Impact of Temperature on the Biochemical Potential of Five Newly Isolated Strains of Microalgae Cultured in a Stirred Tank Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Culture Conditions
2.2. Cell Growth and Biomass Determination
2.3. Lipid Extraction and Purification
2.4. Lipid Fractionation
2.5. Fatty Acid Composition of Cellular Lipids
2.6. Polysaccharide Determination
2.7. Protein Determination
2.8. Pigment Estimation
2.9. Data Treatment and Statistical Analysis
3. Results
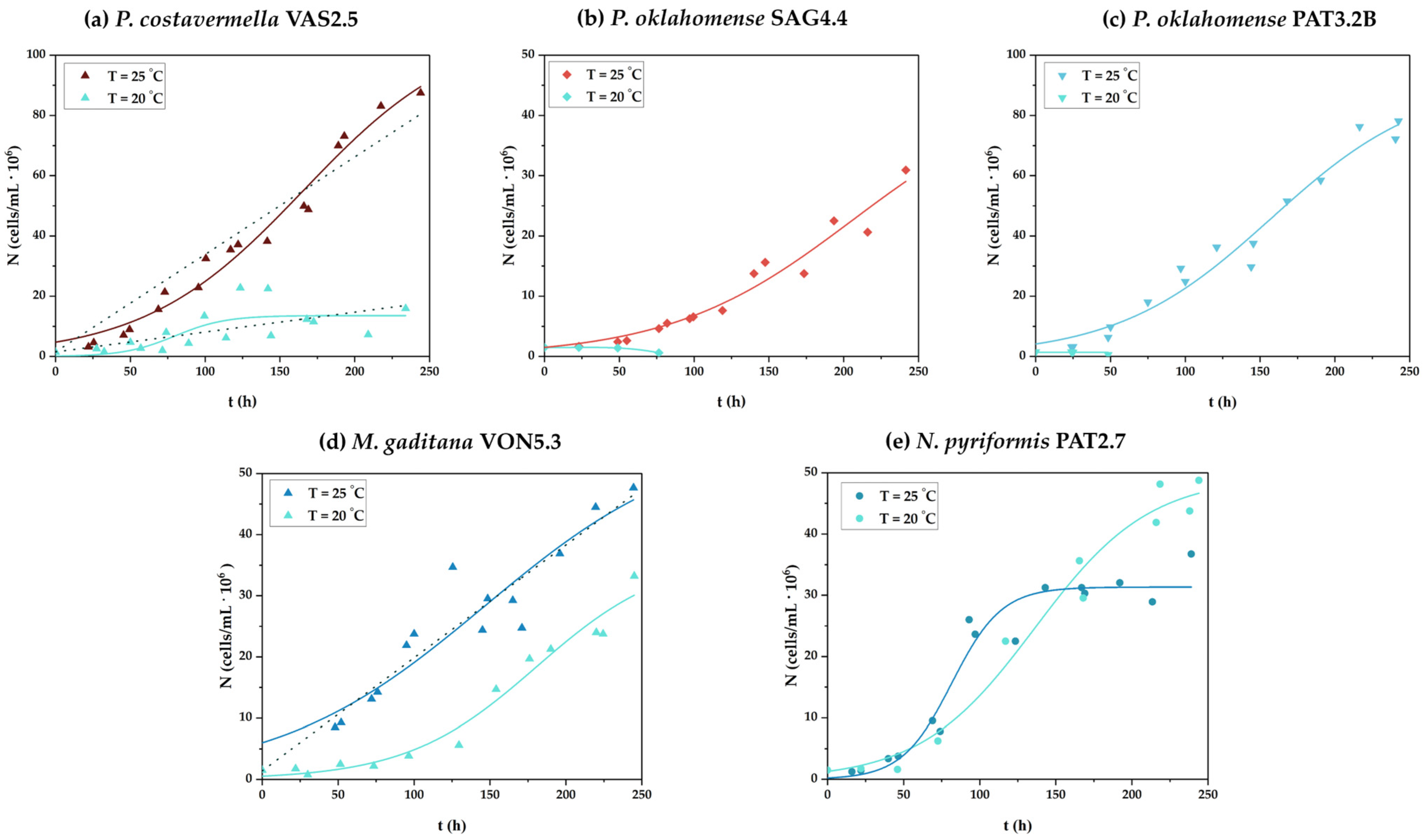
3.1. Cell Growth and Biomass Production
3.2. Accumulation of Storage Materials
3.3. Fatty Acid Composition of Total Lipids and Lipid Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PUFAs | polyunsaturated fatty acids |
| ALA | α-linolenic acid |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| STR | Stirred Tank Reactor |
| mASW | modified Artificial Sea Water |
References
- Chapman, R.L. Algae: The world’s most important “plants”-an introduction. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Raven, J.A. The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. Eur. J. Phycol. 2017, 52, 506–522. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Walcker, R.; Kardol, P.; Geisen, S.; Heger, T.; Lamentowicz, M.; Hamard, S.; Lara, E. Contribution of soil algae to the global carbon cycle. New Phytol. 2022, 234, 64–76. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Korozi, E.; Tsagou, V.; Kefalogianni, I.; Markou, G.; Antonopoulos, D.; Chakalis, L.; Kotzamanis, Y.; Chatzipavlidis, I. Continuous culture of Auxenochlorella protothecoides on biodiesel derived glycerol under mixotrophic and heterotrophic conditions: Growth parameters and biochemical composition. Microorganisms 2022, 10, 541. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef]
- Alhattab, M.; Moorthy, L.S.; Patel, D.; Franco, C.M.M.; Puri, M. Oleaginous microbial lipids’ potential in the prevention and treatment of neurological disorders. Mar. Drugs 2024, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Kalampounias, G.; Dritsas, P.; Karayannis, D.; Androutsopoulou, T.; Gardeli, C.; Papanikolaou, S.; Aggelis, G.; Katsoris, P. Poly-unsaturated fatty acids from Thamnidium elegans and Mortierella alpina suppress prostate cancer cells proliferation and migration. Fermentation 2024, 10, 578. [Google Scholar] [CrossRef]
- Karageorgou, D.; Rova, U.; Christakopoulos, P.; Katapodis, P.; Matsakas, L.; Patel, A. Benefits of supplementation with microbial omega-3 fatty acids on human health and the current market scenario for fish-free omega-3 fatty acid. Trends Food Sci. Technol. 2023, 136, 169–180. [Google Scholar] [CrossRef]
- Sayegh, F.; Elazzazy, A.; Bellou, S.; Moustogianni, A.; Elkady, A.I.; Baeshen, M.N.; Aggelis, G. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 2016, 66, 937–948. [Google Scholar] [CrossRef]
- Dritsas, P.; Asimakis, E.; Lianou, A.; Efstratiou, M.; Tsiamis, G.; Aggelis, G. Microalgae from the Ionian Sea (Greece): Isolation, molecular identification and biochemical features of biotechnological interest. Algal Res. 2023, 74, 103210. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Custódio, L.; Alrokayan, S.; Mouffouk, F.; Varela, J.; Abu-Salah, K.M.; Ben-Hamadou, R. Isolation and fatty acid profile of selected microalgae strains from the Red Sea for biofuel production. Energies 2013, 6, 2773–2783. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Chlorella vulgaris as a green biofuel factory: Comparison between biodiesel, biogas and combustible biomass production. Bioresour. Technol. 2019, 273, 237–243. [Google Scholar] [CrossRef]
- Ryan, L.; Symington, A.M. Algal-oil supplements are a viable alternative to fish-oil supplements in terms of docosahexaenoic acid (22:6n-3; DHA). J. Funct. Foods 2015, 19, 852–858. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Ansari, F.A.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Improving the feasibility of aquaculture feed by using microalgae. Environ. Sci. Pollut. Res. Int. 2021, 28, 43234–43257. [Google Scholar] [CrossRef]
- Gao, S.; Chen, W.; Cao, S.; Sun, P.; Gao, X. Microalgae as fishmeal alternatives in aquaculture: Current status, existing problems, and possible solutions. Environ. Sci. Pollut. Res. Int. 2024, 31, 16113–16130. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Sanchez del Pulgar, J.; Aguzzi, A.; Caproni, R.; Gabrielli, P.; Lombardi-Boccia, G. Chemical characterization and nutritional evaluation of microalgal biomass from large-scale production: A comparative study of five species. Eur. Food Res. Technol. 2020, 246, 323–332. [Google Scholar] [CrossRef]
- Dourou, M.; Dritsas, P.; Baeshen, M.N.; Elazzazy, A.; Al-Farga, A.; Aggelis, G. High-added value products from microalgae and prospects of aquaculture wastewaters as microalgae growth media. FEMS Microbiol. Lett. 2021, 367, fnaa081. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. A critical review on the status and progress of microalgae cultivation in outdoor photobioreactors conducted over 35 years (1986–2021). Energies 2023, 16, 3105. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Sarat Chandra, T.; Deepak, R.S.; Maneesh Kumar, M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: Effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour. Technol. 2016, 207, 430–439. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Venkata Subhash, G.; Rohit, M.V.; Devi, M.P.; Swamy, Y.V.; Venkata Mohan, S. Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresour. Technol. 2014, 169, 789–793. [Google Scholar] [CrossRef]
- Eker-Develi, E. First record of chlorophyte Nephroselmis pyriformis from the north-eastern Mediterranean Sea coast. Mar. Biodivers. Rec. 2015, 8, e65. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of light intensity on antioxidant activity of tropical microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Schoeters, F.; Spit, J.; Van Miert, S. Real-time monitoring of microalgal biomass in pilot-scale photobioreactors using nephelometry. Processes 2021, 9, 1530. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Krishnan, A.; Dahlin, L.R.; Guarnieri, M.T.; Weissman, J.C.; Posewitz, M.C. Small cells with big photosynthetic productivities: Biotechnological potential of the Picochlorum genus. Trends Biotechnol. 2024, 43, 759–772. [Google Scholar] [CrossRef]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J. Biotechnol. 2012, 164, 318–329. [Google Scholar] [CrossRef]
- Dourou, M.; Tsolcha, O.N.; Tekerlekopoulou, A.G.; Bokas, D.; Aggelis, G. Fish farm effluents are suitable growth media for Nannochloropsis gaditana, a polyunsaturated fatty acid producing microalga. Eng. Life Sci. 2018, 18, 851–860. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sumanta, N.; Imranul Haque, C.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Ahn, Y.; Park, S.; Ji, M.K.; Ha, G.S.; Jeon, B.H.; Choi, J. Biodiesel production potential of microalgae, cultivated in acid mine drainage and livestock wastewater. J. Environ. Manag. 2022, 314, 115031. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. The effects of light intensity on the growth rates of green algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sukenin, A.; Carmeli, Y. Regulation of fatty acid composition by irradiance level in the Eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar] [CrossRef]
- de la Vega, M.; Díaz, E.; Vila, M.; León, R. Isolation of a new strain of Picochlorum sp. and characterization of its potential biotechnological applications. Biotechnol. Prog. 2011, 27, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Richmond, A. Microalgal biotechnology at the turn of the millennium: A personal view. J. Appl. Phycol. 2000, 12, 441–451. [Google Scholar] [CrossRef]
- Timm, U.; Klinck, J.M.; Okubo, A. Self-and mutual shading and competition effect on competing algal distributions: Biological implications of the model. Ecol. Model. 1991, 59, 11–36. [Google Scholar] [CrossRef]
- Barten, R.; Kleisman, M.; D’Ermo, G.; Nijveen, H.; Wijffels, R.H.; Barbosa, M.J. Short-term physiologic response of the green microalga Picochlorum sp. (BPE23) to supra-optimal temperature. Sci. Rep. 2022, 12, 3290. [Google Scholar] [CrossRef]
- Dahmen, I.; Chtourou, H.; Jebali, A.; Daassi, D.; Karray, F.; Hassairi, I.; Sayadi, S.; Abdelkafi, S.; Dhouib, A. Optimisation of the critical medium components for better growth of Picochlorum sp. and the role of stressful environments for higher lipid production. J. Sci. Food Agric. 2014, 94, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Šantek, M.I. Bioprospecting of microalgae isolated from the Adriatic Sea: Characterization of biomass, pigment, lipid and fatty acid composition, and antioxidant and antimicrobial activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef]
- Hotos, G.N.; Bekiari, V. Absorption spectra as predictors of algal biomass and pigment content of the cultured microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. Int. J. Plant Biol. 2023, 14, 879–895. [Google Scholar] [CrossRef]
- Ji, M.K.; Yun, H.S.; Hwang, B.S.; Kabra, A.N.; Jeon, B.H.; Choi, J. Mixotrophic cultivation of Nephroselmis sp. using industrial wastewater for enhanced microalgal biomass production. Ecol. Eng. 2016, 95, 527–533. [Google Scholar] [CrossRef]
- Mastropetros, S.G.; Tsigkou, K.; Cladas, Y.; Priya, A.K.; Kornaros, M. Effect of nitrogen, salinity, and light intensity on the biomass composition of Nephroselmis sp.: Optimization of lipids accumulation (including EPA). Mar. Drugs 2023, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Magpusao, J.; Oey, I.; Kebede, B. Chemical, rheological, and volatile profiling of microalgae Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis species. Food Innov. Adv. 2024, 3, 75–87. [Google Scholar] [CrossRef]
- Dambeck, M.; Sandmann, G. Antioxidative activities of algal keto carotenoids acting as antioxidative protectants in the chloroplast. Photochem. Photobiol. 2014, 90, 814–819. [Google Scholar] [CrossRef]
- Manabe, Y.; Takii, Y.; Sugawara, T. Siphonaxanthin, a carotenoid from green algae, suppresses advanced glycation end product-induced inflammatory responses. J. Nat. Med. 2020, 74, 127–134. [Google Scholar] [CrossRef]
- Watanabe, K.; Fujii, K. Isolation of high-level-CO2-preferring Picochlorum sp. strains and their biotechnological potential. Algal Res. 2016, 18, 135–143. [Google Scholar] [CrossRef]
- Mucko, M.; Padisák, J.; Gligora Udovič, M.; Pálmai, T.; Novak, T.; Medić, N.; Gašparović, B.; Peharec Štefanić, P.; Orlić, S.; Ljubešić, Z. Characterization of a high lipid-producing thermotolerant marine photosynthetic pico alga from genus Picochlorum (Trebouxiophyceae). Eur. J. Phycol. 2020, 55, 384–399. [Google Scholar] [CrossRef]
- Zhu, Y.; Dunford, N.T. Growth and biomass characteristics of Picochlorum oklahomensis and Nannochloropsis oculata. JAOCS 2013, 90, 841–849. [Google Scholar] [CrossRef]
- Weissman, J.C.; Likhogrud, M.; Thomas, D.C.; Fang, W.; Karns, D.A.J.; Chung, J.W.; Nielsen, R.; Posewitz, M.C. High-light selection produces a fast-growing Picochlorum celeri. Algal Res. 2018, 36, 17–28. [Google Scholar] [CrossRef]
- Dogaris, I.; Loya, B.; Cox, J.; Philippidis, G. Study of landfill leachate as a sustainable source of water and nutrients for algal biofuels and bioproducts using the microalga Picochlorum oculatum in a novel scalable bioreactor. Bioresour. Technol. 2019, 282, 18–27. [Google Scholar] [CrossRef]
- Mohammady, N. Growth and oil production of Nannochloropsis salina cultivated under multiple stressors. J. Pure Appl. Microbiol. 2014, 8, 2761–2772. [Google Scholar]
- Simionato, D.; Sforza, E.; Corteggiani Carpinelli, E.; Bertucco, A.; Giacometti, G.M.; Morosinotto, T. Acclimation of Nannochloropsis gaditana to different illumination regimes: Effects on lipids accumulation. Bioresour. Technol. 2011, 102, 6026–6032. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The effect of various salinities and light intensities on the growth performance of five locally isolated microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. Red Pappas), Asteromonas gracilis and Dunaliella sp.] in laboratory batch cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Yun, H.S.; Lee, H.; Park, Y.T.; Ji, M.K.; Kabra, A.N.; Jeon, C.; Jeon, B.H.; Choi, J. Isolation of novel microalgae from acid mine drainage and its potential application for biodiesel production. Appl. Biochem. Biotechnol. 2014, 173, 2054–2064. [Google Scholar] [CrossRef]
- Tran, D.; Giordano, M.; Louime, C.; Tran, N.; Vo, T.; Nguyen, D.; Hoang, T. An isolated Picochlorum species for aquaculture, food, and biofuel. N. Am. J. Aquac. 2014, 76, 305–311. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Thinh, L.-V.; Renaud, S.M.; Parry, D.L. Evaluation of recently isolated Australian tropical microalgae for the enrichment of the dietary value of brine shrimp, Artemia nauplii. Aquaculture 1999, 170, 161–173. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D.; Mastropetros, S.G.; Tsigkou, K.; Kouvara, K.; Makridis, P.; Kornaros, M. Isolation, identification, and chemical composition analysis of nine microalgal and cyanobacterial species isolated in lagoons of western Greece. Algal Res. 2023, 69, 102935. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D.; Bekiari, V. Calibration curves of culture density assessed by spectrophotometer for three microalgae (Nephroselmis sp., Amphidinium carterae and Phormidium sp.). Eur. J. Biol. Biotechnol. 2020, 1. [Google Scholar] [CrossRef]
- Makri, A.; Bellou, S.; Birkou, M.; Papatrehas, K.; Dolapsakis, N.P.; Bokas, D.; Papanikolaou, S.; Aggelis, G. Lipid synthesized by micro-algae grown in laboratory- and industrial-scale bioreactors. Eng. Life Sci. 2011, 11, 52–58. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Marxen, K.; Schulz, R.; Vanselow, K.H. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar. Drugs 2010, 8, 2526–2545. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Xu, J.; Cao, J.; Zhou, C.; Yan, X. Isolation of chloroplasts from marine microalga Isochrysis galbana Parke for their lipid composition analysis. J. Ocean Univ. China 2022, 21, 225–235. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Lipids of Cunninghamella echinulata with emphasis to γ-linolenic acid distribution among lipid classes. Appl. Microbiol. Biotechnol. 2006, 73, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Khozin-Goldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Y.; Mu, T.; Ke, A.; Ran, Z.; Xu, J.; Chen, J.; Zhou, C.; Yan, X.; Xu, Q.; et al. Sphingolipids in marine microalgae: Development and application of a mass spectrometric method for global structural characterization of ceramides and glycosphingolipids in three major phyla. Anal. Chim. Acta 2017, 986, 82–94. [Google Scholar] [CrossRef]
- Miazek, K.; Lebecque, S.; Hamaidia, M.; Paul, A.; Danthine, S.; Willems, L.; Frederich, M.; De Pauw, E.; Deleu, M.; Richel, A.; et al. Sphingolipids: Promising lipid-class molecules with potential applications for industry. A review. BASE 2016, 20, 321–336. [Google Scholar] [CrossRef]
- Fidalgo, J.P.; Cid, A.; Torres, E.; Sukenik, A.; Herrero, C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 1998, 166, 105–116. [Google Scholar] [CrossRef]
- Alboresi, A.; Perin, G.; Vitulo, N.; Diretto, G.; Block, M.; Jouhet, J.; Meneghesso, A.; Valle, G.; Giuliano, G.; Maréchal, E.; et al. Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol. 2016, 171, 2468–2482. [Google Scholar] [CrossRef]
- Hu, Q.; Xiang, W.; Dai, S.; Li, T.; Yang, F.; Jia, Q.; Wang, G.; Wu, H. The influence of cultivation period on growth and biodiesel properties of microalga Nannochloropsis gaditana 1049. Bioresour. Technol. 2015, 192, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, C.; Wu, H.; Li, T.; Chen, X.; Wu, H.; Xiang, W. Enhancement in lipid productivity of the marine microalga Nannochloropsis sp. SCSIO-45217 through phosphate adjustment strategies. J. Appl. Phycol. 2023, 35, 1023–1035. [Google Scholar] [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in microalgae: Health benefits, biosynthesis, and metabolic engineering advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, H.Y. Growth and fatty acid profile of the marine microalga Picochlorum sp. grown under nutrient stress conditions. Egypt. J. Aquat. Res. 2013, 39, 233–239. [Google Scholar] [CrossRef]
- Bach, T.M.H.; Takagi, H. Properties, metabolisms, and applications of l-proline analogues. Appl. Microbiol. Biotechnol. 2013, 97, 6623–6634. [Google Scholar] [CrossRef]

| Compound | Supplier | Concentration (g/L) |
|---|---|---|
| NaCl | PENTA (Prague, Czech Republic) | 27.0 |
| MgSO4·7H2O | PanReac AppliChem (Darmstadt, Germany) | 6.6 |
| CaCl2 | PENTA | 1.5 |
| KNO3 | Scharlau (Barcelona, Spain) | 1.0 |
| KH2PO4 | Himedia (Mumbai, India) | 0.07 |
| FeCl3·6H2O | BDH (Poole, England) | 0.014 |
| Na2EDTA | Merck (Darmstadt, Germany) | 0.019 |
| Microelement solution | ||
| ZnSO4·7H2O | Merck | 40.0 |
| H3BO3 | Fluka (Steinheim, Germany) | 600.0 |
| CoCl2·6H2O | Sigma-Aldrich (St. Louis, MO, USA) | 1.5 |
| CuSO4·5H2O | BDH | 40.0 |
| MnCl2 | Sigma-Aldrich | 400.0 |
| (NH4)6MO7O24·4H2O | Sigma-Aldrich | 370.0 |
| Strain | T (°C) | Biomass (x) | Lipids (L) | Polysaccharides (S) | Proteins (P) | Pigments | Growth Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | px | L/x (%) | Lipid Fractions (%) | S/x (%) | P/x (%) | TCh/x (%) | TC/x (%) | Nf | Nmax | μ | R2 | ||||
| (mg/L) | (mg/L∙d) | N | G + S | P | (cells/mL∙106) | (1/d) | |||||||||
| P. costavermella VAS2.5 | 25 | 671.3 ± 17.2 | 67.1 ± 1.7 | 19.3 ± 0.7 | 29.6 ± 3.4 | 57.4 ± 5.4 | 13.1 ± 2.0 | 13.5 ± 0.8 | 25.0 ± 0.2 | 2.6 ± 0.1 | 0.2 ± 0.0 | 87.5 | 110.2 ±12.9 | 0.45 ± 0.05 | 0.98 |
| 20 | 418.4 ± 17.7 | 41.8 ± 2.5 | 5.2 ± 0.3 | UND | UND | UND | 6.7 ± 0.1 | 26.7 ± 0.0 | 2.1 ± 0.2 | 0.8 ± 0.1 | 15.9 | 13.6 ± 2.2 | 1.41 ± 1.04 | 0.51 | |
| P. oklahomense SAG4.4 | 25 | 224.8 ± 5.9 | 22.5 ± 0.6 | 4.1 ± 0.4 | UND | UND | UND | 11.3 ± 0.4 | 28.8 ± 1.0 | 4.9 ± 0.1 | 0.8 ± 0.0 | 30.9 | 45.3 ± 15.6 | 0.39 ± 0.08 | 0.95 |
| P. oklahomense PAT3.2B | 25 | 242.7 ± 13.6 | 24.3 ± 1.4 | 11.5 ± 0.3 | UND | UND | UND | 11.2 ± 1.2 | 50.6 ± 3.0 | 6.1 ± 0.7 | 1.1 ± 0.1 | 20.6 | 91.8 ± 10.8 | 0.47 ± 0.07 | 0.97 |
| M. gaditana VON5.3 | 25 | 527.4 ± 15.0 | 52.7 ± 1.5 | 16.4 ± 2.1 | 55.6 ± 1.6 | 24.7 ± 1.4 | 19.8 ± 0.3 | 8.5 ± 0.0 | 16.4 ± 2.6 | 1.4 ± 0.0 | 1.4 ± 0.0 | 47.7 | 56.7 ± 15.7 | 0.35 ± 0.11 | 0.88 |
| 20 | 509.0 ± 77.8 | 50.9 ± 7.8 | 13.8 ± 0.0 | 22.5 ± 0.2 | 41.3 ± 2.0 | 36.3 ± 2.1 | 9.4 ± 2.1 | 17.5 ± 1.2 | 3.2 ± 0.3 | 1.1 ± 0.1 | 33.3 | 36.8 ± 6.7 | 0.56 ± 0.12 | 0.97 | |
| N. pyriformis PAT2.7 | 25 | 438.8 ± 89.3 | 43.9 ± 8.9 | 3.3 ± 1.7 | 45.8 ± 4.3 | 48.6 ± 5.0 | 6.0 ± 1.0 | 8.0 ± 0.2 | 45.2 ± 13.1 | 1.7 ± 0.3 | 0.3 ± 0.0 | 36.7 | 31.3 ± 1.3 | 1.47 ± 0.34 | 0.95 |
| 20 | 546.7 ± 1.2 | 54.7 ± 0.1 | 9.3 ± 6.1 | 63.5 ± 6.0 | 34.8 ± 4.8 | 2.3 ± 1.2 | 10.7 ± 3.1 | 41.0 ± 10.4 | 1.0 ± 0.0 | 0.3 ± 0.0 | 48.8 | 49.3 ± 3.5 | 0.64 ± 0.12 | 0.98 | |
| Strain | T (°C) | Lipid Fraction | Composition of Total Lipids and Lipid Fractions in Fatty Acids (%, wt/wt) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | Δ9C14:1 | C16:0 | Δ9C16:1 | C17:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ9,12,15C18:3 | Δ6,9,12,15C18:4 | Δ13C20:1 | Δ5,8,11,14,17C20:5 | * Others | |||
| P. costavermella VAS2.5 | 25 | TLs | 6.8 ± 0.3 | 3.5 ± 0.4 | 29.0 ± 0.2 | 28.6 ± 0.3 | <0.5 | <0.5 | 7.0 ± 0.6 | 1.1 ± 0.1 | <0.5 | <0.5 | 3.8 ± 0.1 | 17.0 ± 0.2 | 1.8 ± 0.0 |
| N | 4.5 ± 0.2 | 2.8 ± 1.2 | 34.4 ± 2.4 | 29.0 ± 3.2 | <0.5 | 1.6 ± 0.3 | 15.8 ± 5.7 | 1.4. ± 0.7 | <0.5 | 0.8 ± 0.2 | 1.8 ± 0.1 | 5.4 ± 0.1 | 1.2 ± 0.3 | ||
| G | 9.1 ± 0.3 | 4.3 ± 0.6 | 26.7 ± 2.2 | 29.6 ± 1.8 | <0.5 | <0.5 | 4.9 ± 0.0 | 1.1 ± 0.1 | <0.5 | ND | 3.1 ± 0.0 | 18.6 ± 4.0 | 0.8 ± 0.4 | ||
| P | 3.0 ± 0.3 | 0.7 ± 0.0 | 21.5 ± 1.0 | 20.1 ± 1.4 | 0.7 ± 0.1 | <0.5 | 10.1 ± 0.1 | 3.2 ± 1.3 | 1.3 ± 0.1 | ND | 7.9 ± 1.0 | 30.3 ± 4.9 | 1.0 ± 0.2 | ||
| 20 | TLs | 6.3 ± 0.5 | 4.1 ± 0.1 | 14.3 ± 0.6 | 26.8 ± 0.9 | <0.5 | <0.5 | 4.7 ± 0.3 | 1.4 ± 0.3 | ND | ND | 3.7 ± 0.7 | 35.9 ± 0.7 | 2.3 ± 0.1 | |
| P. oklahomense SAG4.4 | 25 | TLs | 2.4 ± 0.2 | 8.5 ± 0.5 | 12.2 ± 2.1 | 4.7 ± 0.6 | 3.7 ± 0.2 | 7.2 ± 0.0 | 18.4 ± 2.4 | 8.2 ± 0.2 | 6.3 ± 0.6 | 18.9 ± 3.6 | ND | ND | 9.5 ± 0.3 |
| P. oklahomense PAT3.2B | 25 | TLs | 2.3 ± 0.2 | 9.3 ± 0.3 | 15.2 ± 0.1 | 4.4 ± 0.4 | ND | 6.4 ± 0.7 | 14.4 ± 0.8 | 25.3 ± 0.0 | 17.9 ± 1.0 | ND | ND | ND | 4.8 ± 0.0 |
| Strain | T (°C) | Lipid Fraction | Composition of Total Lipids and Lipid Fractions in Fatty Acids (%, wt/wt) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | Δ9C14:1 | C16:0 | Δ9C16:1 | C17:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ9,12,15C18:3 | Δ6,9,12,15C18:4 | Δ13C20:1 | Δ5,8,11,14,17C20:5 | * Others | |||
| Microchloropsis gaditana VON5.3 | 25 | TLs | 7.2 ± 0.6 | 3.7 ± 0.7 | 24.1 ± 1.3 | 30.5 ± 0.8 | <0.5 | <0.5 | 5.5 ± 1.3 | 2.0 ± 0.0 | <0.5 | ND | 3.4 ± 0.5 | 19.9 ± 0.1 | 2.9 ± 0.7 |
| N | 6.6 ± 0.9 | 3.5 ± 0.5 | 27.5 ± 1.1 | 34.3 ± 0.6 | 0.6 ± 0.1 | 0.7 ± 0.1 | 6.7 ± 1.4 | 1.7 ± 0.3 | <0.5 | ND | 3.1 ± 0.6 | 10.4 ± 1.9 | 2.1 ± 0.2 | ||
| G | 10.1 ± 1.0 | 5.3 ± 0.3 | 22.6 ± 1.8 | 28.7 ± 0.6 | <0.5 | <0.5 | 5.0 ± 1.4 | 2.0 ± 0.7 | 0.6 ± 0.0 | ND | 2.5 ± 0.5 | 19.6 ± 2.7 | 2.4 ± 0.1 | ||
| P | 2.8 ± 0.0 | 1.7 ± 0.1 | 36.3 ± 1.0 | 8.4 ± 0.1 | <0.5 | 17.6 ± 0.9 | 13.0 ± 0.6 | 1.6 ± 0.6 | 0.6 ± 0.0 | ND | 5.9 ± 1.4 | 10.9 ± 0.2 | 1.1 ± 0.1 | ||
| 20 | TLs | 7.0 ± 0.5 | 3.4 ± 0.5 | 27.1 ± 1.7 | 30.4 ± 0.3 | ND | 1.0 ± 0.4 | 6.0 ± 2.8 | 1.8 ± 0.5 | 2.2 ± 1.4 | 0.6 ± 0.0 | 2.8 ± 0.9 | 16.0 ± 6.9 | 1.8 ± 0.1 | |
| N | 5.5 ± 0.3 | 2.5 ± 1.3 | 32.4 ± 1.2 | 37.8 ± 1.5 | <0.5 | 0.9 ± 0.1 | 5.9 ± 0.4 | 1.1 ± 0.1 | 0.7 ± 0.0 | 1.5 ± 0.6 | 2.0 ± 0.0 | 8.2 ± 0.1 | 0.9 ± 0.2 | ||
| G | 9.0 ± 0.2 | 5.6 ± 0.8 | 24.3 ± 0.7 | 28.1 ± 0.7 | <0.5 | <0.5 | 2.4 ± 0.3 | 1.2 ± 0.0 | <0.5 | <0.5 | 1.4 ± 0.1 | 18.7 ± 2.3 | 2.2 ± 0.2 | ||
| P | 2.6 ± 0.0 | 1.1 ± 0.1 | 17.6 ± 0.8 | 28.9 ± 1.7 | <0.5 | <0.5 | 5.1 ± 0.2 | 2.6 ± 0.0 | 1.0 ± 0.1 | ND | 7.5 ± 1.0 | 29.5 ± 3.3 | 0.8 ± 0.1 | ||
| Strain | T (°C) | Lipid Fraction | Composition of Total Lipids and Lipid Fractions in Fatty Acids (%, wt/wt) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | Δ9C14:1 | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | * Others | |||
| Nephroselmis pyriformis PAT2.7 | 25 | TLs | 31.0 ± 0.8 | 7.2 ± 1.2 | 9.2 ± 0.6 | 40.1 ± 0.1 | 2.0 ± 0.7 | 3.7 ± 2.0 | 2.2 ± 0.3 | 4.8 ± 3.2 |
| N | 36.0 ± 1.4 | 6.0 ± 0.3 | 9.4 ± 0.6 | 43.0 ± 1.9 | 0.7 ± 0.3 | 1.6 ± 0.8 | 1.4 ± 0.0 | 1.6 ± 0.5 | ||
| G | 31.9 ± 0.2 | 5.4 ± 0.7 | 8.2 ± 0.4 | 39.8 ± 0.4 | 2.2 ± 1.1 | 3.2 ± 0.7 | <0.5 | 8.8 ± 1.9 | ||
| P | 15.5 ± 0.7 | 4.4 ± 3.9 | 14.3 ± 0.6 | 29.8 ± 0.2 | 5.8 ± 1.1 | 19.4 ± 2.3 | 1.9 ± 0.5 | 9.1 ± 0.1 | ||
| 20 | TLs | 30.8 ± 1.0 | 5.9 ± 0.1 | 8.4 ± 0.2 | 39.2 ± 0.9 | 1.2 ± 0.1 | 1.4 ± 0.3 | ND | 13.1 ± 1.2 | |
| N | 37.4 ± 1.8 | 6.3 ± 0.2 | 8.8 ± 0.8 | 44.8 ± 0.9 | <0.5 | 0.8 ± 0.0 | ND | 0.9 ± 0.2 | ||
| G | 30.3 ± 0.4 | 17.3 ± 0.0 | 7.6 ± 0.4 | 22.1 ± 0.0 | 2.5 ± 0.1 | 4.7 ± 0.5 | ND | 7.8 ± 1.2 | ||
| P | 20.3 ± 3.4 | 1.5 ± 0.2 | 14.5 ± 0.5 | 15.1 ± 1.3 | 8.7 ± 0.2 | 20.2 ± 5.7 | ND | 10.5 ± 0.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dritsas, P.; Aggelis, G. Impact of Temperature on the Biochemical Potential of Five Newly Isolated Strains of Microalgae Cultured in a Stirred Tank Reactor. Microorganisms 2025, 13, 1155. https://doi.org/10.3390/microorganisms13051155
Dritsas P, Aggelis G. Impact of Temperature on the Biochemical Potential of Five Newly Isolated Strains of Microalgae Cultured in a Stirred Tank Reactor. Microorganisms. 2025; 13(5):1155. https://doi.org/10.3390/microorganisms13051155
Chicago/Turabian StyleDritsas, Panagiotis, and George Aggelis. 2025. "Impact of Temperature on the Biochemical Potential of Five Newly Isolated Strains of Microalgae Cultured in a Stirred Tank Reactor" Microorganisms 13, no. 5: 1155. https://doi.org/10.3390/microorganisms13051155
APA StyleDritsas, P., & Aggelis, G. (2025). Impact of Temperature on the Biochemical Potential of Five Newly Isolated Strains of Microalgae Cultured in a Stirred Tank Reactor. Microorganisms, 13(5), 1155. https://doi.org/10.3390/microorganisms13051155








