossRef]
- Huang, L.X.; Hu, J.; Su, Y.Q.; Qin, Y.X.; Kong, W.D.; Zhao, L.M.; Ma, Y.; Xu, X.J.; Lin, M.; Zheng, J.; et al. Genome-wide detection of predicted non-coding RNAs related to the adhesion process in Vibrio alginolyticus using high-throughput sequencing. Front. Microbiol. 2016, 7, 619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markevich, N.I.; Galimova, M.H.; Markevich, L.N. Hysteresis and bistability in the succinate-CoQ reductase activity and reactive oxygen species production in the mitochondrial respiratory complex II. Redox. Biol. 2020, 37, 101630. [Google Scholar] [CrossRef] [PubMed]
- Hoffelder, M.; Raasch, K.; Ooyen, J.V.; Eggeling, L. The E2 domain of OdhA of Corynebacterium glutamicum has succinyltransferase activity dependent on lipoyl residues of the acetyltransferase AceF. J. Bacteriol. 2010, 192, 5203–5211. [Google Scholar] [CrossRef]
- Cenigaonandia-Campillo, A.; Serna-Blasco, R.; Gómez-Ocabo, L.; Solanes-Casado, S.; Baños-Herraiz, N.; Puerto-Nevado, L.D.; Cañas, J.A.; Aceñero, M.J.; García-Foncillas, J.; Aguilera, O. Vitamin C activates pyruvate dehydrogenase (PDH) targeting the mitochondrial tricarboxylic acid (TCA) cycle in hypoxic KRAS mutant colon cancer. Theranostics 2021, 11, 3595–3606. [Google Scholar] [CrossRef]
- Qi, J.J.; Wang, Y.; Li, H.R.; Shang, Y.B.; Gao, S.; Ding, C.; Liu, X.H.; Wang, S.H.; Li, T.; Tian, M.X.; et al. Mycoplasma synoviae dihydrolipoamide dehydrogenase is an immunogenic fibronectin/plasminogen binding protein and a putative adhesin. Vet. Microbiol. 2022, 265, 109328. [Google Scholar] [CrossRef]
- Feeney, T.; Hartwig, F.P.; Davies, N.M. How to use directed acyclic graphs: Guide for clinical researchers. BMJ 2025, 388, e078226. [Google Scholar] [CrossRef]
- Almet, A.A.; Tsai, Y.C.; Watanabe, M.; Nie, Q. Inferring pattern-driving intercellular flows from single-cell and spatial transcriptomics. Nat. Methods 2024, 21, 1806–1817. [Google Scholar] [CrossRef]
- Naiyer, S.; Singh, S.S.; Kaur, D.; Mukherjee, A.; Singh, Y.P.; Bhattacharya, A.; Bhattacharya, S. Transcriptomic analysis of ribosome biogenesis and pre-rRNA processing during growth stress in Entamoeba histolytica. Exp. Parasitol. 2022, 239, 108308. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yonekura, K.; Namba, K. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J. Mol. Biol. 2004, 337, 105–113. [Google Scholar] [CrossRef]
- Brown, M.T.; Steel, B.C.; Silvestrin, C.; Wilkinson, D.A.; Delalez, N.J.; Lumb, C.N.; Obara, B.; Armitage, J.P.; Berry, R.M. Flagellar hook flexibility is essential for bundle formation in swimming Escherichia coli cells. J. Bacteriol. 2012, 194, 3495–3501. [Google Scholar] [CrossRef]
- Dong, T.G.; Mekalanos, J.J. Characterization of the RpoN regulon reveals differential regulation of T6SS and new flagellar operons in Vibrio cholerae O37 strain V52. Nucleic. Acids. Res. 2012, 40, 7766–7775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Garcia-Alcala, M.; Balleza, E.; Cluzel, P. Stochastic transcriptional pulses orchestrate flagellar biosynthesis in Escherichia coli. Sci. Adv. 2020, 6, eaax0947. [Google Scholar] [CrossRef]
- Shao, S.; Li, C.L.; Zhao, L.Y.; Zhang, Y.X.; Yin, K.Y.; Wang, Q.Y. Interplay between ferric uptake regulator Fur and horizontally acquired virulence regulator Esr B coordinates virulence gene expression in Edwardsiella piscicida. Microbiol. Res. 2021, 253, 126892. [Google Scholar] [CrossRef]
- Brauer, M.J.; Huttenhower, C.; Airoldi, E.M.; Rosenstein, R.; Matese, J.C.; Gresham, D.; Boer, V.M.; Troyanskaya, O.G.; Botstein, D. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol. Biol. Cell. 2008, 19, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Starosta, A.L.; Lassak, J.; Jung, K.; Wilson, D.N. The bacterial translation stress response. FEMS Microbiol. Rev. 2014, 38, 1172–1201. [Google Scholar] [CrossRef]
- Wilson, D.N.; Blaha, G.; Connell, S.R.; Ivanov, P.V.; Jenke, H.; Stelzl, U.; Teraoka, Y.; Nierhaus, K.H. Protein synthesis at atomic resolution: Mechanistics of translation in the light of highly resolved structures for the ribosome. Curr. Protein Pept. Sci. 2002, 3, 1–53. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhang, D.J.; Chen, Z.; Xu, R.M.; Nierhaus, K.H.; Gong, W.M.; Qin, Y. A conserved proline switch on the ribosome facilitates the recruitment and binding of trGTPases. Nat. Struct. Mol. Biol. 2012, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.W.; Nishikino, T.; Hu, B.; Kojima, S.; Homma, M.; Liu, J. Molecular architecture of the sheathed polar flagellum in Vibrio alginolyticus. Proc. Natl. Acad. Sci. USA 2017, 114, 10966–10971. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.F.; Wang, Y.S.; Yang, Q.; Xiao, Y.L.; Cai, H.R.; Xie, C.M. Using ROS as a second messenger, NADPH oxidase 2 mediates macrophage senescence via interaction with NF- κB during Pseudomonas aeruginosa infection. Oxid. Med. Cell. Longev. 2018, 2018, 9741838. [Google Scholar] [CrossRef]
- Crisan, C.V.; Chande, A.T.; Williams, K.; Raghuram, V.; Rishishwar, L.; Steinbach, G.; Watve, S.S.; Yunker, P.; Jordan, I.K.; Hammer, B.K. Analysis of Vibrio cholerae genomes identifies new type VI secretion system gene clusters. Genome. Biol. 2019, 20, 163. [Google Scholar] [CrossRef]


| Primer | Sequences (5′–3′) a |
|---|---|
| 8F | AGAGTTTGATCCTGGCTCAG |
| 1492R | GGTTACCTTGTTACGACTT |
| VsfurF | CTCGAGATGTCAGACAATAATCAAGCG (Xho I) |
| VsfurR | GGATCCAGTTACAATGCCAGCATC (BamH I) |
| VsFldARTF | CTTGTTGCTATCTTTGGTTGTGG |
| VsFldARTR | ATTGGCTGTCATCGCCTTCA |
| VsSecARTF | AGGTTGGTTAGACGAAGACGACA |
| VsSecARTR | CGCGAACTTTAGAAAGGATGGT |
| VsDusBRTF | ATGGCTGGCGTAACGGATAG |
| VsDusBRTR | ACTGAACGAATGCCCGACTC |
| VsAspARTF | AACCGCAGCCAATCTACAAA |
| VsAspARTR | CCGTCATAGGCACAGCATCTT |
| VsCsrARTF | TGTTGGCGAAACACTGATGAT |
| VsCsrARTR | GGTGAACAGATACTTCTTTAGGTGC |
| VsPurBRTF | CCCAGAAGTTGAATGGCACC |
| VsPurBRTR | TTACGAAGAACCGTAGAGTCAGTAAG |
| VsFlgCRTF | TGGTTCTGCGATGAGTGCTG |
| VsFlgRTR | GCTAATGGGTGATCTGGGTTGTA |
| VsCarBRTF | ACTCGATCACAGTGGCTCCG |
| VsCarBRTR | CATAACTTCACCAACCGACTTCAT |
| 933F | GCACAAGCGGTGGAGCATGTGG |
| 16SRTR1 | CGTGTGTAGCCCTGGTCGTA |
| Sample Name | Raw Reads | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| H1 | 18,511,874 | 17,811,848 | 2.58 G | 0.04 | 98.70% | 96.69% | 46.64% |
| H2 | 15,482,650 | 14,958,380 | 2.17 G | 0.03 | 98.73% | 96.79% | 45.75% |
| L1 | 18,457,720 | 17,780,906 | 2.56 G | 0.04 | 98.70% | 96.71% | 47.09% |
| L2 | 18,578,108 | 17,928,076 | 2.59 G | 0.03 | 98.68% | 96.67% | 46.05% |
| N1 | 17,714,246 | 17,072,464 | 2.47 G | 0.04 | 98.61% | 96.41% | 46.90% |
| N2 | 18,152,356 | 17,497,854 | 2.50 G | 0.04 | 98.69% | 96.71% | 46.20% |
| C1 | 60,946,986 | 58,627,030 | 8.44 G | 0.04 | 98.58% | 96.08% | 54.91% |
| C2 | 62,823,344 | 60,461,710 | 8.60 G | 0.04 | 98.61% | 96.14% | 53.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Hu, L.; Zhu, S.; Liang, W.; Yang, L.; Li, C. RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus. Microorganisms 2025, 13, 1173. https://doi.org/10.3390/microorganisms13061173
Liao C, Hu L, Zhu S, Liang W, Yang L, Li C. RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus. Microorganisms. 2025; 13(6):1173. https://doi.org/10.3390/microorganisms13061173
Chicago/Turabian StyleLiao, Changyu, Lincheng Hu, Si Zhu, Weikang Liang, Lei Yang, and Chenghua Li. 2025. "RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus" Microorganisms 13, no. 6: 1173. https://doi.org/10.3390/microorganisms13061173
APA StyleLiao, C., Hu, L., Zhu, S., Liang, W., Yang, L., & Li, C. (2025). RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus. Microorganisms, 13(6), 1173. https://doi.org/10.3390/microorganisms13061173






