Evaluation of Growth Performance, Biochemical Composition, and Polyhydroxyalkanoates Production of Four Cyanobacterial Species Grown in Cheese Whey
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Whey
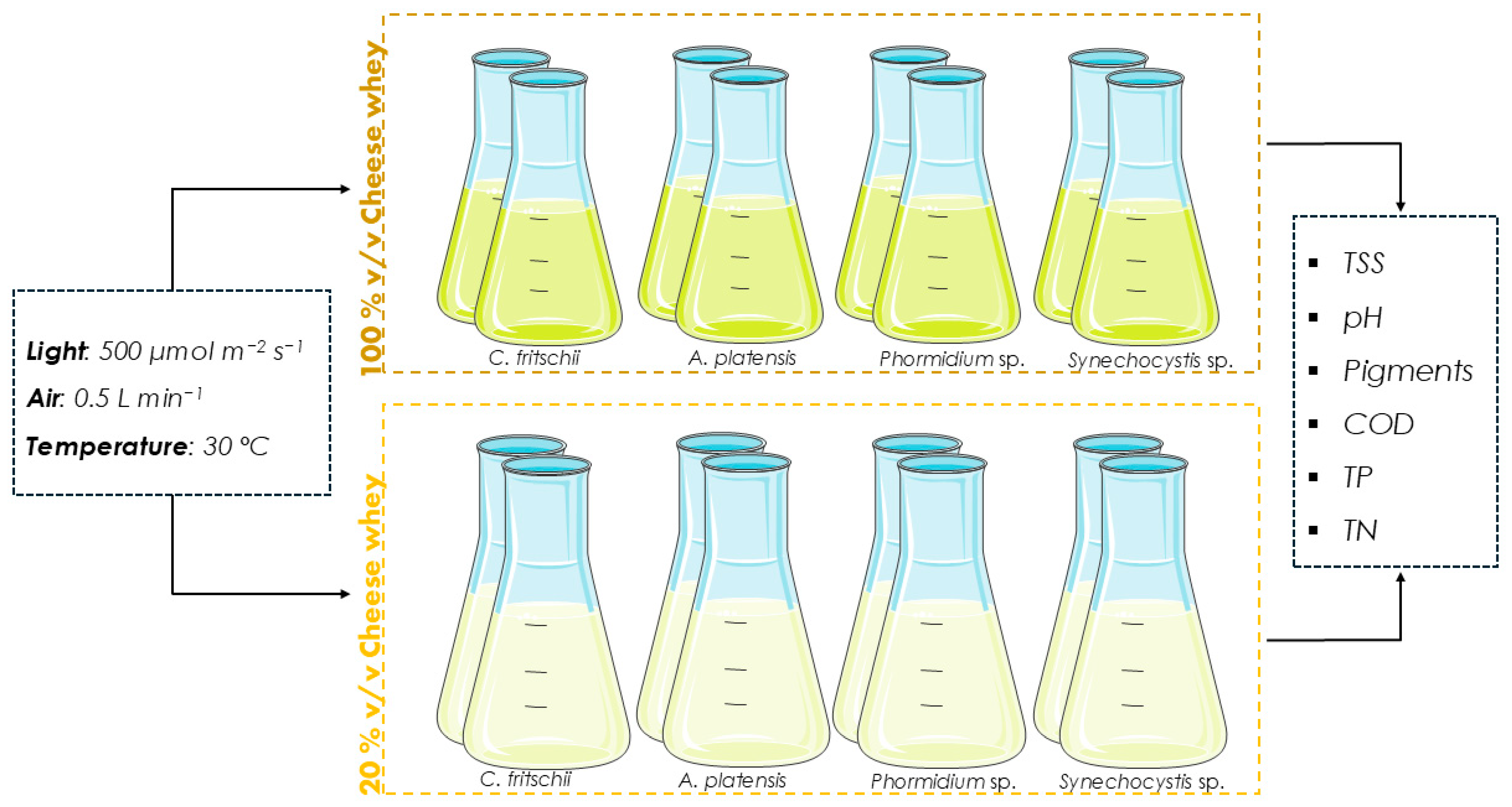
2.2. Cyanobacteria Species and Cultivation Conditions
2.3. Analytical Methods
2.3.1. Biomass Growth
2.3.2. Cyanobacterial Pigments
2.3.3. CW Remediation
2.3.4. Biomass Compositional Analysis
2.3.5. Statistical Analysis
3. Results and Discussion
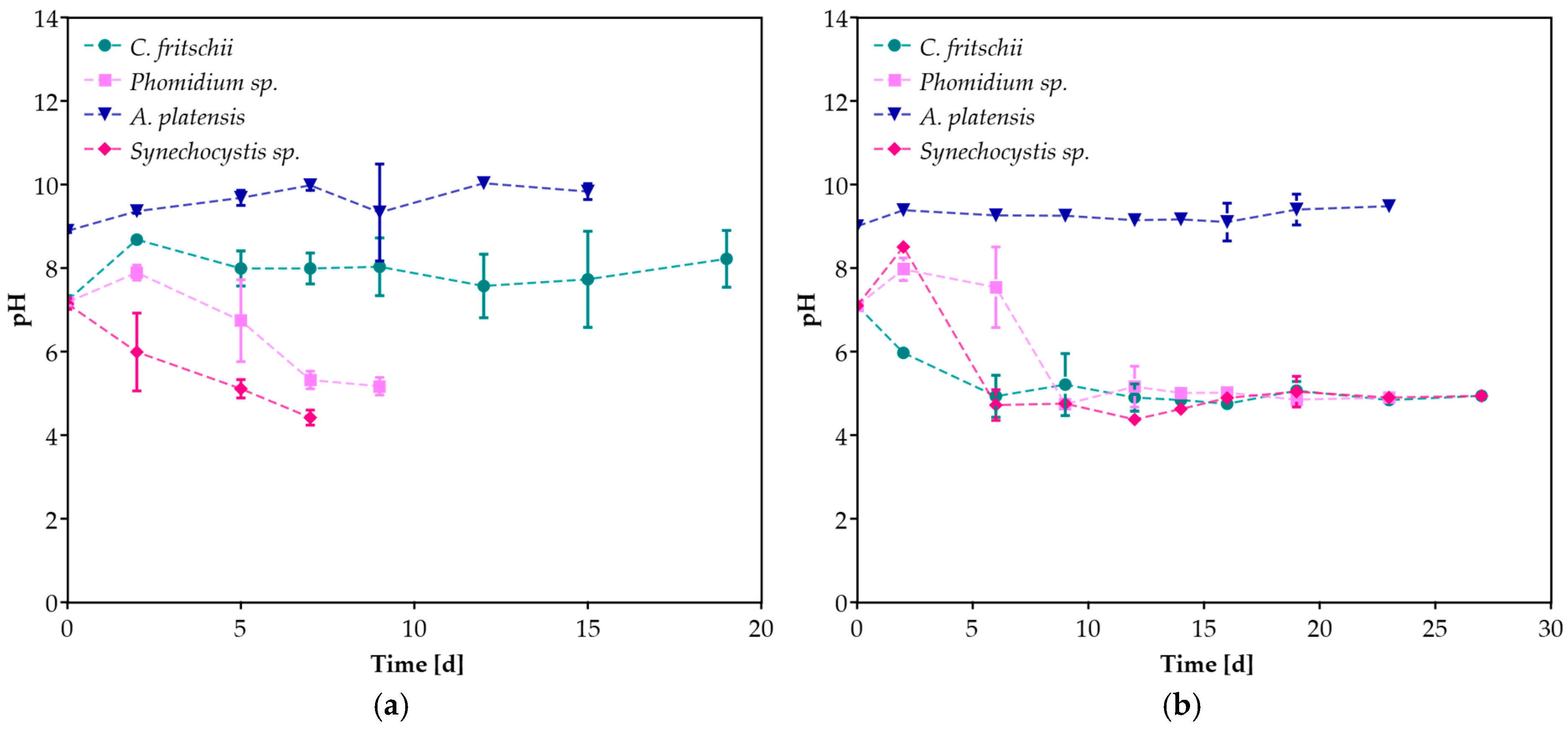
3.1. Cyanobacteria Growth
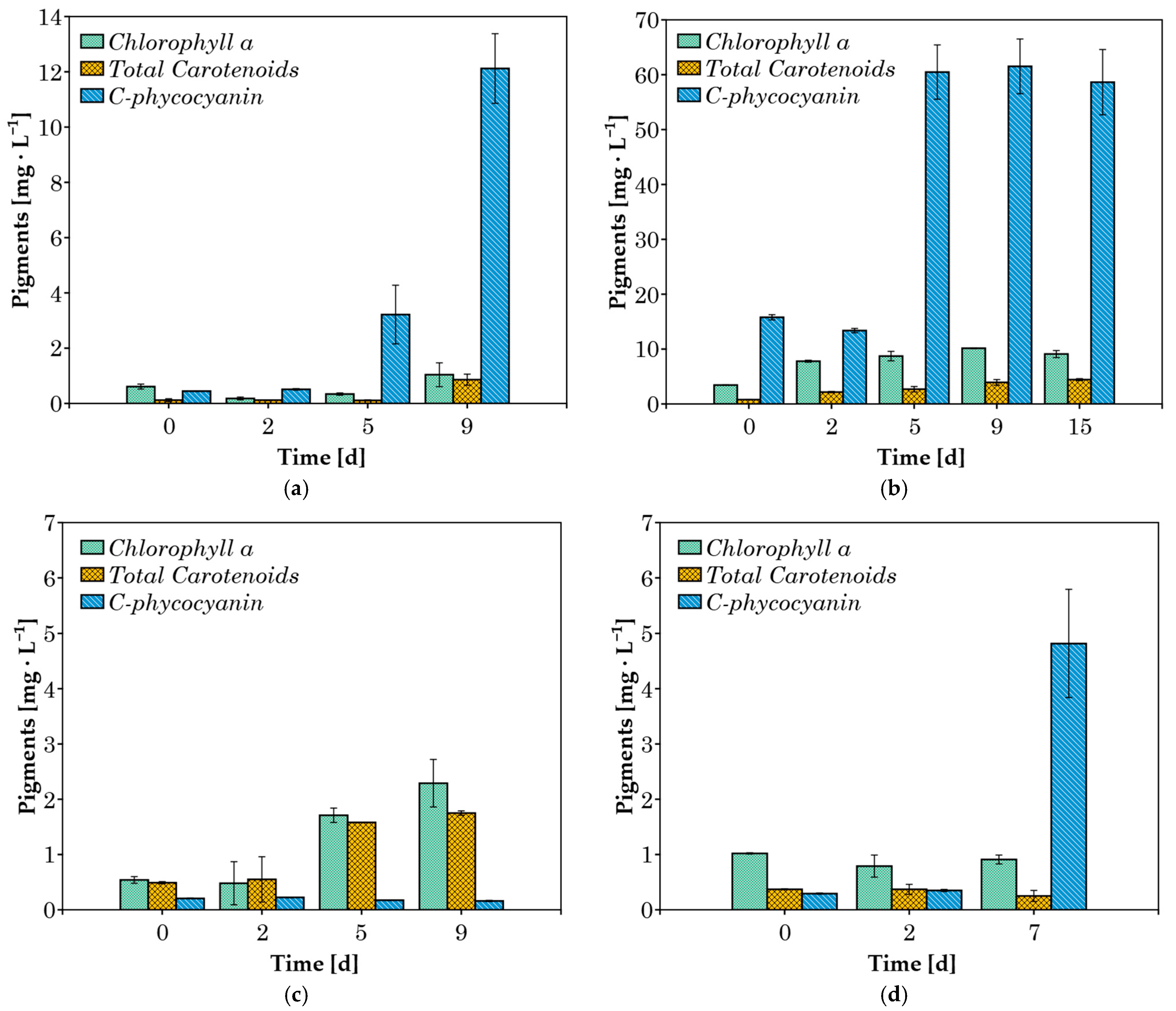
3.2. Pigments
3.3. CW Bioremediation
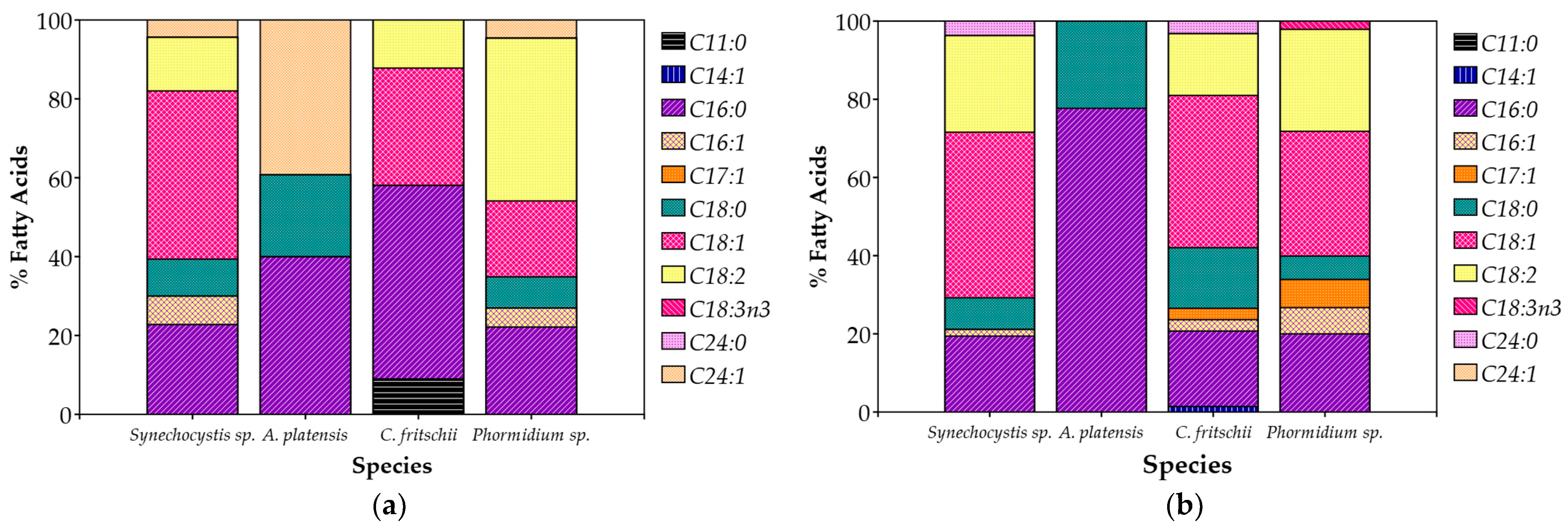
3.4. Biomass Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOD | Biological oxygen demand |
| COD | Chemical oxygen demand |
| CW | Cheese whey |
| DW | Dry weight |
| FAMEs | Fatty acid methyl ester |
| FAs | Fatty acids |
| PHAs | Polyhydroxyalkanoates |
| PHB | Polyhydroxybutyrate |
| TKN | Total Kjeldahl nitrogen |
| TN | Total nitrogen |
| TP | Total phosphorus |
| TS | Total solids |
| TSS | Total suspended solids |
| VS | Volatile solids |
References
- Fujita, Y.; Uesaka, K. Nitrogen Fixation in Cyanobacteria. In Cyanobacterial Physiology: From Fundamentals to Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 29–45. [Google Scholar] [CrossRef]
- Agarwal, P.; Soni, R.; Kaur, P.; Madan, A.; Mishra, R.; Pandey, J.; Singh, S.; Singh, G. Cyanobacteria as a Promising Alternative for Sustainable Environment: Synthesis of Biofuel and Biodegradable Plastics. Front. Microbiol. 2022, 13, 939347. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, A.; Torre, S.; Usai, L.; Casula, M.; Fais, G.; Nieri, P.; Concas, A.; Lutzu, G.A. Effect of Cheese Whey on Phycobiliproteins Production and FAME Profile by Arthrospira platensis (Spirulina): Promoting the Concept of a Circular Bio-Economy. Sustain. Chem. Pharm. 2024, 40, 101625. [Google Scholar] [CrossRef]
- Zanolla, V.; Biondi, N.; Niccolai, A.; Abiusi, F.; Adessi, A.; Rodolfi, L.; Tredici, M.R. Protein, Phycocyanin, and Polysaccharide Production by Arthrospira Platensis Grown with LED Light in Annular Photobioreactors. J. Appl. Phycol. 2022, 34, 1189–1199. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Otero, A.; Coutinho, P. Application of Microalgae and Microalgal Bioactive Compounds in Skin Regeneration. Algal Res. 2021, 58, 102395. [Google Scholar] [CrossRef]
- Baraldi, L.; Usai, L.; Torre, S.; Fais, G.; Casula, M.; Dessi, D.; Nieri, P.; Concas, A.; Lutzu, G.A. Dairy Wastewaters to Promote Mixotrophic Metabolism in Limnospira (Spirulina) platensis: Effect on Biomass Composition, Phycocyanin Content, and Fatty Acid Methyl Ester Profile. Life 2025, 15, 184. [Google Scholar] [CrossRef]
- Nawaz, T.; Gu, L.; Hu, Z.; Fahad, S.; Saud, S.; Zhou, R. Advancements in Synthetic Biology for Enhancing Cyanobacterial Capabilities in Sustainable Plastic Production: A Green Horizon Perspective. Fuels 2024, 5, 394–438. [Google Scholar] [CrossRef]
- Fayshal, M.A. Current Practices of Plastic Waste Management, Environmental Impacts, and Potential Alternatives for Reducing Pollution and Improving Management. Heliyon 2024, 10, e40838. [Google Scholar] [CrossRef]
- Abdo, S.M.; Ali, G.H. Analysis of Polyhydroxybutrate and Bioplastic Production from Microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and Challenges in Producing Polyhydroxyalkanoate Biopolymers from Cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Tanweer, S.; Mishra, P.; Dash, K.; Panda, B. Two Decades Research on Cyanobacterial PHB: Challenges and Opportunities. In Polyhydroxyalkanoates: Sustainable Production and Biotechnological Applications I: Microbial Biodiversity, Biowastes, and Bioprocesses; Springer: Singapore, 2025; pp. 71–102. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Luis, C.-C.; Otiniano, N.M.; De La Cruz-Noriega, M.; Gallozzo-Cardenas, M. Utilization of Cheese Whey for Energy Generation in Microbial Fuel Cells: Performance Evaluation and Metagenomic Analysis. Fermentation 2025, 11, 176. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; ur Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Uporaba Sirutke: Održivo Iskorištenje i Smanjenje Štetnog Utjecaja Na Okoliš. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of Cheese Whey, a by-Product from the Dairy Industry. In Food Industry; IntechOpen: London, UK, 2013. [Google Scholar]
- Vlyssides, A.G.; Tsimas, E.S.; Barampouti, E.M.P.; Mai, S.T. Anaerobic Digestion of Cheese Dairy Wastewater Following Chemical Oxidation. Biosyst. Eng. 2012, 113, 253–258. [Google Scholar] [CrossRef]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic Cultivation of Green Microalgae Scenedesmus Obliquus on Cheese Whey Permeate for Biodiesel Production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Tsotsouli, K.; Didos, S.; Koukaras, K.; Argiriou, A. Mixotrophic Cultivation of Dunaliella Tertiolecta in Cheese Whey Effluents to Enhance Biomass and Exopolysaccharides (EPS) Production: Biochemical and Functional Insights. Mar. Drugs 2025, 23, 120. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Charalambous, P.; Vyrides, I. Dairy Wastewater Management in EU: Produced Amounts, Existing Legislation, Applied Treatment Processes and Future Challenges. J. Environ. Manage 2022, 303, 114152. [Google Scholar] [CrossRef] [PubMed]
- Dumpler, J.; Kulozik, U. Heat-Induced Coagulation of Concentrated Skim Milk Heated by Direct Steam Injection. Int. Dairy. J. 2016, 59, 62–71. [Google Scholar] [CrossRef]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium Sp. in Various Salinity and Light Regimes and Their Influence on Its Phycocyanin and Other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Markou, G. Fed-Batch Cultivation of Arthrospira and Chlorella in Ammonia-Rich Wastewater: Optimization of Nutrient Removal and Biomass Production. Bioresour. Technol. 2015, 193, 35–41. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Washington, DC, USA, 2012. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Pispas, K.; Manthos, G.; Sventzouri, E.; Geroulia, M.; Mastropetros, S.G.; Ali, S.S.; Kornaros, M. Optimizing Phycocyanin Extraction from Cyanobacterial Biomass: A Comparative Study of Freeze–Thaw Cycling with Various Solvents. Mar. Drugs 2024, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D.; Jaeschke, D.P. Saline Extraction Assisted by Ultrasound: A Method to Obtain Purified Phycocyanin. J. Biotechnol. 2024, 384, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, K.; Kornaros, M. Development of a High-Rate Anaerobic Thermophilic Upflow Packed Bed Reactor for Efficient Bioconversion of Diluted Three-Phase Olive Mill Wastewater into Methane. Fuel 2022, 310, 122263. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of Microalgal Biomass Profiles as Novel Functional Ingredient for Food Products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Koutra, E.; Grammatikopoulos, G.; Kornaros, M. Selection of Microalgae Intended for Valorization of Digestate from Agro-Waste Mixtures. Waste Manag. 2018, 73, 123–129. [Google Scholar] [CrossRef]
- Levine, R.B.; Costanza-Robinson, M.S.; Spatafora, G.A. Neochloris Oleoabundans Grown on Anaerobically Digested Dairy Manure for Concomitant Nutrient Removal and Biodiesel Feedstock Production. Biomass Bioenergy 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Braunegg, G.; Sonnleitner, B.Y.; Lafferty, R.M. A Rapid Gas Chromatographic Method for the Determination of Poly-β-Hydroxybutyric Acid in Microbial Biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Casá, N.E.; Lois-Milevicich, J.; Alvarez, P.; Mateucci, R.; de Escalada Pla, M. Chlorella Vulgaris Cultivation Using Ricotta Cheese Whey as Substrate for Biomass Production. J. Appl. Phycol. 2022, 34, 745–756. [Google Scholar] [CrossRef]
- de Andrade, A.F.; e Silva, P.E.D.C.; de Melo, R.G.; do Nascimento Ferreira, M.P.; Porto, A.L.F.; Bezerra, R.P. Microalgal Production under Mixotrophic Conditions Using Cheese Whey as Substrate. Acta Sci. Biol. Sci. 2022, 44. [Google Scholar] [CrossRef]
- Bonett, J.E.A.; de Sousa Geraldino, P.; Cardoso, P.G.; de Freitas Coelho, F.; Duarte, W.F. Isolation of Freshwater Microalgae and Outdoor Cultivation Using Cheese Whey as Substrate. Biocatal. Agric. Biotechnol. 2020, 29, 101799. [Google Scholar] [CrossRef]
- Pereira, M.I.B.; Chagas, B.M.E.; Sassi, R.; Medeiros, G.F.; Aguiar, E.M.; Borba, L.H.F.; Silva, E.P.E.; Neto, J.C.A.; Rangel, A.H.N. Mixotrophic Cultivation of Spirulina platensis in Dairy Wastewater: Effects on the Production of Biomass, Biochemical Composition and Antioxidant Capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, V.; Klontza, E.E.; Dimitriou-Christidis, P.; Fountoulakis, M.; Lekkas, D.F. Evaluation of Arthrospira (Spirulina) platensis Growth on Cheese Whey in the Context of Circular Economy. Sustain. Chem. Pharm. 2023, 34, 101173. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Development and Cost-Benefit Analysis of a Novel Process for Biofuel Production from Microalgae Using Pre-Treated High-Strength Fresh Cheese Whey Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23963–23980. [Google Scholar] [CrossRef]
- Salah, A.; Sany, H.; El-Sayed, A.E.-K.B.; El-Bahbohy, R.M.; Mohamed, H.I.; Amin, A. Growth Performance and Biochemical Composition of Desmodesmus Sp. Green Alga Grown on Agricultural Industries Waste (Cheese Whey). Water Air Soil. Pollut. 2023, 234, 770. [Google Scholar] [CrossRef]
- Deng, X.; Xue, C.; Chen, B.; Amoah, P.K.; Li, D.; Hu, X.; Gao, K. Glucose Addition-induced Changes in the Growth and Chemical Compositions of a Freshwater Microalga Chlorella Kessleri. J. Chem. Technol. Biotechnol. 2019, 94, 1202–1209. [Google Scholar] [CrossRef]
- Youssef, A.M.; Gomaa, M.; Mohamed, A.K.S.H.; El-Shanawany, A.-R.A. Enhancement of Biomass Productivity and Biochemical Composition of Alkaliphilic Microalgae by Mixotrophic Cultivation Using Cheese Whey for Biofuel Production. Environ. Sci. Pollut. Res. 2024, 31, 42875–42888. [Google Scholar] [CrossRef]
- Le, T.T.; Corato, A.; Gerards, T.; Gérin, S.; Remacle, C.; Franck, F. Heterotrophy Compared to Photoautotrophy for Growth Characteristics and Pigment Compositions in Batch Cultures of Four Green Microalgae. Plants 2024, 13, 1182. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.-J. Heterotrophic Cultivation of Microalgae for Pigment Production: A Review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Tosuner, Z.V.; Ürek, R.Ö. Cultivation of Arthrospira Platensis in Heterotrophic and Mixotrophic Conditions with Different Concentrations of Whey. Aquat. Res. 2022, 5, 146–153. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, S.; Chen, H.; Pei, H. Enhanced Phycocyanin Production from Spirulina subsalsa via Freshwater and Marine Cultivation with Optimized Light Source and Temperature. Bioresour. Technol. 2023, 378, 129009. [Google Scholar] [CrossRef]
- Mastropetros, S.G.; Pispas, K.; Zagklis, D.; Tsigkou, K.; Ali, S.S.; Ariyadasa, T.U.; Kornaros, M. Effect of a Dark-Colored Substrate on the Production of Phycocyanin by the Cyanobacterium Phormidium Sp. J. Environ. Chem. Eng. 2023, 11, 110580. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-Biorefinery with Cascading Resource Recovery Design Associated to Dairy Wastewater Treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef]
- Stratigakis, N.C.; Nazos, T.T.; Chatzopoulou, M.; Mparka, N.; Spantidaki, M.; Lagouvardou-Spantidaki, A.; Ghanotakis, D.F. Cultivation of a Naturally Resilient Chlorella Sp.: A Bioenergetic Strategy for Valorization of Cheese Whey for High Nutritional Biomass Production. Algal Res. 2024, 82, 103616. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Villaró-Cos, S.; Ciardi, M.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Lafarga, T. Assessment of the Mixotrophic Production of Chlorella Vulgaris Using Milk Whey as a Nutrient Source. J. Appl. Phycol. 2024, 36, 87–100. [Google Scholar] [CrossRef]
- Panda, B.; Sharma, L.; Mallick, N. Poly-β-Hydroxybutyrate Accumulation in Nostoc Muscorum and Spirulina platensis under Phosphate Limitation. J. Plant Physiol. 2005, 162, 1376–1379. [Google Scholar] [CrossRef]
- Koutra, E.; Mastropetros, S.G.; Ali, S.S.; Tsigkou, K.; Kornaros, M. Assessing the Potential of Chlorella Vulgaris for Valorization of Liquid Digestates from Agro-Industrial and Municipal Organic Wastes in a Biorefinery Approach. J. Clean. Prod. 2021, 280, 124352. [Google Scholar] [CrossRef]
- López, C.V.G.; García, M.D.C.C.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein Measurements of Microalgal and Cyanobacterial Biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.H.; Nguyen, T.L.A.; Duong, T.T.; Doan, O.T.; Tran, H.T.T.; Tran, L.T.T. Selection of Microalgae and Cyanobacteria to Produce Polyhydroxyalkanoates (PHAs)-A Case Study in Vietnam. Case Stud. Chem. Environ. Eng. 2024, 10, 100808. [Google Scholar] [CrossRef]
- Zhang, S.; Bryant, D.A. Biochemical Validation of the Glyoxylate Cycle in the Cyanobacterium Chlorogloeopsis Fritschii Strain PCC 9212. J. Biol. Chem. 2015, 290, 14019–14030. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Mean Value ± SD |
|---|---|
| pH | 5.3 ± 0.1 |
| Total suspended solids (TSS) [g L−1] | 6.5 ± 0.7 |
| Volatile suspended solids (VSS) [g L−1] | 0.6 ± 0.1 |
| Total solids (TS) [g L−1] | 56.5 ± 0.4 |
| Volatile solids (VS) [g L−1] | 51.9 ± 1.3 |
| Chemical oxygen demand (COD) [g L−1] | 65.0 ± 5.0 |
| Carbohydrates * [g L−1] | 43.3 ± 1.2 |
| Total nitrogen (TN) [g L−1] | 0.9 ± 0.1 |
| Total phosphorus (TP) [g L−1] | 0.4 ± 0.0 |
| C. fritschii | A. platensis | Phormidium sp. | Synechocystis sp. | |||||
|---|---|---|---|---|---|---|---|---|
| 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | |
| Maximum Biomass [g L−1] | 3.18 ± 0.29 d,e | 4.36 ± 0.48 c,d | 2.48 ± 0.39 e | 4.90 ± 0.46 b,c | 2.75 ± 0.14 d,e | 6.41 ± 0.59 b | 2.19 ± 0.40 e | 10.90 ± 0.49 a |
| μmax [d−1] | 0.21 ± 0.03 b | 0.08 ± 0.01c | 0.11 ± 0.01 c | 0.08 ± 0.01 c | 0.18 ± 0.01 b | 0.11 ± 0.00 c | 0.28 ± 0.02 a | 0.08 ± 0.01 c |
| Productivity [g L−1 d−1] | 0.24 ± 0.05 b,c | 0.38 ± 0.02 a,b | 0.17 ± 0.03 c | 0.36 ± 0.08 a,b,c | 0.29 ± 0.01 a,b,c | 0.48 ± 0.02 a | 0.27 ± 0.10 b,c | 0.38 ± 0.02 a,b |
| C. fritschii | A. platensis | Phormidium sp. | Synechocystis sp. | |||||
|---|---|---|---|---|---|---|---|---|
| 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | |
| CODremoval [%] | 51.4 ± 0.5 a | 52.3 ± 11.5 a | 11.1 ± 1.6 c | 17.1 ± 3.0 b,c | 47.9 ± 7.9 a | 42.9 ± 11.2 a,b | 49.4 ± 5.3 a | 57.9 ± 3.9 a |
| TP removal [%] | 32.8 ± 5.8 a,b,c | 41.2 ± 10.1 a,b | 41.6 ± 1.7 a,b | 11.6 ± 2.2 c | 27.5 ± 6.1 a,b,c | 37.1 ± 5.8 a,b | 19.1 ± 7.7 b,c | 44.6 ± 6.6 a |
| TN removal [%] | 53.1 ± 2.0 a,b | 30.4 ± 4.6 b,c | 55.9 ± 2.5 a | 40.0 ± 10.2 a,b,c | 41.2 ± 6.6 a,b,c | 48.3 ± 5.1 a,b | 18.4 ± 5.2 c | 59.0 ± 9.3 a |
| C. fritschii | A. platensis | Phormidium sp. | Synechocystis sp. | |||||
|---|---|---|---|---|---|---|---|---|
| 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | 20% CW | 100% CW | |
| Carbohydrates [%] | 38.1 ± 2.4 a | 28.7 ± 2.7 a | 24.7 ± 10.1 a | 16.7 ± 5.7 a | 19.6 ± 6.7 a | 29.4 ± 1.9 a | 35.9 ± 4.8 a | 34.2 ± 5.3 a |
| Proteins [%] | 22.2 ± 3.4 b | 41.6 ± 5.3 a,b | 34.0 ± 0.1 a,b | 38.4 ± 1.0 a,b | 45.5 ± 2.9 a | 42.4 ± 1.7 a,b | 47.9 ± 13.3 a | 39.3 ± 1.8 a,b |
| Lipids [%] | 1.0 ± 0.1 b | 16.8 ± 1.2 a | 10.8 ± 3.2 a,b | 1.7 ± 0.7 b | 3.8 ± 1.7 b | 7.4 ± 2.7 a,b | 4.2 ± 2.8 a,b | 11.1 ± 7.5 a,b |
| PHAs [%] | 10.7 ± 2.1 a | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.9 ± 0.4 b | 0.4 ± 0.1 b | 1.4 ± 0.1 b | 2.8 ± 1.4 b | 0.5 ± 0.0 b |
| Ash [%] | 8.5 ± 1.9 b,c | 4.7 ± 0.8 c | 22.5 ± 2.4 a,b,c | 28.1 ± 5.3 a | 26.0 ± 8.5 a,b | 13.3 ± 5.9 a,b,c | 8.4 ± 2.3 b,c | 9.0 ± 4.2 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sventzouri, E.; Pispas, K.; Kournoutou, G.G.; Geroulia, M.; Giakoumatou, E.; Ali, S.S.; Kornaros, M. Evaluation of Growth Performance, Biochemical Composition, and Polyhydroxyalkanoates Production of Four Cyanobacterial Species Grown in Cheese Whey. Microorganisms 2025, 13, 1157. https://doi.org/10.3390/microorganisms13051157
Sventzouri E, Pispas K, Kournoutou GG, Geroulia M, Giakoumatou E, Ali SS, Kornaros M. Evaluation of Growth Performance, Biochemical Composition, and Polyhydroxyalkanoates Production of Four Cyanobacterial Species Grown in Cheese Whey. Microorganisms. 2025; 13(5):1157. https://doi.org/10.3390/microorganisms13051157
Chicago/Turabian StyleSventzouri, Eirini, Konstantinos Pispas, Georgia G. Kournoutou, Maria Geroulia, Eleni Giakoumatou, Sameh Samir Ali, and Michael Kornaros. 2025. "Evaluation of Growth Performance, Biochemical Composition, and Polyhydroxyalkanoates Production of Four Cyanobacterial Species Grown in Cheese Whey" Microorganisms 13, no. 5: 1157. https://doi.org/10.3390/microorganisms13051157
APA StyleSventzouri, E., Pispas, K., Kournoutou, G. G., Geroulia, M., Giakoumatou, E., Ali, S. S., & Kornaros, M. (2025). Evaluation of Growth Performance, Biochemical Composition, and Polyhydroxyalkanoates Production of Four Cyanobacterial Species Grown in Cheese Whey. Microorganisms, 13(5), 1157. https://doi.org/10.3390/microorganisms13051157







