Label-Free Flow Cytometry: A Powerful Tool to Rapidly and Accurately Assess the Efficacy of Chemical Disinfectants
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Disinfectants
2.2. Bacterial Isolates, Growth and Culture Conditions
2.3. Standard Qualitative/Quantitative Suspension Tests
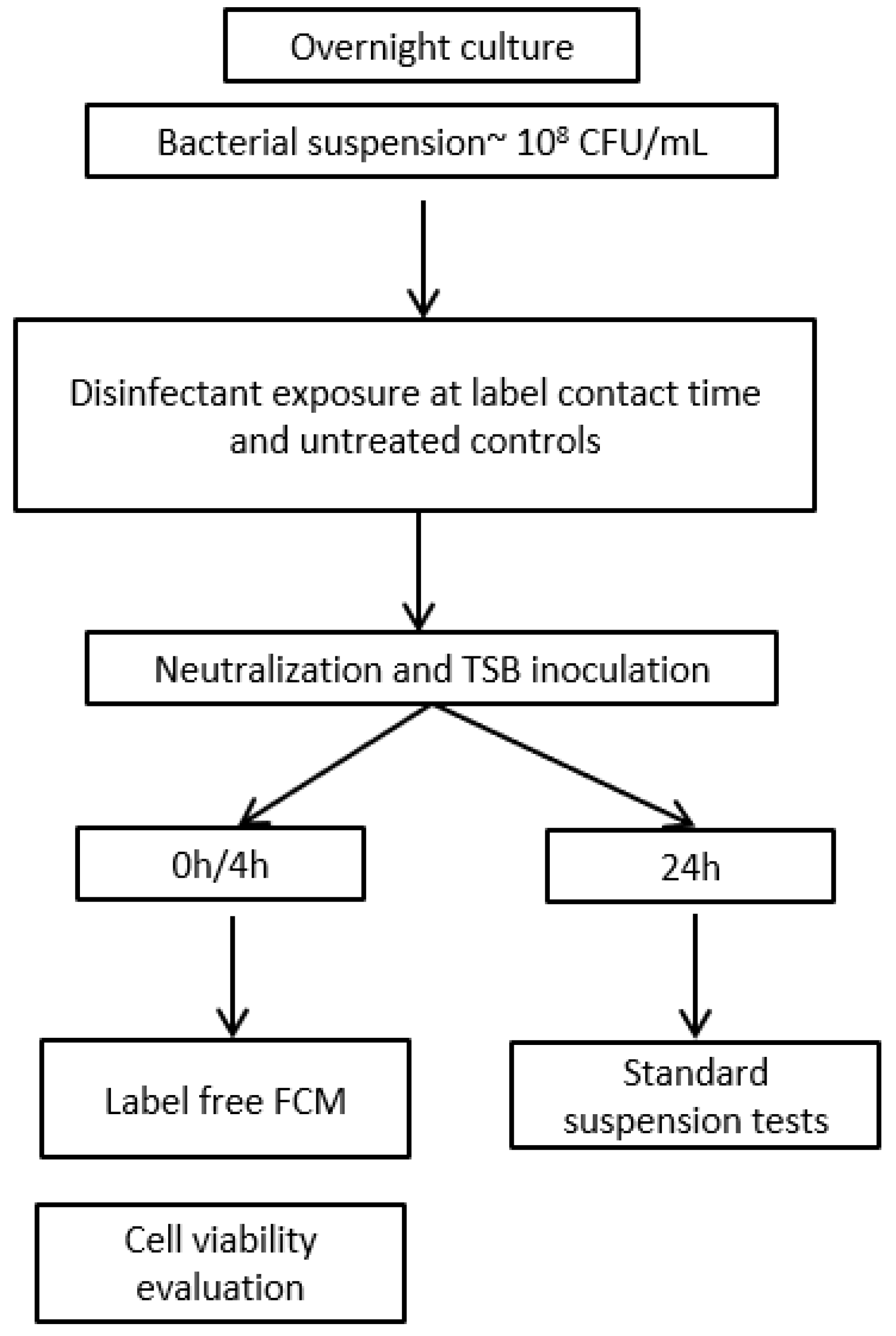
2.4. Disinfectant Efficacy Testing Using Label-Free FCM
2.4.1. Sample Preparation for Label-Free FCM
2.4.2. Detection of Bacteria Using Label-Free FCM
2.4.3. Instrument Settings
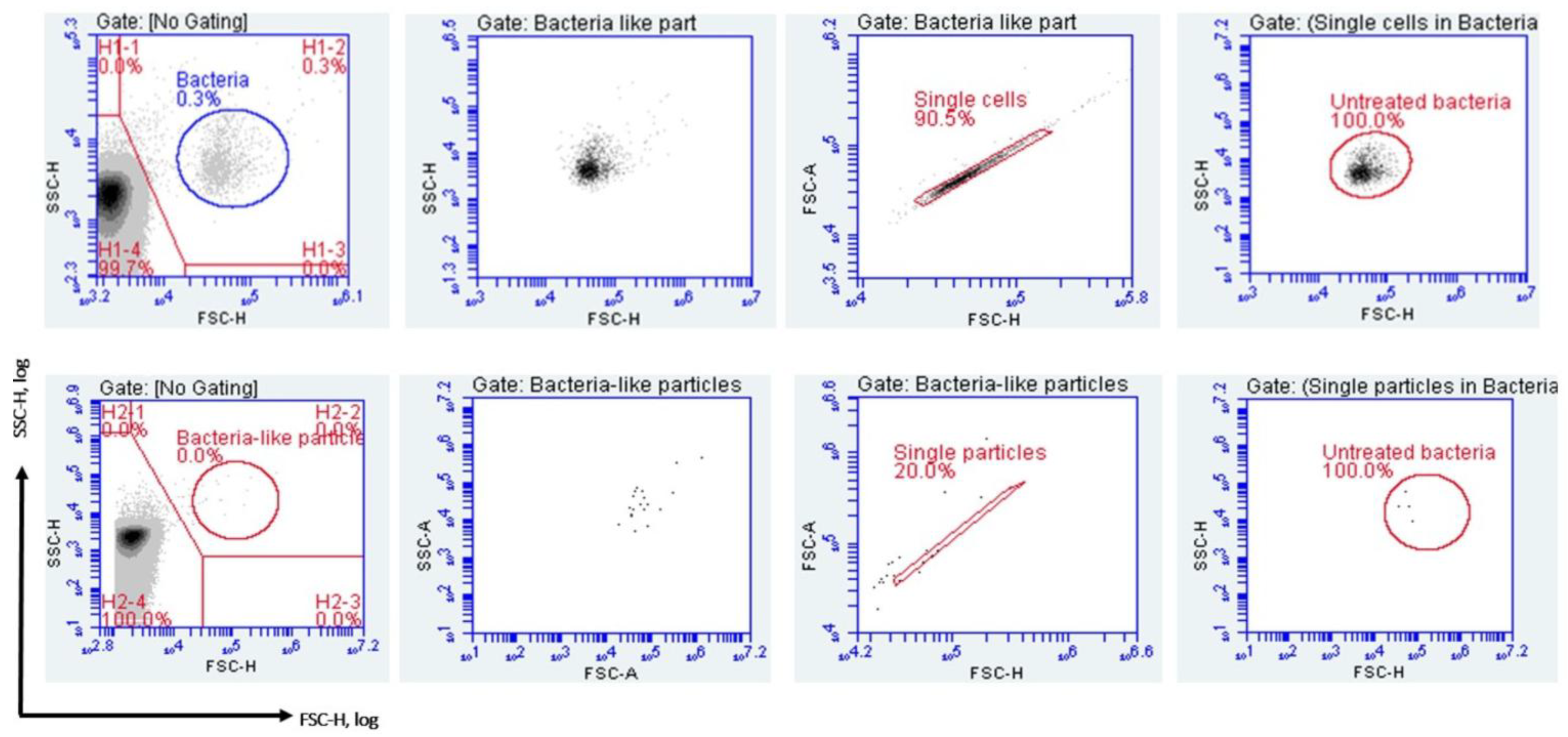
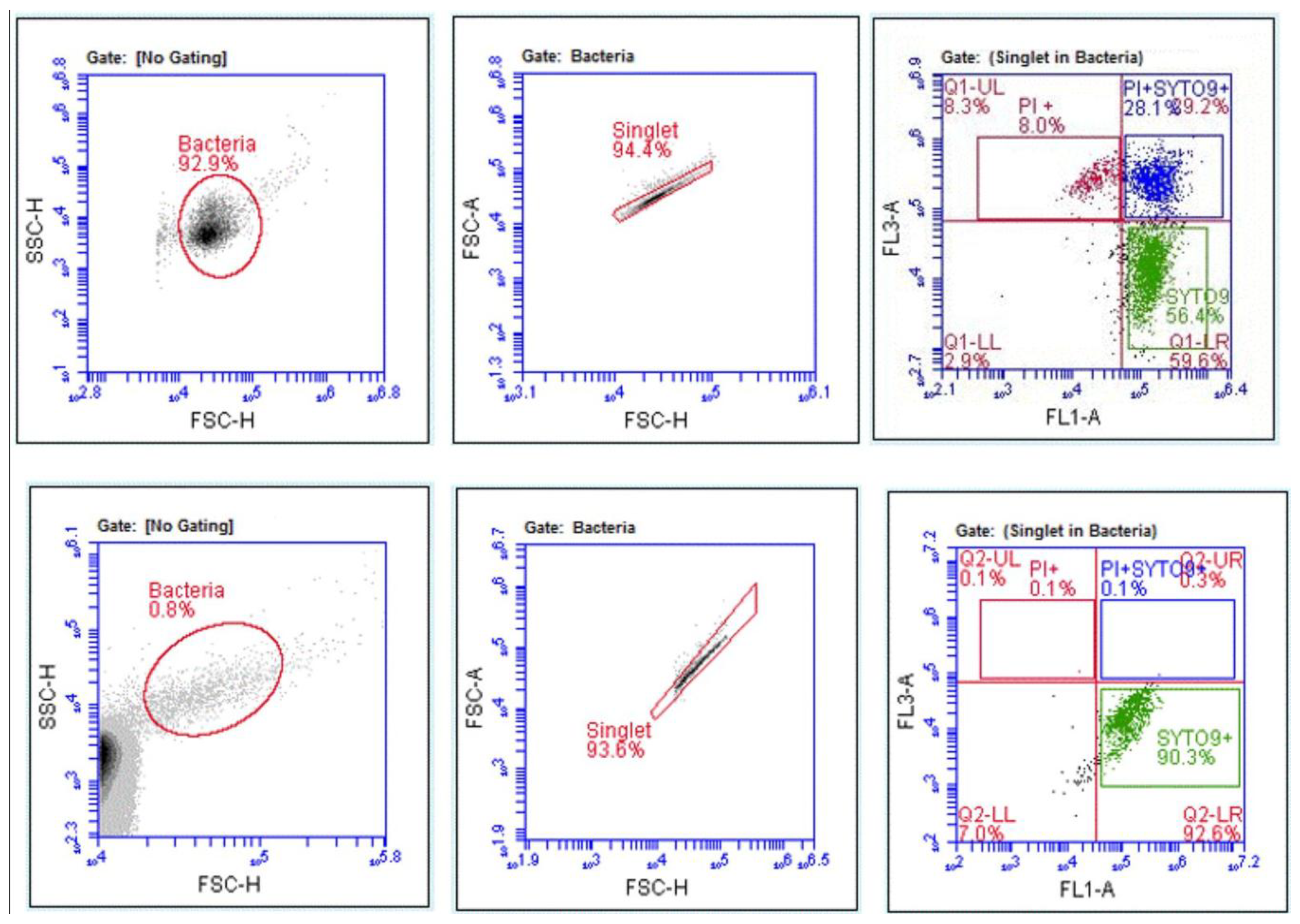
2.4.4. Gating Strategy and Data Analysis
2.5. Assessment of Disinfectant-Induced VBNC State in Bacteria Using FCM
2.6. Statistical Analysis
3. Results
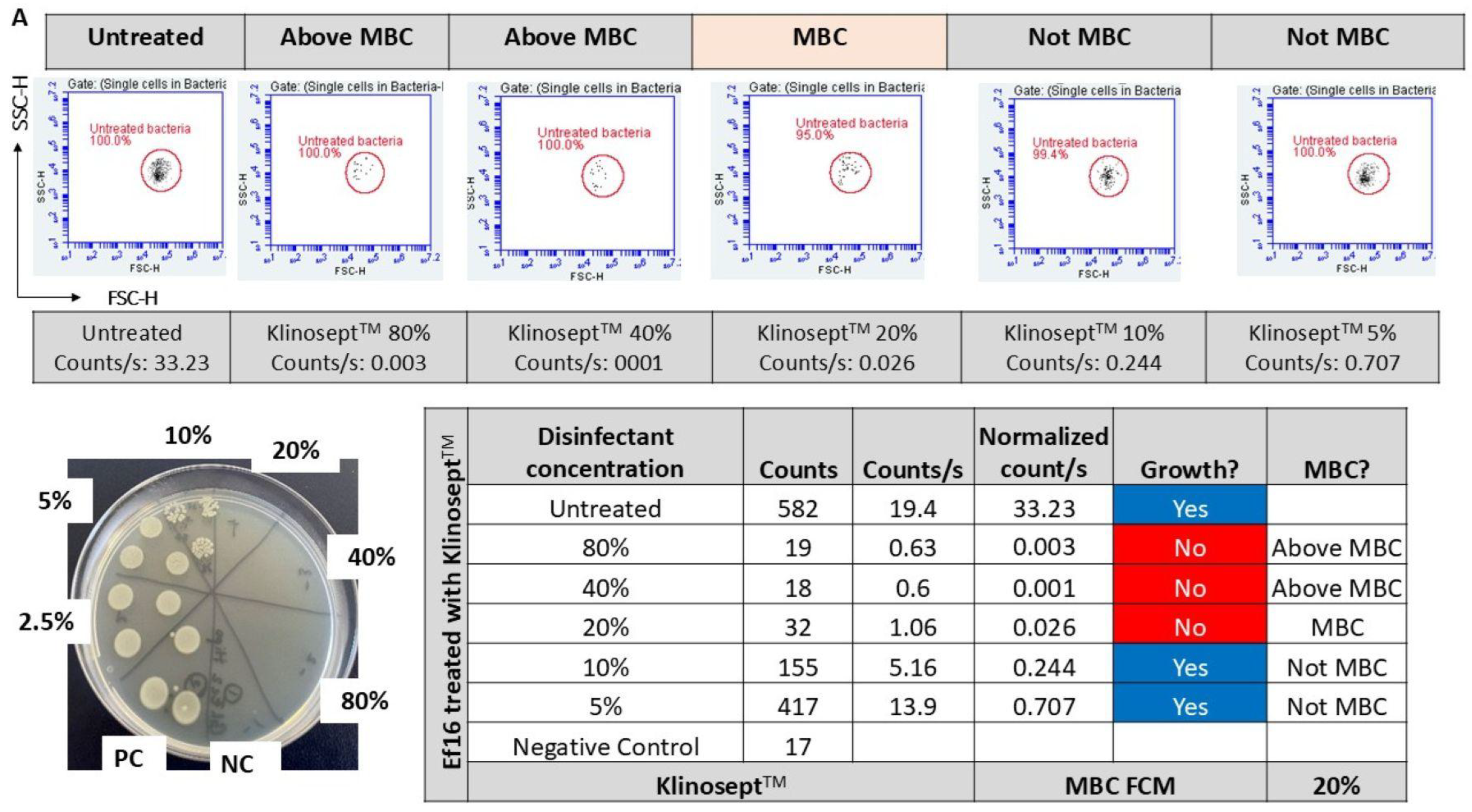
3.1. Minimum Inhibitory and Bactericidal Concentrations
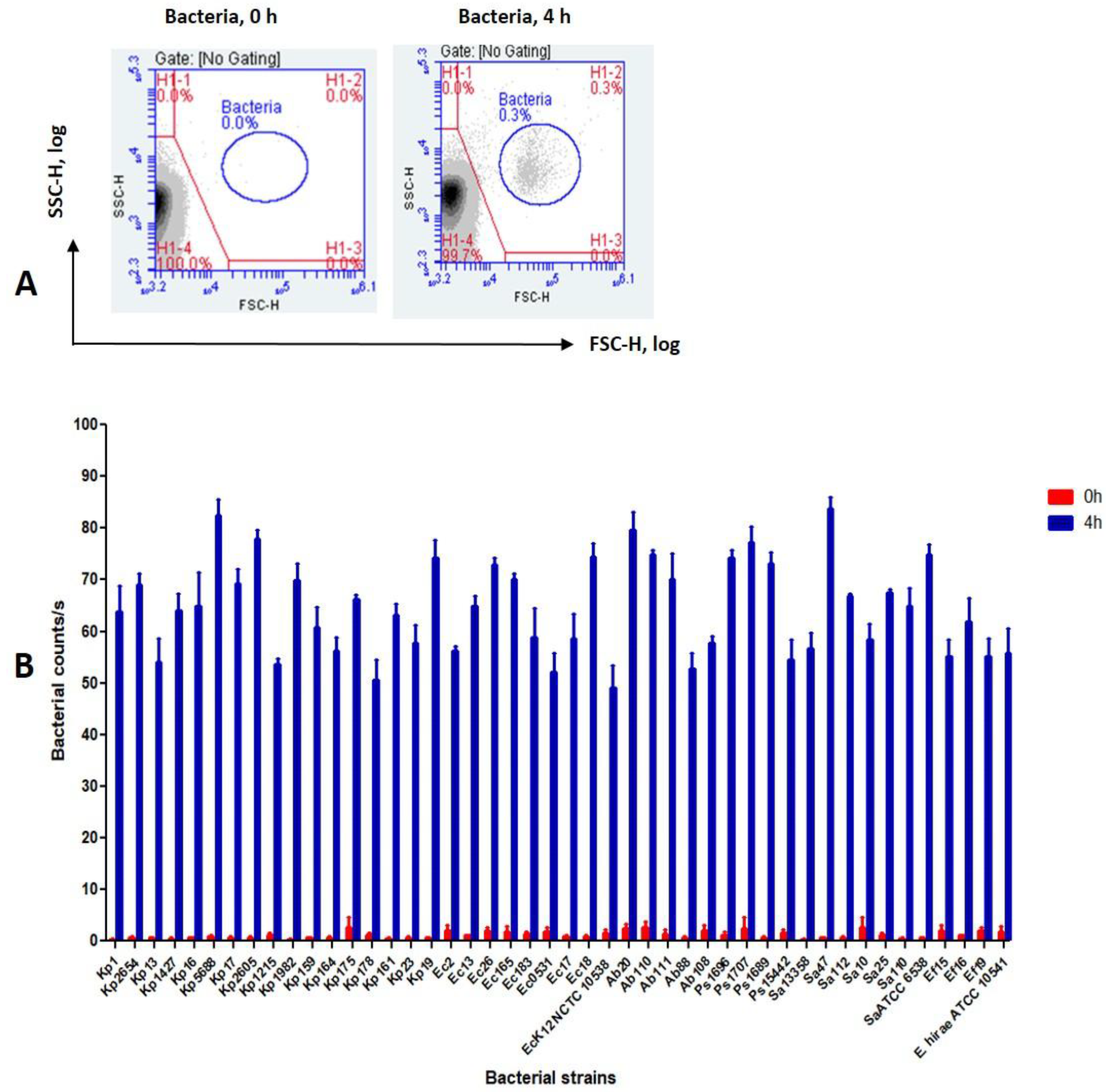
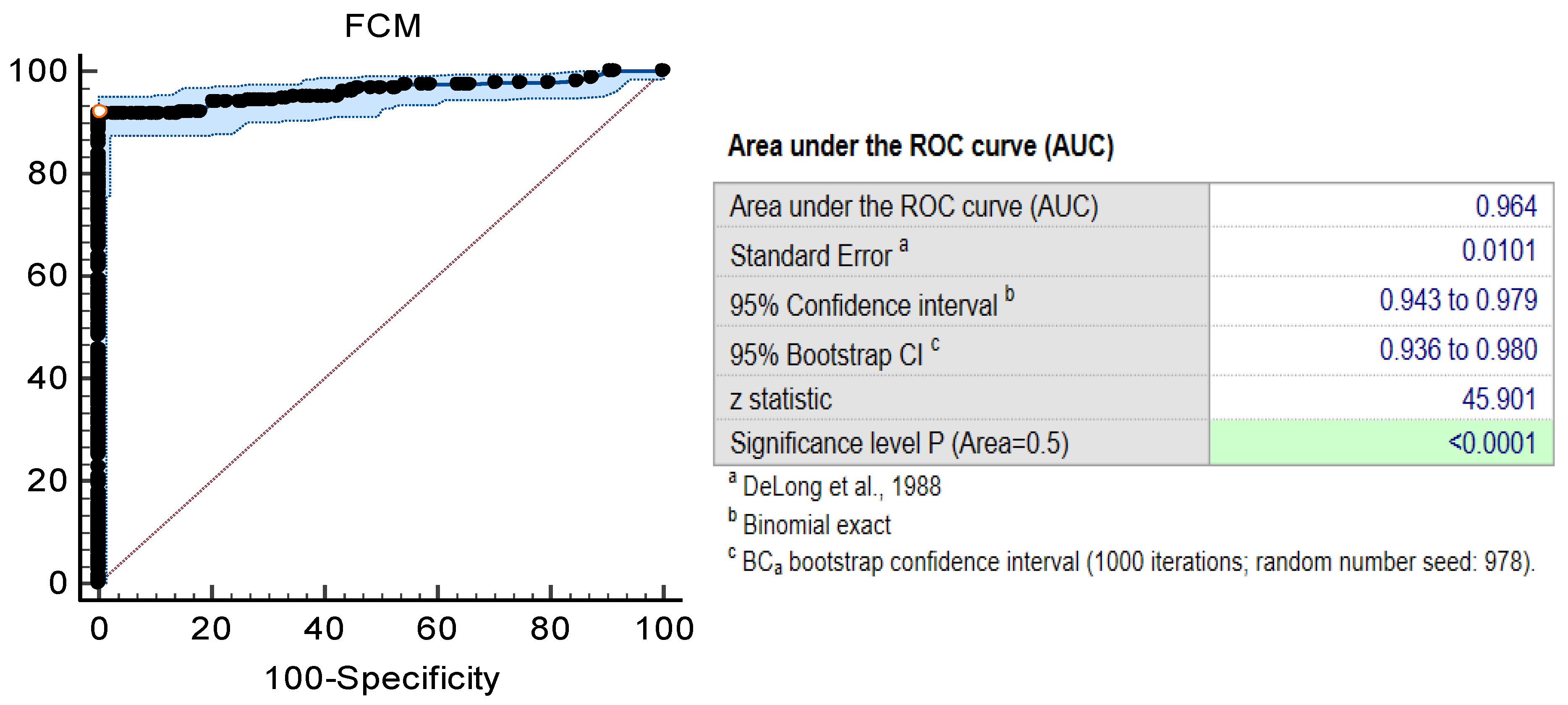
3.2. Detection of Bacteria Using the FCM Assay
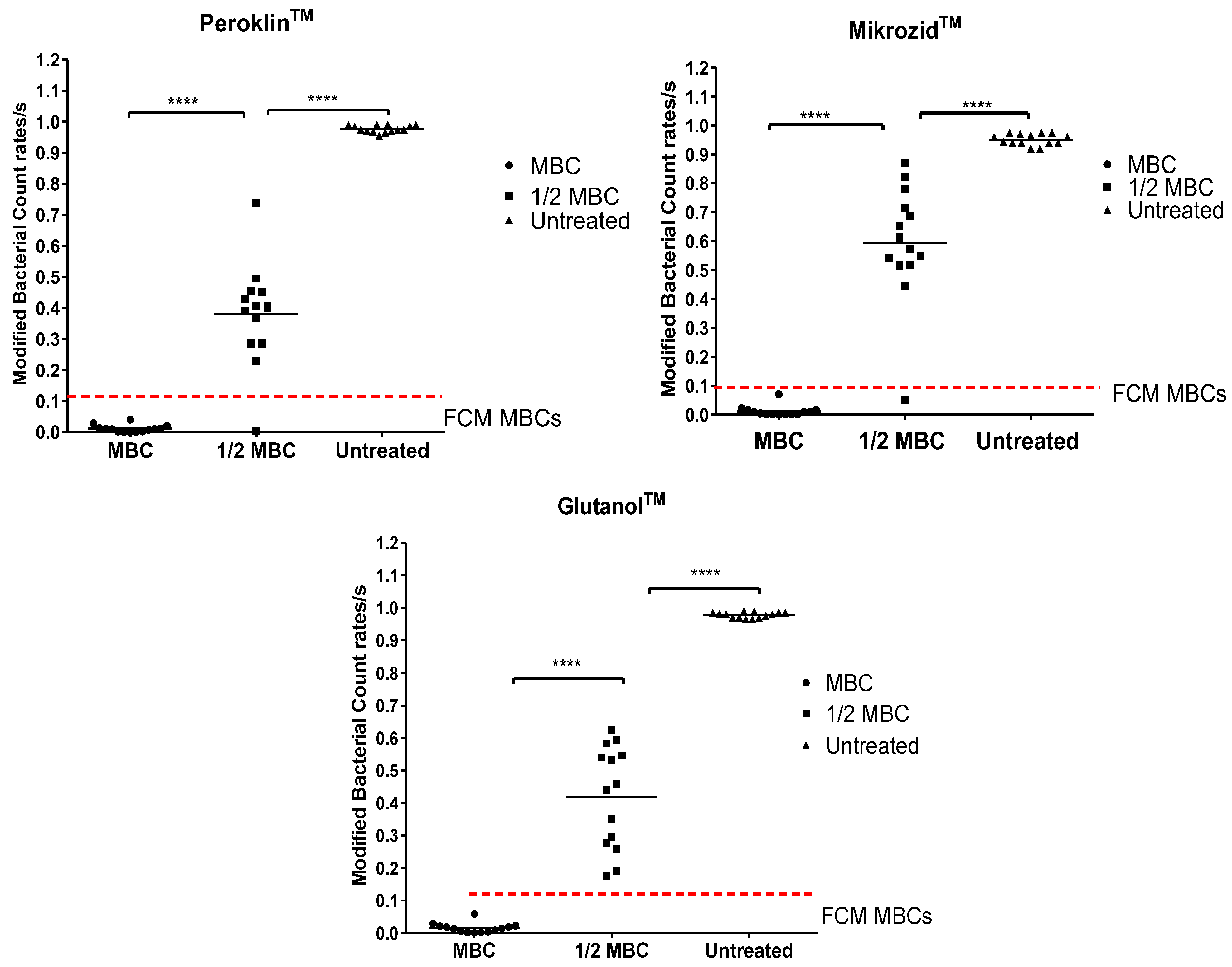
3.3. Disinfectant Efficacy Using the Label-Free FCM Method
3.4. Disinfectant-Induced VBNC Bacteria Determined by FCM with Fluorescent Labelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Randolph, K.C.; Osborn, S.L.; Tyler, H.L. Culture-dependent and culture-independent methods for bacterial community analysis in the environment. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Oliver, J.D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Giao, M.S.; Wilks, S.A.; Azevedo, N.F.; Vieira, M.J.; Keevil, C.W. Comparison between flow cytometry and culture-based methods for detection of Legionella pneumophila in water. Cytom. Part. B Clin. Cytom. 2009, 76, 117–125. [Google Scholar] [CrossRef]
- Servain-Viel, S.; Aknin, M.L.; Domenichini, S.; Perlemuter, G.; Cassard, A.M.; Schlecht-Louf, G.; Moal, V.L. A flow cytometry method for safe detection of bacterial viability. Cytom. Part. A 2024, 105, 146–156. [Google Scholar] [CrossRef]
- Párraga-Niño, N.; Cortès-Tarragó, R.; Quero, S.; Garcia-Núñez, M.; Arqué, E.; Sabaté, S.; Ramirez, D.; Gavaldà, L. Persistence of viable but nonculturable Legionella pneumophila state in hospital water systems: A hidden enemy? Sci. Total Environ. 2024, 927, 172410. [Google Scholar] [CrossRef]
- Barry Schroeder, A.L.; Reed, A.M.; Radwan, O.; Bowen, L.L.; Ruiz, O.N.; Gunasekera, T.S.; Hoffmann, A. Identification of Pseudomonas protegens and Bacillus subtilis Antimicrobials for Mitigation of Fuel Biocontamination. Biomolecules 2025, 15, 227. [Google Scholar] [CrossRef]
- Clausen, C.H.; Dimaki, M.; Bertelsen, C.V.; Skands, G.E.; Rodriguez-Trujillo, R.; Thomsen, J.D.; Svendsen, W.E. Bacteria Detection and Differentiation Using Impedance Flow Cytometry. Sensors (Basel) 2018, 18, 3496. [Google Scholar] [CrossRef]
- Pîndaru, A.M.; Măruțescu, L.; Popa, M.; Chifiriuc, M.C. A Label-Free Optical Flow Cytometry Based-Method for Rapid Assay of Disinfectants’ Bactericidal Activity. Int. J. Mol. Sci. 2024, 25, 7158. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Makuła, M.; Włodarczyk-Makuła, M.; Wołejko, E.; Wydro, U.; Serra-Majem, L.; Wiater, J. Inanimate Surfaces as a Source of Hospital Infections Caused by Fungi, Bacteria and Viruses with Particular Emphasis on SARS-CoV-2. Int. J. Environ. Res. Public. Health. 2022, 19, 8121. [Google Scholar] [CrossRef]
- Zahornacký, O.; Porubčin, Š.; Rovňáková, A.; Jarčuška, P. Gram-Negative Rods on Inanimate Surfaces of Selected Hospital Facilities and Their Nosocomial Significance. Int. J. Environ. Res. Public. Health 2022, 19, 6039. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.; Sultan, O.; Mitchell, B.G.; Jenney, A.; Kiernan, M.; Brewster, D.J.; Russo, P.L. How long do nosocomial pathogens persist on inanimate surfaces? A scoping review. J. Hosp. Infect. 2024, 147, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhang, Z.; Shen, R.; Liu, X.; Li, X.; Chen, B.; Wu, X.; Li, H.; Xie, X.; Huang, S. Disinfection Strategies for Carbapenem-Resistant Klebsiella pneumoniae in a Healthcare Facility. Antibiotics 2022, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef]
- Brunke, M.S.; Konrat, K.; Schaudinn, C.; Piening, B.; Pfeifer, Y.; Becker, L.; Schwebke, I.; Arvand, M. Tolerance of biofilm of a carbapenem-resistant Klebsiella pneumoniae involved in a duodenoscopy-associated outbreak to the disinfectant used in reprocessing. Antimicrob. Resist. Infect. Control 2022, 11, 81. [Google Scholar] [CrossRef]
- Hanczvikkel, A.; Tóth, Á.; Kopcsóné Németh, I.A.; Bazsó, O.; Závorszky, L.; Buzgó, L.; Lesinszki, V.; Göbhardter, D.; Ungvári, E.; Damjanova, I.; et al. Nosocomial outbreak caused by disinfectant-resistant Serratia marcescens in an adult intensive care unit, Hungary, February to March 2022. Euro Surveill. 2024, 29, 2300492. [Google Scholar] [CrossRef]
- Everall, I.; Nogueira, C.L.; Bryant, J.M.; Sánchez-Busó, L.; Chimara, E.; Duarte, R.D.S.; Ramos, J.P.; Lima, K.V.B.; Lopes, M.L.; Palaci, M.; et al. Genomic epidemiology of a national outbreak of post-surgical Mycobacterium abscessus wound infections in Brazil. Microb. Genom. 2017, 3, e000111. [Google Scholar] [CrossRef]
- Maillard, J.Y. Testing the Effectiveness of Disinfectants and Sanitizers. In Handbook of Hygiene Control in the Food Industry, 2nd ed.; Woodhead Publishing Series in Food Science; Lelieveld, H., Holah, J., Gabrić, D., Eds.; Technology and Nutrition: Cambridge, UK, 2016; pp. 569–586. [Google Scholar]
- Maillard, J.Y.; Centeleghe, I. How biofilm changes our understanding of cleaning and disinfection. Antimicrob. Resist. Infect. Control 2023, 12, 95. [Google Scholar] [CrossRef]
- Ledwoch, K.; Maillard, J.-Y. Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials 2019, 12, 18. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Pascoe, M. Disinfectants and antiseptics: Mechanisms of action and resistance. Nat. Rev. Microbiol. 2024, 22, 4–17. [Google Scholar] [CrossRef]
- Mima, T.; Joshi, S.; Gomez-Escalada, M.; Schweizer, H.P. Identification and Characterization of TriABC-OpmH, a Triclosan Efflux Pump of Pseudomonas aeruginosa Requiring Two Membrane Fusion Proteins. J. Bacteriol. 2007, 189, 7600–7609. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.M.; Xu, W.; Loewen, P.C.; Zhanel, G.G.; Kumar, A. Triclosan Can Select for an AdeIJK-Overexpressing Mutant of Acinetobacter baumannii ATCC 17978 That Displays Reduced Susceptibility to Multiple Antibiotics. Antimicrob. Agents Chemother. 2014, 58, 6424–6431. [Google Scholar] [CrossRef]
- Yeung, Y.W.S.; Ma, Y.; Liu, S.Y.; Pun, W.H.; Chua, S.L. Prevalence of Alcohol-Tolerant and Antibiotic-Resistant Bacterial Pathogens on Public Hand Sanitizer Dispensers. J. Hosp. Infect. 2022, 127, 26–33. [Google Scholar] [CrossRef]
- Mehdipour, M.; Gholipour, S.; Mohammadi, F.; Hatamzadeh, M.; Nikaeen, M. Incidence of Co-Resistance to Antibiotics and Chlorine in Bacterial Biofilm of Hospital Water Systems: Insights into the Risk of Nosocomial Infections. J. Infect. Public. Health 2023, 16, 210–216. [Google Scholar] [CrossRef]
- Mavri, A.; Smole Možina, S. Development of Antimicrobial Resistance in Campylobacter jejuni and Campylobacter coli Adapted to Biocides. Int. J. Food Microbiol. 2013, 160, 304–312. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Wang, X.K.; Tagkopoulos, I. Biocide-Induced Emergence of Antibiotic Resistance in Escherichia coli. Front. Microbiol. 2021, 12, 640923. [Google Scholar]
- Kuznetsova, M.V.; Nesterova, L.Y.; Mihailovskaya, V.S.; Selivanova, P.A.; Kochergina, D.A.; Karipova, M.O.; Valtsifer, I.V.; Averkina, A.S.; Starčič Erjavec, M. Nosocomial Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus: Sensitivity to Chlorhexidine-Based Biocides and Prevalence of Efflux Pump Genes. Int. J. Mol. Sci. 2025, 26, 355. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Le Jeune, A.; Le Gall-David, S.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Molecular mechanisms of higher MICs of antibiotics and quaternary ammonium compounds for Escherichia coli isolated from bacteraemia. J. Antimicrob. Chemother. 2012, 67, 2837–2842. [Google Scholar] [CrossRef]
- Nordholt, N.; Kanaris, O.; Schmidt, S.B.I.; Schreiber, F. Persistence against benzalkonium chloride promotes rapid evolution of tolerance during periodic disinfection. Nat. Commun. 2021, 12, 6792. [Google Scholar] [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing Tolerance of Hospital Enterococcus faecium to Handwash Alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef]
- McMurry, L.M.; McDermott, P.F.; Levy, S.B. Genetic Evidence That InhA of Mycobacterium smegmatis Is a Target for Triclosan. Antimicrob. Agents Chemother. 1999, 43, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Svetlíková, Z.; Skovierová, H.; Niederweis, M.; Gaillard, J.-L.; McDonnell, G.; Jackson, M. Role of Porins in the Susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to Aldehyde-Based Disinfectants and Drugs. Antimicrob. Agents Chemother. 2009, 53, 4015–4018. [Google Scholar] [CrossRef]
- Dulyayangkul, P.; Satapoomin, N.; Avison, M.B.; Charoenlap, N.; Vattanaviboon, P.; Mongkolsuk, S. Over-Expression of Hypochlorite Inducible Major Facilitator Superfamily (MFS) Pumps Reduces Antimicrobial Drug Susceptibility by Increasing the Production of MexXY Mediated by ArmZ in Pseudomonas aeruginosa. Front. Microbiol. 2021, 11, 592153. [Google Scholar] [CrossRef]
- Gholipour, S.; Nikaeen, M.; Mehdipour, M.; Mohammadi, F.; Rabbani, D. Occurrence of Chlorine-Resistant Pseudomonas aeruginosa in Hospital Water Systems: Threat of Waterborne Infections for Patients. Antimicrob. Resist. Infect. Control 2024, 13, 111. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201–e01218. [Google Scholar] [CrossRef]
- Lee, J.; Iwasaki, T.; Ohtani, S.; Matsui, H.; Nejima, R.; Mori, Y.; Kagaya, F.; Yagi, A.; Yoshimura, A.; Hanaki, H.; et al. Benzalkonium Chloride Resistance in Staphylococcus epidermidis on the Ocular Surface of Glaucoma Patients Under Long-Term Administration of Eye Drops. Transl. Vis. Sci. Technol. 2020, 9, 9. [Google Scholar] [CrossRef]
- Mangalappalli-Illathu, A.K.; Vidovic, S.; Korber, D.R. Differential adaptive response and survival of Salmonella enterica serovar Enteritidis planktonic and biofilm cells exposed to benzalkonium chloride. Antimicrob. Agents Chemother. 2008, 52, 3669–3680. [Google Scholar] [CrossRef]
- EN 13727; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity in the Medical Area. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- Centeleghe, I.; Norville, P.; Hughes, L.; Maillard, J.Y. Klebsiella pneumoniae survives on surfaces as a dry biofilm. Am. J. Infect. Control 2023, 51, 1157–1162. [Google Scholar] [CrossRef]
- Chiang, E.L.C.; Lee, S.; Medriano, C.A.; Li, L.; Bae, S. Assessment of physiological responses of bacteria to chlorine and UV disinfection using a plate count method, flow cytometry and viability PCR. J. Appl. Microbiol. 2022, 132, 1788–1801. [Google Scholar] [CrossRef]
- Hu, Z.; Bai, X. Self-Repair and Resuscitation of Viable Injured Bacteria in Chlorinated Drinking Water: Achromobacter as an Example. Water Res. 2023, 245, 120585. [Google Scholar] [CrossRef]
- Highmore, C.J.; Warner, J.C.; Rothwell, S.D.; Wilks, S.A.; Keevil, C.W. Viable-but-Nonculturable Listeria monocytogenes and Salmonella enterica Serovar Thompson Induced by Chlorine Stress Remain Infectious. mBio 2018, 9, e00540-18. [Google Scholar] [CrossRef] [PubMed]
- Orta de Velásquez, M.T.; Yáñez Noguez, I.; Casasola Rodríguez, B.; Román Román, P.I. Effects of Ozone and Chlorine Disinfection on VBNC Helicobacter pylori by Molecular Techniques and FESEM Images. Environ. Technol. 2017, 38, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Deng, X.; Zheng, S.; Zhang, W.; He, J.Z.; Yu, X.; Feng, M.; Ye, C. Viable but non-culturable state for-mation and resuscitation of different antibiotic-resistant Escherichia coli induced by UV/chlorine. Water Res. 2024, 261, 122011. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ye, C.; Chen, S.; Zhang, S.; Yu, X. Viable but Non-Culturable E. coli Induced by Low-Level Chlorination Have Higher Persistence to Antibiotics than Their Culturable Counterparts. Environ. Pollut. 2017, 230, 242–249. [Google Scholar] [CrossRef]
- Arvaniti, M.; Orologas-Stavrou, N.; Tsitsilonis, O.E.; Skandamis, P. Induction into Viable but Non Culturable State and Out-Growth Heterogeneity of Listeria monocytogenes Is Affected by Stress History and Type of Growth. Int. J. Food Microbiol. 2024, 421, 110786. [Google Scholar] [CrossRef]
- Brauge, T.; Faille, C.; Leleu, G.; Denis, C.; Hanin, A.; Midelet, G. Treatment with Disinfectants May Induce an Increase in Viable but Non-Culturable Populations of Listeria monocytogenes in Biofilms Formed in Smoked Salmon Processing Environments. Food Microbiol. 2020, 92, 103548. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Allende, A. Peroxyacetic Acid and Chlorine Dioxide Unlike Chlorine Induce Viable but Non-Culturable (VBNC) Stage of Listeria monocytogenes and Escherichia coli O157:H7 in Wash Water. Food Microbiol. 2021, 100, 103866. [Google Scholar] [CrossRef]
- Liao, X.; Xia, X.; Yang, H.; Zhu, Y.; Deng, R.; Ding, T. Bacterial Drug-Resistance and Viability Phenotyping upon Disinfectant Exposure Revealed by Single-Nucleotide Resolved-Allele Specific Isothermal RNA Amplification. J. Hazard. Mater. 2023, 448, 130800. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Ren, H.; Li, C.; Zhang, B.; Shi, R.; Wang, Y.; Lu, S.; Li, Y.; Lu, Q.; et al. Preliminary Study of the Characterization of the Viable but Noncultivable State of Yersinia enterocolitica Induced by Chloride and UV Irradiation. Microorganisms 2024, 12, 1778. [Google Scholar] [CrossRef]
- Casini, B.; Baggiani, A.; Totaro, M.; Mansi, A.; Costa, A.L.; Aquino, F.; Miccoli, M.; Valentini, P.; Bruschi, F.; Lopalco, P.L.; et al. Detection of Viable but Non-Culturable Legionella in Hospital Water Network Following Monochloramine Disinfection. J. Hosp. Infect. 2018, 98, 46–52. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Mafu, A.A.; Massicotte, R.; Pichette, G.; Ahmad, D. Importance of mechanical action in a terminal disinfection process for decontamination of Clostridium difficile spores on hospital inert contact surfaces. Int. J. Infect. Control 2015, 11, 1–9. [Google Scholar]
- Filbrun, A.B.; Richardson, J.C.; Khanal, P.C.; Tzeng, Y.L.; Dickson, R.M. Rapid, label-free antibiotic susceptibility determined directly from positive blood culture. Cytometry. Part A 2022, 101, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Velican, A.M.; Măruţescu, L.; Kamerzan, C.; Cristea, V.C.; Banu, O.; Borcan, E.; Chifiriuc, M.C. Rapid detection and antibiotic susceptibility of uropathogenic Escherichia coli by flow cytometry. Microorganisms 2020, 8, 1233. [Google Scholar] [CrossRef]
- Broeren, M.A.C.; Maas, Y.; Retera, E.; Arents, N.L.A. Antimicrobial susceptibility testing in 90 min by bacterial cell count monitoring. Clin. Microbiol Infect 2013, 19, 286–291. [Google Scholar] [CrossRef]
- Gruchado, P.; Gil, M.I.; Larrosa, M.; Allende, A. Detection and Quantification Methods for Viable but Non-culturable (VBNC) Cells in Process Wash Water of Fresh-Cut Produce: Industrial Validation. Front. Microbiol. 2020, 11, 673. [Google Scholar]
- Cheswick, R.; Nocker, A.; Moore, G.; Jefferson, B.; Jarvis, P. Exploring the use of flow cytometry for understanding the efficacy of disinfection in chlorine contact tanks. Water Res. 2022, 217, 118420. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Chen, T.J.; Kotecha, N. Cytobank: Providing an analytics platform for community cytometry data analysis and collaboration. Curr. Top. Microbiol. Immunol. 2014, 377, 127–157. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Kjellerup, B.V.; Xu, Z. Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends Microbiol. 2023, 10, 1013–1023. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Xia, X.; Li, B.; Hung, Y.C. Viability assay of E. coli O157: H7 treated with electrolyzed oxidizing water using flow cytometry. Food Control. 2018, 88, 47–53. [Google Scholar] [CrossRef]
- Teixeira, P.; Fernandes, B.; Silva, A.M.; Dias, N.; Azeredo, J. Evaluation by Flow Cytometry of Escherichia coli Viability in Lettuce after Disinfection. Antibiotics 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Pellegrino, R. MAPK is implicated in sepsis, immunity, and inflammation. Int. J. Infect. 2024, 8, 100–104. [Google Scholar]
- Toniato, E. IL-37 is an inhibitory cytokine that could be useful for treating infections. Int. J. Infect. 2024, 8, 1–2. [Google Scholar]
- Massicotte, R.; Mafu, A.A.; Ahmad, D.; Deshaies, F.; Pichette, G.; Belhumeur, P. Comparison between flow fytometry and traditional culture methods for efficacy assessment of six disinfectant agents against nosocomial bacterial species. Front. Microbiol. 2017, 8, 112. [Google Scholar] [CrossRef]
- Wozniak-Kosek, A.; Kawiak, J. Flow cytometry analysis of the activity of disinfecting agents tested with Staphylococcus aureus and Escherichia coli. Pol. J. Microbiol. 2005, 54, 21–26. [Google Scholar]
- Phe, M.H.; Dossot, M.; Guilloteau, H.; Block, J.C. Highly chlorinated Escherichia coli cannot be stained by propidium iodide. Can. J. Microbiol. 2007, 53, 664–670. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wang, Y.; Zeng, J.; Ye, C.; Li, X.; Guo, L.; Zhang, S.; Yu, X. Induction of Escherichia coli into a VBNC state through chlorina-tion/chloramination and differences in characteristics of the bacterium between states. Water Res. 2018, 142, 279–288. [Google Scholar] [CrossRef]
- Ye, C.; Chen, C.; Feng, M.; Ou, R.; Yu, X. Emerging Contaminants in the Water Environment: Disinfection-Induced Viable but Non-Culturable Waterborne Pathogens. J. Hazard. Mater. 2024, 461, 132666. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, H.; Wan, K.; Ye, C.; Yu, X. Quantification and antibiotic resistance risk assessment of chlorination-residual viable/VBNC Escherichia coli and Enterococcus in on-site hospital wastewater treatment system. Sci. Total Environ. 2023, 872, 162139. [Google Scholar] [CrossRef]
- Faria-Ramos, I.; Espinar, M.J.; Rocha, R.; Santos-Antunes, J.; Rodrigues, A.G.; Cantón, R.; Pina-Vaz, C. A novel flow cytometric assay for rapid detection of extended-spectrum beta-lactamases. Clin Microbiol Infect. 2013, 19, E8–E15. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization: An overview. Am. J. Infect. Control. 2016, 44, e1–e6. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), Chemical Disinfectants. 2020. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed on 28 February 2025).
- Alsco 2023. Top 8 Types of Disinfectants Used in Hospitals. Available online: https://alsco.com/resources/top-8-types-of-disinfectants-used-in-hospitals/ (accessed on 28 February 2025).
- Maillard, J.Y. Resistance of bacteria to biocides. Microbiol. Spectr. 2018, 6, ARBA-0006-2017. [Google Scholar] [CrossRef]


| Bacterial Species | Chemical Disinfectant | Mechanisms of Acquired Disinfectant Resistance | References |
|---|---|---|---|
| Acinetobacter baumannii | Triclosan | Efflux pumps FabI, AdelIJK | [23] |
| Bacillus cereus | Alcohol | Biofilms | [24] |
| Bacillus and Staphylococcus | Chlorine | ND | [25] |
| Campylobacter spp. | Benzalkonium chloride Chlorhexidine; Cetylpyridinium chloride | Efflux pumps | [26] |
| Escherichia coli | Hydrogen peroxide | RNA polymerase (rpo) | [27] |
| E. coli | Benzalkonium chloride | Efflux pumps qacE∆1gene | [28,29] |
| E. coli | Benzalkonium chloride | Membrane alterations | [30] |
| Enteroccocus faecium | Alcohol | ND | [31] |
| E. coli | Chlorophene Povidone-iodine | Porins OmpR; EnvZ | [27] |
| Mycobacterium smegmatis | Triclosan | Lipid metabolism (InhA) | [32] |
| Mycobacterium chelonae | Glutaraldehyde | Porins Msp | [33] |
| P. aeruginosa | Sodium hypochlorite | Efflux pumps MuxABC–OpmBa | [34] |
| P. aeruginosa | Chlorine | Efflux pumps, class 1 integrons intI1 gene | [35] |
| P. aeruginosa | Benzalkonium chloride | Efflux pumps sugE-A | [36] |
| Staphylococcus epidermidis | Benzalkonium chloride | Efflux pumps qacC/smr | [37] |
| Salmonella enterica serovar enteritidis | Benzalkonium chloride | Membrane alterations | [38] |
| Bacterial Species | Chemical Disinfectants | References |
|---|---|---|
| E. coli, P. aeruginosa E. faecalis B. sphaericus Achromobacter Listeria monocytogenes Salmonella enterica Helicobacter pylori | Chlorine | [41,42,43,44,45,46] |
| L. monocytogenes | Peracetic acid Hydrogen peroxide Quaternary ammonium | [47] [48] |
| L. monocytogenes E. coli | Peroxyacetic acid Chlorine dioxide | [49] |
| Salmonella enterica | Quaternary ammonium salt 75% ethanol Peracetic acid | [50] |
| Yersinia enterocolitica | Chloride | [51] |
| Legionella spp. | Monochloramine | [52] |
| Chemical Disinfectants Tested | Active Substances | Label Concentration | Label Contact Time |
|---|---|---|---|
| MikrozidTM | 25% ethanol, 35% Propan-1-ol | RTU | 1 min |
| KlinoseptTM | 85% ethanol | RTU | 1 min |
| GlutanolTM | 2.4% glutaraldehyde, 15% ethanol | RTU | 5 min |
| PeroklinTM | 6.0% hydrogen peroxide | RTU | 5 min |
| Clor2KlinTM | 1.5% chlorine | 1–10% | 15 min |
| Species | Strain Code | Source | Antibiotic Resistance Patterns |
|---|---|---|---|
| K. pneumoniae | Kp16 | Sputum | CZ, AMP, E, MEM, IMP, CRO, CXM, FEP, AMC, FOX, CN, TE, CIP, TOB, SXT |
| E. coli | Ec2 | Urine | AMC |
| E. coli | Ec17 | Urine | CZ, PRL, AMP, CTX, ATM, CXM, FEP, AMC, TE, CIP, SXT |
| E. coli | Ec10538 | ATCC reference strain | - |
| A. baumannii | Ab88 | Sputum | SAM, IMP, MEM, DOR, FEP, ATM, CN, AK, CIP, CAZ |
| A. baumannii | Ab108 | Skin infection | IMP, MEM, DOR, FEP, CN, AK, CIP, CAZ |
| P. aeruginosa | Ps1696 | Secretion | TZP, MEM, IMP, FEP, ATM, CAZ, AK, DOR, CN, CIP, TOB |
| P. aeruginosa | Ps1707 | Tracheal secretion | CAZ, ATM, FEP, MEM, IMP, AK, TOB, CIP, CN, DOR |
| P. aeruginosa | Ps15442 | ATCC reference strain | - |
| S. aureus | Sa13358 | Blood culture | FOX, AZM, P, E, TE |
| S. aureus | Sa47 | Skin infection | FOX, AZM, P, CN, E, TE, LZD |
| S. aureus | Sa6538 | ATCC reference strain | - |
| E. faecium | Ef16 | Tracheal secretion | TE, CN, CIP, P, VA |
| E. hirae | Eh10541 | ATCC reference strain | - |
| Bacterial Strains | Chemical Disinfectant | ||||
|---|---|---|---|---|---|
| MikrozidTM (%) | KlinoseptTM (%) | GlutanolTM (%) | PeroklinTM (%) | Clor2KlinTM (%) | |
| Kp16 | 30 | 30 | 1.25 | 0.07 | 0.004 |
| Ec2 | 30 | 60 | 2.5 | 0.15 | 0.003 |
| Ec17 | 30 | 60 | 2.5 | 0.15 | 0.003 |
| Ec10538 | 30 | 40 | 1.25 | 0.15 | 0.004 |
| Ab88 | 15 | 30 | 2.5 | 0.07 | 0.001 |
| Ab108 | 15 | 30 | 2.5 | 0.07 | 0.001 |
| Ps1696 | 10 | 20 | 1.25 | 0.47 | 0.003 |
| Ps1707 | 10 | 20 | 1.25 | 1.87 | 0.003 |
| Ps15442 | 30 | 40 | 2.5 | 0.07 | 0.003 |
| Sa13358 | 40 | 40 | 0.62 | 0.07 | 0.006 |
| Sa47 | 40 | 60 | 1.25 | 0.07 | 0.003 |
| Sa6538 | 60 | 40 | 2.5 | 0.07 | 0.025 |
| Ef16 | 30 | 30 | 5 | 0.07 | 0.001 |
| Eh10541 | 30 | 40 | 2.5 | 0.07 | 0.003 |
| Bacterial Strains | Chemical Disinfectants | |||||
|---|---|---|---|---|---|---|
| MikrozidTM | KlinoseptTM | GlutanolTM | PeroklinTM | Clor2KlinTM | ||
| Kp16 | ||||||
| Ec2 | ||||||
| Ec17 | ||||||
| EcK12 NCTC 10538 | ||||||
| Ab88 | ||||||
| Ab108 | ||||||
| Ps1696 | ||||||
| Ps1707 | ||||||
| Ps15442 | ||||||
| Sa13358 | ||||||
| Sa47 | ||||||
| SaATCC 6538 | ||||||
| Ef16 | ||||||
| Eh ATCC 10541 | ||||||
| Agreement | 12/14 (85.71%) | 11/14 (78.57%) | 14/14 100 (%) | 13/14 (92.85%) | 14/14 (100%) | |
| Agreement within ±1 dilution | 14/14 (100%) | 13/14 (92.85%) | 14/14 (100%) | 14/14 (100%) | 14/14 (100%) | |
| Agreement within ±2 dilution | 14/14 (100%) | 14/14 (100%) | 14/14 (100%) | 14/14 (100%) | 14/14 (100%) | |
| Agreement | ||||||
| Error is within ±1 dilution | ||||||
| Error is within ±2 dilution | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pîndaru, A.; Măruțescu, L.G.; Popa, M.; Lambert, C.; Chifiriuc, M.-C. Label-Free Flow Cytometry: A Powerful Tool to Rapidly and Accurately Assess the Efficacy of Chemical Disinfectants. Microorganisms 2025, 13, 1156. https://doi.org/10.3390/microorganisms13051156
Pîndaru A, Măruțescu LG, Popa M, Lambert C, Chifiriuc M-C. Label-Free Flow Cytometry: A Powerful Tool to Rapidly and Accurately Assess the Efficacy of Chemical Disinfectants. Microorganisms. 2025; 13(5):1156. https://doi.org/10.3390/microorganisms13051156
Chicago/Turabian StylePîndaru, Andreea, Luminița Gabriela Măruțescu, Marcela Popa, Claude Lambert, and Mariana-Carmen Chifiriuc. 2025. "Label-Free Flow Cytometry: A Powerful Tool to Rapidly and Accurately Assess the Efficacy of Chemical Disinfectants" Microorganisms 13, no. 5: 1156. https://doi.org/10.3390/microorganisms13051156
APA StylePîndaru, A., Măruțescu, L. G., Popa, M., Lambert, C., & Chifiriuc, M.-C. (2025). Label-Free Flow Cytometry: A Powerful Tool to Rapidly and Accurately Assess the Efficacy of Chemical Disinfectants. Microorganisms, 13(5), 1156. https://doi.org/10.3390/microorganisms13051156







