Abstract
Bacterial tolerance, especially in Staphylococcus aureus (S. aureus), may arise under intermittent antibiotic regimens and act as a stepping stone toward resistance development. However, the transition from tolerance to resistance and its contributing factors remain poorly understood. This study explores the role of the efflux pump gene abcA in this process. abcA mutants (overexpression, knockout, and complementation) were constructed via homologous recombination. These strains were subjected to 21 cycles of intermittent exposure to oxacillin at 20× MIC, and the resistance evolution was monitored. Spontaneous mutation frequencies and survival abilities in these mutants were also measured to determine their involvement in resistance development. The abcA overexpression mutant exhibited a faster development of resistance compared to the wildtype strain. Conversely, the abcA knockout mutant maintained susceptibility to oxacillin, with no significant changes in the relative MIC. Increased mutation frequency and enhanced survival were observed in the overexpression strain, whereas both were reduced in the knockout. abcA overexpression significantly accelerated the development of oxacillin resistance in S. aureus by promoting spontaneous mutations and bacterial survival. Disrupting abcA may offer a novel strategy to prevent the evolution of antibiotic resistance.
1. Introduction
Antimicrobial resistance (AMR) is rapidly spreading among opportunistic pathogens, posing a growing threat to global public health. Predictive models estimate that, in 2019 alone, AMR contributed to around 4.95 million deaths (range 3.62–6.57 million) [1], and the annual death toll is expected to soar to 10 million by 2050 [2]. Among the various pathogens contributing to AMR, Staphylococcus aureus (S. aureus) stands out due to its role in severe infections such as endocarditis, sepsis, and pyemia. In 2019, S. aureus accounted for approximately 26.1% of all AMR-related deaths, among the six key pathogens identified by the World Health Organization (WHO) [1]. The rise of β-lactam antibiotic resistance, including resistance to methicillin, oxacillin, and penicillin, underscores the urgency of this issue. These antibiotics are primary treatments for severe infections, yet over 70% of AMR-related mortalities are attributed to resistance against them and fluoroquinolones. In response, the WHO has identified methicillin-resistant Staphylococcus aureus MRSA as one of the critical pathogens requiring urgent antibiotic development efforts [3,4]. A thorough understanding of the evolutionary mechanisms behind β-lactam resistance is critical to combating the escalating AMR challenge by disrupting the process of resistance development.
Bacteria adopt two primary strategies to develop resistance. Under sustained exposure to low levels of antibiotics, especially at sub-lethal concentrations, pathogens rapidly acquire antibiotic resistance [5,6,7,8]. This resistance may arise through multiple strategies, including enzymatic breakdown of the drug, structural alterations of antibiotic targets, or impaired intracellular accumulation due to limited membrane permeability or activation of efflux systems [9]. By contrast, exposure to transiently elevated antibiotic levels—often exceeding the Minimum Inhibitory Concentration (MIC)—can select for tolerant phenotypes, enabling bacteria to persist temporarily under lethal drug concentrations [10,11]. For instance, dormancy-induced tolerance has been found to protect bacteria from the attack of various antibiotics that necessitate bacterial growth to be effective [12]. Both genetic and environmental factors can induce tolerance [13,14]. Despite substantial progress in resistance research, interest has only recently shifted to understanding how tolerance contributes to resistance, especially in persistent infection settings [15,16,17,18]. Studies have shown that bacterial tolerance can rapidly evolve into resistance, as observed in Escherichia coli (E. coli) and S. aureus [19,20,21]. The rapid evolution from tolerance to resistance indicates that certain factors within tolerance are promoting this resistance acquisition. However, the specific contributors that facilitate the transition from tolerance to resistance are still not inadequately elucidated.
Efflux pumps, ubiquitous transporter proteins found in both prokaryotic and eukaryotic cells, are recognized as a key resistance mechanism by extruding antibiotics from bacterial cells [22]. Although resistance mechanisms have been extensively studied, how efflux pumps influence the progression from bacterial tolerance to full resistance remains insufficiently understood. Given that tolerance is known to accelerate the subsequent evolution of resistance, as documented by Levin-Reisman et al. [19], the observed upregulation of multiple multidrug efflux genes in tolerant cells suggests a potential role for efflux pumps as contributors to the progression from tolerance to resistance [12]. Our previous work revealed that the NorA efflux pump promoted resistance development against ciprofloxacin [16]. However, the involvement of other efflux pumps, such as the AbcA pump from the ATP-binding cassette family [23], remain unclear.
AbcA is important in bacterial physiology, such as antibiotic resistance and virulence. AbcA can extrude β-lactam drugs, including oxacillin, to confer β-lactam resistance [24,25]. AbcA also contributes to S. aureus virulence by secreting phenol-soluble modulins [26,27]. A recent study highlighted AbcA’s participation in tolerance formation, where tolerant cell levels decreased in an abcA knockout mutant under exposure to 25× the MIC of nafcillin [28]. However, the specific involvement of AbcA in mediating the shift from a tolerant to a resistant phenotype has not been investigated.
This study aimed to elucidate the role of the AbcA efflux pump in the development of antibiotic resistance, specifically through the lens of bacterial tolerance. We previously conducted in vitro evolutional experiments with the wildtype Newman. The resultant oxacillin tolerance strain OXA.C13.T5 was used in this study to investigate the role of AbcA in the development of resistance from tolerance. This was achieved through engineering overexpression, knockout, and complement mutants of abcA and monitoring resistance development in these mutants during evolutionary experiments. Our second objective was to uncover how AbcA influenced—if it had an effect—this resistance development by assessing the mutation and survival rates of these abcA mutants.
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Methods
The four tolerant S. aureus strains (Table S1) used in this study were derived from the wildtype Newman strain. These tolerant strains were developed through periodic 5 h exposures to 20× MIC concentrations of different antibiotics—oxacillin, imipenem, flucloxacillin, and meropenem—as detailed in the evolutionary adaptation procedure in the Supplementary Materials. S. aureus strains were grown either in a TSB liquid medium with shaking or on solid TSB agar plates at 37 °C. The cultivation of E. coli was performed using Luria-Bertani (LB) medium under comparable conditions. For long-term preservation, bacterial cultures were combined with glycerol to a final concentration of 50% v/v and stored at −80 °C.
2.2. Construction of AbcA Mutants
The abcA mutants (overexpression and knockout) were created according to the protocol established by Li et al. (2020) [29]. The wildtype Newman strain, with unaltered abcA expression, served as a control. Overexpression was achieved by introducing the pSE1 vector, which carries the abcA gene, into the Newman strain. For the knockout study, abcA was knocked out in the OXA.C13.T5 strains using the pKZ2 vector, resulting in the ∆abcA mutant. Restoration of abcA in the knockout mutant was accomplished using the shuttle plasmids pSC1 containing the abcA gene. More detailed mutant-construction procedures, including vector preparation and the primers used (Table S2), are shown in the Supplementary Materials.
2.3. In Vitro Adaptive Evolution Experiment
The in vitro adaptive evolution strategy was adapted from a previously established protocol [11] and our prior methodology, involving repeated high-dose (20× MIC) exposure of the abcA mutants (overexpression, knockout, and complemented strains) to antibiotics. This cyclic process was performed over 7 to 21 rounds until resistance was detected. Each evolution cycle included the following three phases: antibiotic challenge, drug withdrawal, and recovery growth. Specifically, 20 μL of overnight culture was transferred into 2 mL of TSB containing 20× MIC of the corresponding antibiotic. The culture was incubated at 37 °C with agitation for 5 h, followed by two PBS washes and centrifugation at 1500× g for 20 min to eliminate residual antibiotic. The resulting pellet was resuspended in 2 mL of fresh TSB and cultured overnight. Half of the overnight culture was used to initiate the next evolution cycle, while the remainder—after verifying purity based on colony morphology on TSA—was cryopreserved at −80 °C for downstream analyses.
2.4. Mutation Rate Estimation
Mutation frequency was measured following the procedure described by El Meouche et al. [30], with minor adaptations. Frozen S. aureus stocks were streaked on Mueller–Hinton II (MH2) agar to obtain isolated colonies. Three representative colonies with uniform morphology were selected per strain and inoculated into TSB. After 24 h of incubation, the cultures were serially diluted (107-fold) to reduce the potential impact of pre-existing mutants from the stationary phase. Each colony-derived dilution was used to establish eight replicate cultures, which were incubated overnight at 37 °C. To estimate population density, 5 μL from each replicate was diluted (107–108-fold) and plated onto plain TSB agar. In parallel, 100 μL aliquots were spread onto rifampicin-supplemented agar (100 mg/L). Non-selective plates were incubated for 24 h, and selective plates for 48–72 h, both at 37 °C. Mutation frequency was calculated as the ratio of colony-forming units (CFUs) on the rifampicin plates to those on the drug-free plates.
2.5. Survival Ability Assessment Under Antibiotic Pressure
Overnight S. aureus cultures originating from a single colony were diluted 1:100 into fresh TSB. Antibiotics were then added at concentrations equivalent to 20× MIC. At defined time intervals (12, 24, and 36 h), 500 μL aliquots were withdrawn, serially diluted, and spread onto TSB agar. To eliminate residual antibiotic interference, cells were washed with PBS prior to plating. Plates were incubated for a minimum of 48 h to allow for visible colony formation. Each experiment was performed independently at least three times, with multiple technical replicates, to ensure the robustness and reproducibility of the findings.
2.6. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism (v9.0.0, Mac OS). Statistical significance was assessed via one-way ANOVA, followed by LSD or Dunnett’s T3 multiple comparisons test. Two-tailed p values less than 0.05 were interpreted as statistically significant (*), while values below 0.01 were considered highly significant (**).
3. Results
3.1. Tolerant Strain OXA.C13.T5 Developed Resistance Faster Than the Wildtype Strain Newman
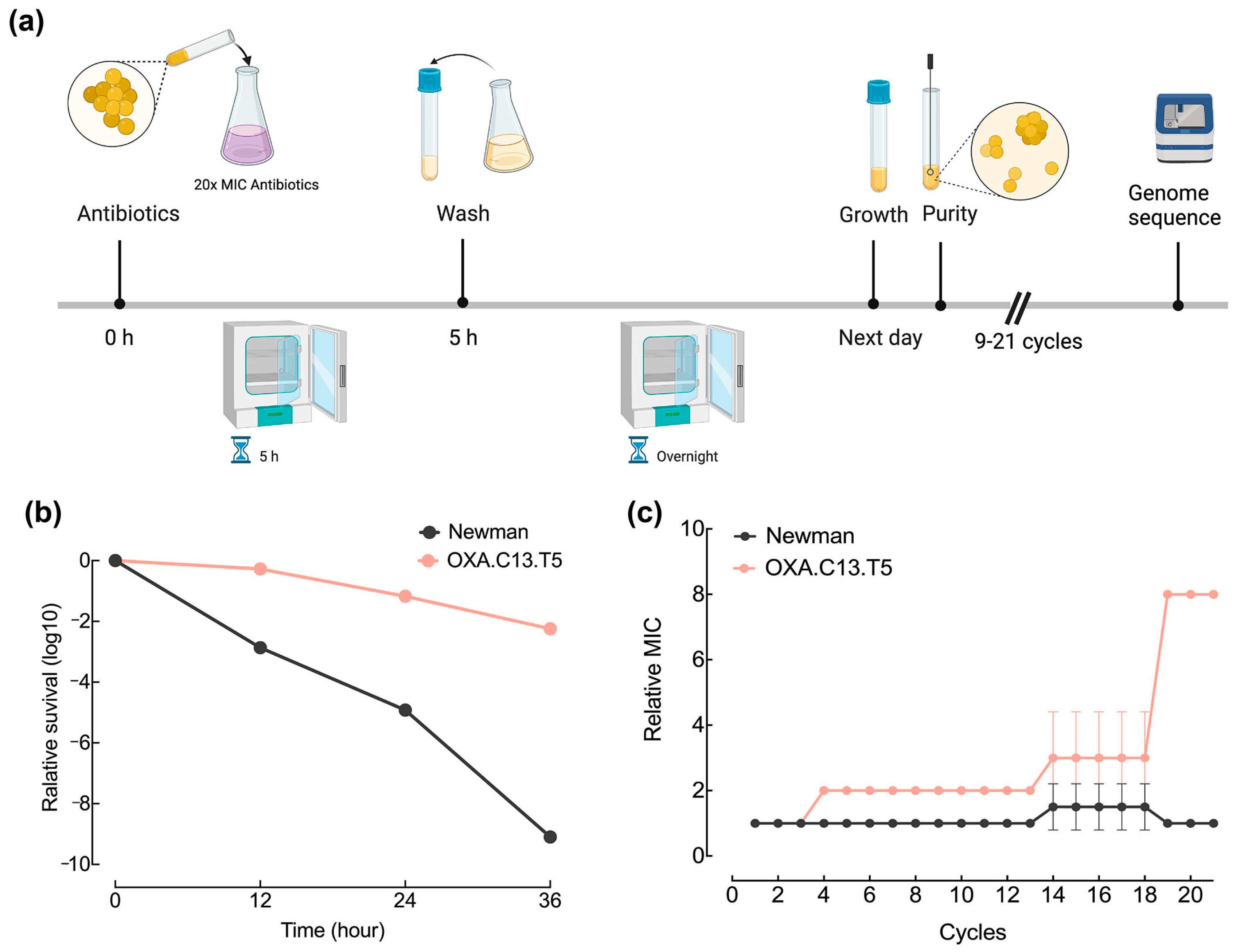
We previously acquired four tolerant strains by the intermittent exposure of the wildtype strain Newman to four β-lactam antibiotics (oxacillin, imipenem, flucloxacillin, and meropenem) (Table S1). All four tolerant strains possessed mutations in genes such as gdpp encoding c-di-AMP phosphodiesterase for OXA.C13.T5, pth encoding peptidyl-tRNA hydrolase for IMI.C9.T5, 545 encoding unknown 545 for FLUC.C20.T5, and map encoding MHC class II analog protein for FLUC.C20.T5. The gdpp mutation was responsible for the tolerance phenotype of the OXA.C13.T5 strain to oxacillin, as the OXA.C13.T5 strain exhibited a characteristic tolerance phenotype with delayed killing under 20× MIC of oxacillin (Figure 1b). On the other hand, a similar killing time was observed between the gdpp-restored OXA.C13.T5 strain and the Newman strain (unpublished data). To illustrate whether the tolerance background impacted the development from tolerance to resistance, the wildtype strain Newman and the tolerant strain OXA.C13.T5 were intermitted challenged with 20× MIC oxacillin for 5 h and continued for 21 cycles (Figure 1a). Changes in the MIC during exposure indicated that the tolerant strain OXA.C13.T5 became resistant against oxacillin at the 19th cycle, while the Newman strain remained susceptible to oxacillin until the 21st cycle (Figure 1c), suggesting that the existence of some factors in tolerant strains contributed to resistance development.

Figure 1.
Tolerant strain OXA.C13.T5 developed oxacillin resistance faster than wildtype Newman. (a) Schematic presentation of intermittent oxacillin challenge cycles for the tolerant strain OXA.C13.T5 and the wildtype Newman strain; (b) Killing kinetics over a 36 h treatment period with 20× MIC oxacillin; viable cells were quantified at 12 h intervals via colony counts. (c) MIC progression in evolved strains, compared to their respective baseline MICs at cycle 0.
3.2. Increased Expression of Efflux Pump AbcA Across Oxacillin Tolerant Strains
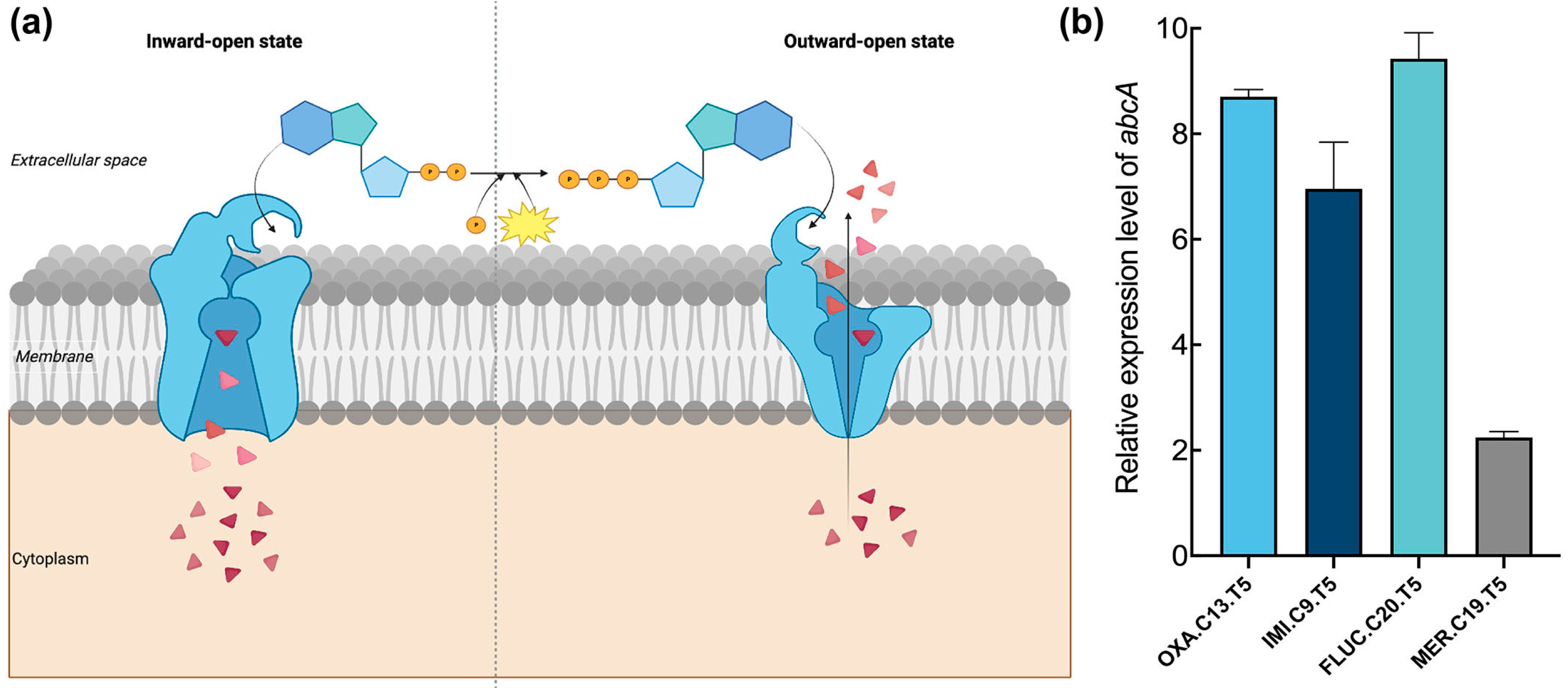
It is well known that ABC transporters contribute to bacterial pathogenesis and virulence, as well as multidrug resistance, through exporting xenobiotics [31]. We investigated whether ABC transporters contribute to the transition from a tolerant phenotype to a resistant one. Therefore, the expression level of abcA, which encodes an ATP-binding cassette (ABC) efflux transporter operating via ATP-driven conformational cycling between inward- and outward-facing states (Figure 2a), was quantified in four tolerant strains using real-time PCR. An elevated relative expression level of abcA was observed in all selected tolerant strains, with increases of over 6-fold for OXA.C13.T5, IMI.C9.T5, and FLUC.C20.T5 strains relative to Newman (Figure 2b). Although FLUC.C20.T5 showed the least change, its relative expression level of abcA still increased two-fold. The increased expression of abcA across the tolerant strains suggests that it may play an important role in promoting the development of resistance from tolerance.

Figure 2.
Expression of abcA in tolerant strains. (a) ABC efflux pumps extrude antibiotics by changing inward-open state to outward-open state driven by ATP. The left part is the inward-open state, which changes into the outward-open state (right part) after acquiring ATP. As the arrow shows, this transformation changes pumps antibiotics out from the inside to the outside. Red triangles represent antibiotic molecules, orange circles represent ATP, and the yellow burst indicates the energy release that drives the conformational change. (b) The relative expression level of abcA. Each tolerant strain’s expression is relative to the expression level of the wildtype strain.
3.3. Increased Expression of AbcA Accelerated Resistance Development Against Oxacillin
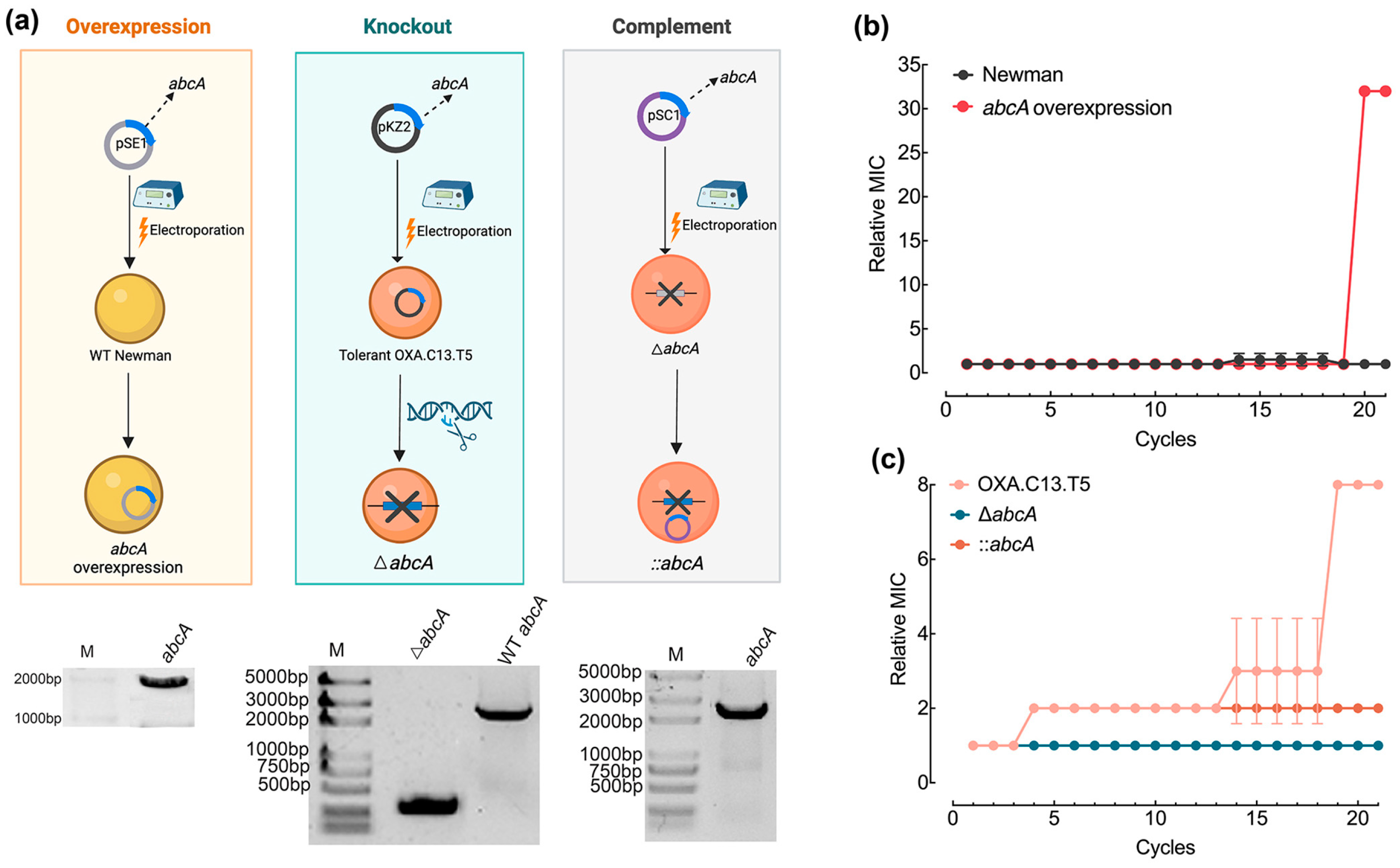
To assess whether elevated abcA expression facilitates the transition from tolerance to resistance, the gene was introduced into the wildtype Newman strain to generate an overexpression mutant (Figure 3a). Meanwhile, abcA was deleted from the tolerant OXA.C13.T5 background, and a complementation strain was also established (Figure 3a). All constructed mutants underwent 21 cycles of oxacillin exposure at 20× MIC, following the protocol outlined in Figure 1a. abcA overexpression enabled the wildtype Newman strain to acquire oxacillin resistance by the 20th cycle, reaching a relative MIC of 30, whereas the control strain remained susceptible throughout (Figure 3b). In contrast, the knockout of abcA in the tolerant OXA.C13.T5 background prevented further resistance acquisition, and the strain retained susceptibility (Figure 3c). Additionally, the abcA complement failed to restore the development of oxacillin resistance (Figure 3c). These results collectively confirm that increased abcA expression facilitated resistance development against oxacillin.

Figure 3.
Effect of abcA overexpression and deficiency on resistance development. (a) Experiment scheme and gel electrophoresis confirmation for constructing abcA mutants. (b) Relative MIC dynamics for the abcA-overexpressing strain (red) versus wildtype Newman (gray). (c) Resistance development of abcA knockout strain (blue line), tolerant strain (salmon lines), and abcA complementary strain (tangerine line). Relative MIC represents the MIC value of each cycle relative to that of the first cycle.
3.4. Elevated AbcA Expression Increased the Emergence of Resistance Mutations
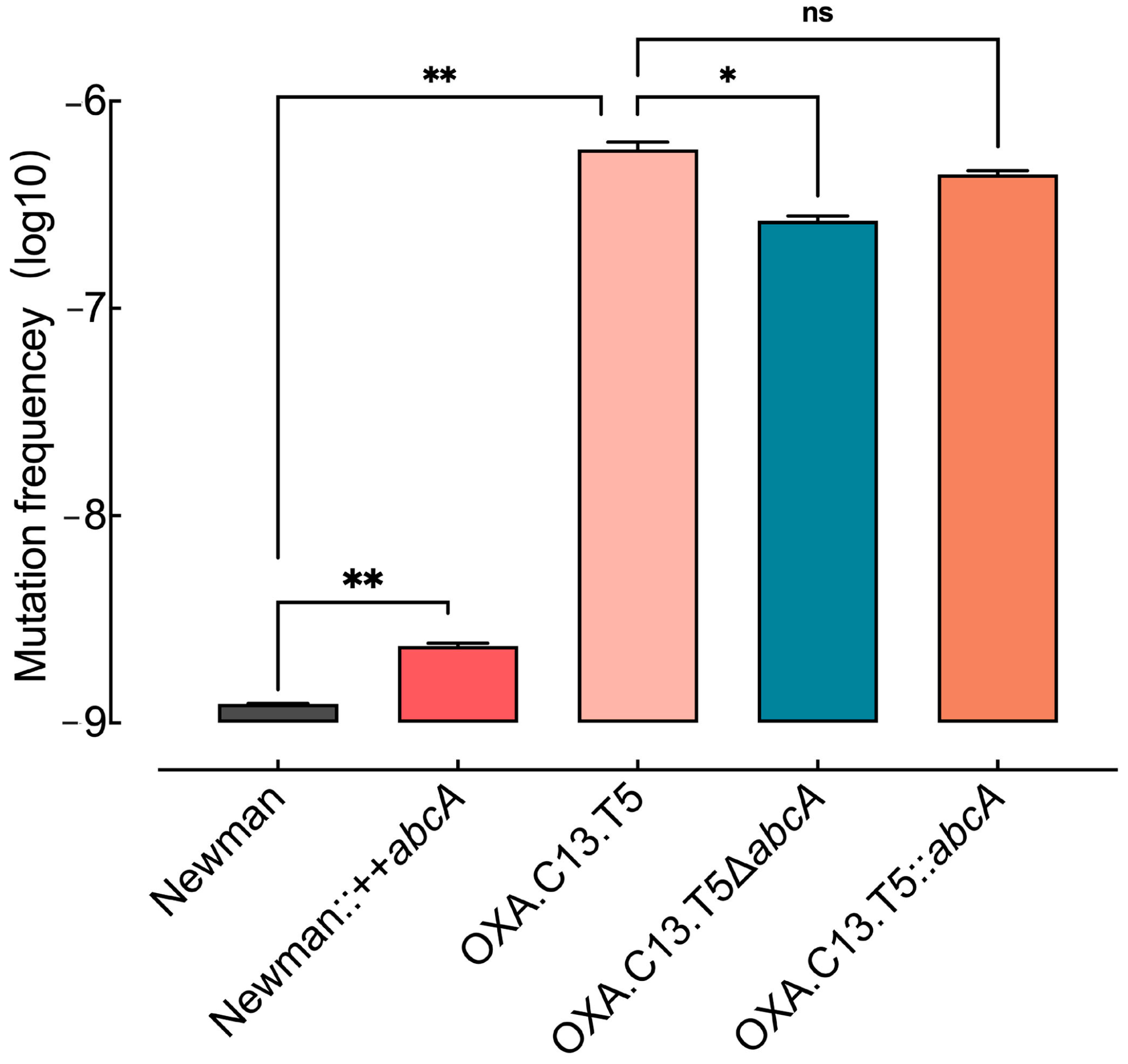
To understand how the increased abcA expression facilitated the development of resistance against oxacillin, we hypothesized that increased mutation rates might promote emergence of resistance mutation. To test this hypothesis, spontaneous mutation frequencies were assessed across different abcA mutants. As illustrated in Figure 4, the abcA overexpression strain exhibited a markedly higher mutation frequency than the control, whereas the knockout of abcA led to a notable decrease relative to the tolerant background. The reintroduction of abcA restored the mutation rate to levels comparable with the parental tolerant strain. These findings suggest that the upregulation of abcA promotes mutational emergence, thereby increasing the likelihood of resistance-conferring variants.

Figure 4.
Random mutation rates of either abcA overexpression, knockout, and complement mutants. Random mutation rates were evaluated across abcA-related mutants, including the wildtype, overexpression, knockout, and complemented strains, as well as the tolerant OXA.C13.T5. Statistical comparisons are based on ≥3 independent experiments. Error bars indicate standard deviation. ns not significant, * p < 0.05, ** p < 0.01.
3.5. Elevated AbcA Expression Increased Bacterial Survival Ability
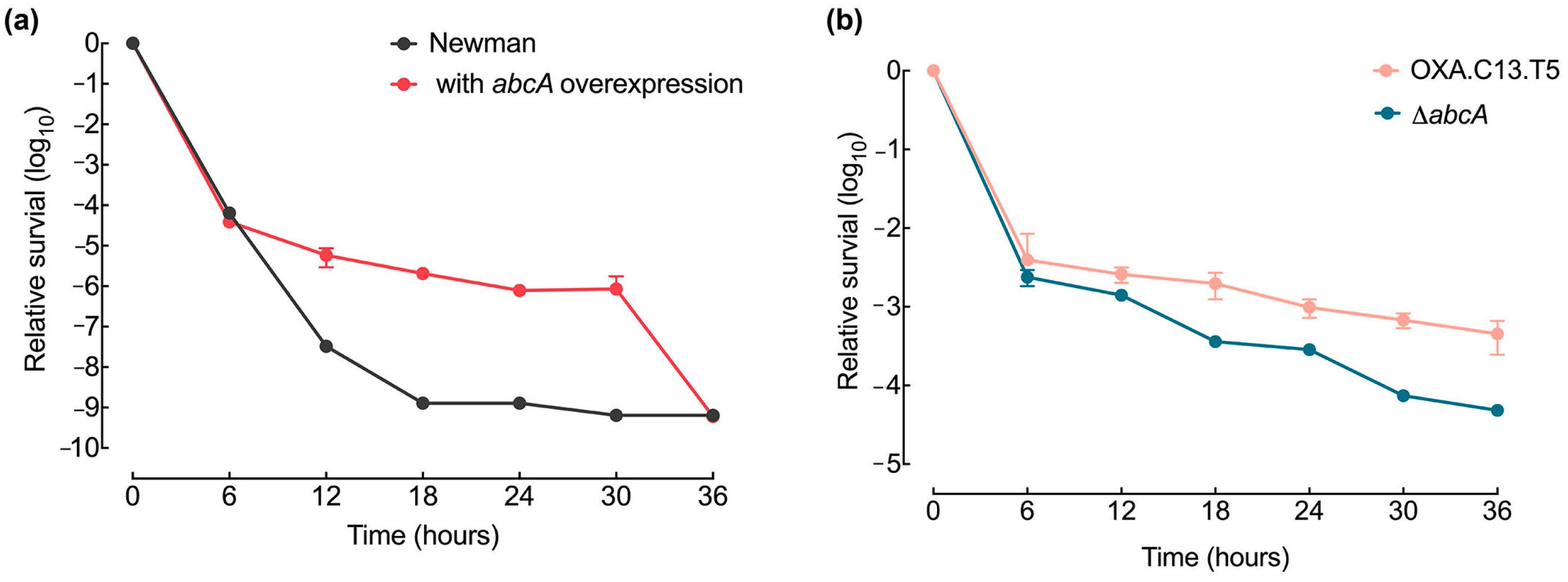
Even if resistance mutation emerged, they would disappear without robust survival capacities. To assess whether elevated abcA expression enhances bacterial persistence, we tracked the viability of the abcA mutant strains during a 36 h exposure to oxacillin at 20× MIC. The overexpression of abcA led to a higher survival ability than the wildtype strain under the oxacillin treatment (Figure 5a), while the abcA knockout strain showed a decrease in relative survival compared to the control strain OXA.C13.T5 (Figure 5b). These results demonstrate that elevated abcA expression increased survival capacities, ensuring the spread of the emerging resistance mutations throughout the population.
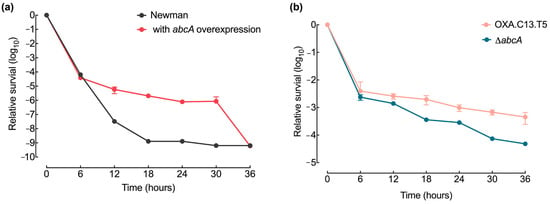
Figure 5.
Effect of abcA mutants on survival ability. (a) Time-dependent survival profile of the abcA-overexpressing strain (red), compared with the control Newman strain (gray). CFU values were normalized to initial values. (b) Survival curves of the tolerant OXA.C13.T5 (salmon) and abcA knockout mutant (blue) during exposure to oxacillin at 20× MIC. Data represent mean ± standard deviation from a minimum of three independent experiments.
4. Discussion
Tolerance (including persistence) and resistance are distinct strategies by which bacteria withstand antibiotic stress. Both strategies reduce antibiotic efficacy, but how tolerance promotes resistance remains incompletely understood. Levin-Reisman et al. [32] showed that tolerance can synergize with resistance mutations to enhance survival, yet the progression from tolerance to resistance is still poorly characterized. In this study, we explored this transition, highlighting the role of the efflux pump abcA. The tolerant strain OXA.C13.T5 acquired oxacillin resistance earlier than the wildtype Newman (Figure 1), suggesting that tolerance background accelerates resistance development. This observation was consistent across S. aureus and E. coli [11,33] and under different treatment conditions, including intermittent β-lactam exposure [19] and drug combinations [34], where tolerance mutations preceded resistance acquisition. These findings support a broader model in which tolerance facilitates resistance evolution, posing a clinical threat by potentially leading to the ultimate failure of antibiotics and underscoring the need to disrupt this trajectory through targeted interventions.
Although tolerance has been linked to the emergence of resistance, the molecular drivers underlying this transition remain unclear. Our findings identify the efflux pump abcA as a key factor mediating this progression. AbcA transporters are implicated in diverse physiological roles across organisms, including multidrug resistance in cancer [35,36]. In S. aureus, AbcA contributes both to β-lactam resistance and virulence via cytolytic toxin secretion [24,27]. Here, we propose a the following third role: facilitating the shift from tolerance—a major cause of recurrent infection—to full resistance, thereby compromising antibiotic efficacy. We observed significantly elevated abcA expression in tolerant strains (OXA.C13.T5, IMI.C9.T5, and FLUC.C20.T5) compared to the wildtype strain (Figure 2b), consistent with the findings of Pu et al. (2016), where there was increased efflux gene expression in E. coli persisters [12]. These findings support a role for efflux systems in establishing tolerance under β-lactam exposure. Furthermore, previous studies on norA and tolC have shown that the deletion of efflux components reduces tolerance [16,30], while norA upregulation accelerates ciprofloxacin resistance [16,37]. Based on these observations, we hypothesized that abcA facilitates the tolerance-to-resistance transition. This was supported by our data showing faster resistance emergence in abcA-overexpressing strains and the complete failure of resistance acquisition in abcA knockout strains (Figure 3b,c). Parallel patterns observed in norA mutants suggest that efflux-mediated transitions may represent a broader adaptive mechanism across efflux families.
Our findings indicate that tolerance promotes the evolution of antibiotic resistance by enhancing both survival and mutation rates. Repeated oxacillin exposure in the wildtype Newman strain led to tolerance but not resistance (Figure 1b). The whole-genome sequencing of the tolerant OXA.C13.T5 strain revealed a single mutation in gdpp, unrelated to survival or mutagenesis (Table S1). Functional comparisons showed that abcA overexpression significantly increased mutation frequency and survival under oxacillin treatment (Figure 4 and Figure 5), while knockout impaired both. These results suggest that abcA facilitates resistance evolution by simultaneously improving the likelihood of resistance mutations and supporting the survival of emerging mutants. Notably, although abcA complementation restored mutation frequency, it did not result in resistance acquisition. This indicates that while elevated mutability increases the chance of resistance mutations, it is insufficient to establish the resistance phenotype. In particular, plasmid-based abcA expression may lack the stress-inducible regulation necessary for resistance development and fixation under intermittent antibiotic exposure. Mechanistically, our results align with Windels et al.’s model in E. coli, which proposed that enhanced survival and mutation jointly drive resistance [17]. We extend this concept by demonstrating that the abcA-mediated increase in both traits enables the transition from tolerance to resistance. Similar trends observed with norA [16] and elevated abcA expression in multiple tolerant strains (Figure 2b) suggest a broader role for efflux pumps. Furthermore, our intermittent antibiotic exposure model reflects clinical treatment regimens [38], supporting the translational relevance of abcA-driven adaptation.
To our knowledge, this study is the first to demonstrate that abcA plays a direct role in promoting oxacillin resistance from a tolerant state. While prior research has broadly linked tolerance to resistance, our findings uniquely identify AbcA as a mechanistic driver of this transition. This insight presents AbcA as a potential therapeutic target. For example, combining antibiotics with AbcA inhibitors could reduce resistance emergence and improve treatment efficacy. Such a strategy may enable both simultaneous bacterial clearance and suppression of resistance development. These results also underscore the importance of monitoring efflux pump activity as part of resistance management in clinical settings and aligning with the antibiotic stewardship principles that advocate for informed drug use. To model clinical exposure, we employed intermittent exposure to 20× MIC oxacillin based on the established in vitro evolution protocols [11,19]. Although 20× MIC exceeds the standard plasma levels, such concentrations are achievable in local administration settings. For instance, cefazolin irrigation during surgery produces wound site concentrations > 4000 µg/mL [39], and the intramammary infusion of cloxacillin can maintain concentrations > 30 µg/mL in the milk for days [40]. These local exposures impose strong selective pressure, supporting the clinical relevance of our experimental model. Despite the robustness of our in vitro findings, limitations remain. The simplified laboratory context cannot fully replicate host environments, and further in vivo validation is needed. Future research should also explore whether other efflux systems share this tolerance-to-resistance bridging function. Understanding such mechanisms may inform broader strategies to delay or prevent resistance evolution.
5. Conclusions
In conclusion, our data map an evolutionary pathway from tolerance to resistance, demonstrating that the increased expression of efflux pump abcA not only accelerated the generation of resistance mutations but also enhanced the survival of resistant mutants. This comprehensive understanding of the evolution from tolerance to resistance is crucial for developing more effective antibiotic treatment strategies for combating the growing challenge of antibiotic inefficiency due to the emergence of resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13051140/s1, Table S1: Staphylococcus aureus strains used in this study; Table S2: Primers used in this study.
Author Contributions
X.Y. and X.Z designed the study; M.L., L.Z. and P.L. contributed to collecting data; X.Y. and Z.H. analyzed and interpreted the data; X.Y. drafted the article; and X.Z. critically revised the draft paper. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partially supported by Natural Sciences and Engineering Research Council of Canada [RGPIN-2022-03884].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| MIC | Minimum Inhibitory Concentration |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- WHO. WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; p. 655. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Laumen, J.G.E.; Van Dijck, C.; Manoharan-Basil, S.S.; Abdellati, S.; De Baetselier, I.; Cuylaerts, V.; De Block, T.; Van den Bossche, D.; Xavier, B.B.; Malhotra-Kumar, S.; et al. Sub-Inhibitory Concentrations of Chlorhexidine Induce Resistance to Chlorhexidine and Decrease Antibiotic Susceptibility in Neisseria Gonorrhoeae. Front. Microbiol. 2021, 12, 776909. [Google Scholar] [CrossRef] [PubMed]
- Trampari, E.; Holden, E.R.; Wickham, G.J.; Ravi, A.; Martins, L.d.O.; Savva, G.M.; Webber, M.A. Exposure of Salmonella Biofilms to Antibiotic Concentrations Rapidly Selects Resistance with Collateral Tradeoffs. Npj Biofilms Microbiomes 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Cai, Y.; Wang, Z.; Li, G.; An, T. Sub-Lethal Photocatalysis Promotes Horizontal Transfer of Antibiotic Resistance Genes by Conjugation and Transformability. Water Res. 2022, 221, 118808. [Google Scholar] [CrossRef]
- Li, X.; Gu, A.Z.; Zhang, Y.; Xie, B.; Li, D.; Chen, J. Sub-Lethal Concentrations of Heavy Metals Induce Antibiotic Resistance via Mutagenesis. J. Hazard. Mater. 2019, 369, 9–16. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of Lag Time Underlies Antibiotic Tolerance in Evolved Bacterial Populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A.B.; et al. Enhanced Efflux Activity Facilitates Drug Tolerance in Dormant Bacterial Cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef]
- Helaine, S.; Conlon, B.P.; Davis, K.M.; Russell, D.G. Host Stress Drives Tolerance and Persistence: The Bane of Anti-Microbial Therapeutics. Cell Host Microbe 2024, 32, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Deventer, A.T.; Stevens, C.E.; Stewart, A.; Hobbs, J.K. Antibiotic Tolerance among Clinical Isolates: Mechanisms, Detection, Prevalence, and Significance. Clin. Microbiol. Rev. 2024, 37, e0010624. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Hao, Z.H.; Liu, P.L.; Liu, M.M.; Zhao, L.L.; Zhao, X. Increased Expression of Efflux Pump norA Drives the Rapid Evolutionary Trajectory from Tolerance to Resistance against Ciprofloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2022, 66, e00594-22. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; Fauvart, M.; Wenseleers, T.; Van den Bergh, B.; Michiels, J. Bacterial Persistence Promotes the Evolution of Antibiotic Resistance by Increasing Survival and Mutation Rates. ISME J. 2019, 13, 1239–1251. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic Tolerance Facilitates the Evolution of Resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Michiels, J.E.; Wenseleers, T.; Windels, E.M.; Boer, P.V.; Kestemont, D.; De Meester, L.; Verstrepen, K.J.; Verstraeten, N.; Fauvart, M.; et al. Frequency of Antibiotic Application Drives Rapid Evolutionary Adaptation of Escherichia Coli Persistence. Nat. Microbiol. 2016, 1, 16020. [Google Scholar] [CrossRef]
- Barrett, T.C.; Mok, W.W.K.; Murawski, A.M.; Brynildsen, M.P. Enhanced Antibiotic Resistance Development from Fluoroquinolone Persisters After a Single Exposure to Antibiotic. Nat. Commun. 2019, 10, 1177. [Google Scholar] [CrossRef]
- McGowen, K.; Funck, T.; Wang, X.; Zinga, S.; Wolf, I.D.; Akusobi, C.; Denkinger, C.M.; Rubin, E.J.; Sullivan, M.R. Efflux Pumps and Membrane Permeability Contribute to Intrinsic Antibiotic Resistance in Mycobacterium abscessus. PLoS Pathog. 2025, 21, e1013027. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Domanski, T.L.; Bayles, K.W. Analysis of Staphylococcus aureus Genes Encoding Penicillin-Binding Protein 4 and an ABC-Type Transporter. Gene 1995, 167, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Jang, S. Multidrug Efflux Pumps in Staphylococcus aureus and Their Clinical Implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schrader-Fischer, G.; Berger-Bächi, B. The AbcA Transporter of Staphylococcus aureus Affects Cell Autolysis. Antimicrob. Agents Chemother. 2001, 45, 407–412. [Google Scholar] [CrossRef]
- Yoshikai, H.; Kizaki, H.; Saito, Y.; Omae, Y.; Sekimizu, K.; Kaito, C. Multidrug-Resistance Transporter AbcA Secretes Staphylococcus aureus Cytolytic Toxins. J. Infect. Dis. 2016, 213, 295–304. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Wang, Y.; Ferrer-Espada, R.; Reedy, J.L.; Martens, A.T.; Goulev, Y.; Paulsson, J.; Vyas, J.M.; Hooper, D.C. Staphylococcus aureus AbcA Transporter Enhances Persister Formation under β-Lactam Exposure. Antimicrob. Agents Chemother. 2024, 68, e0134023. [Google Scholar] [CrossRef]
- Li, F.; Zhai, D.; Wu, Z.; Zhao, Y.; Qiao, D.; Zhao, X. Impairment of the Cell Wall Ligase, LytR-CpsA-Psr Protein (LcpC), in Methicillin Resistant Staphylococcus aureus Reduces Its Resistance to Antibiotics and Infection in a Mouse Model of Sepsis. Front. Microbiol. 2020, 11, 557. [Google Scholar] [CrossRef]
- El Meouche, I.; Dunlop, M.J. Heterogeneity in Efflux Pump Expression Predisposes Antibiotic-Resistant Cells to Mutation. Science 2018, 362, 686–690. [Google Scholar] [CrossRef]
- Akhtar, A.A.; Turner, D.P.J. The Role of Bacterial ATP-Binding Cassette (ABC) Transporters in Pathogenesis and Virulence: Therapeutic and Vaccine Potential. Microb. Pathog. 2022, 171, 105734. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between Antibiotic Tolerance, Persistence, and Resistance Mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef]
- Mechler, L.; Herbig, A.; Paprotka, K.; Fraunholz, M.; Nieselt, K.; Bertram, R. A Novel Point Mutation Promotes Growth Phase-Dependent Daptomycin Tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of Tolerance on the Evolution of Antibiotic Resistance Under Drug Combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC Transporters: From Microorganisms to Man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC Subfamily A Transporters: Multifaceted Players with Incipient Potentialities in Cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux Pump Activity Potentiates the Evolution of Antibiotic Resistance across S. aureus Isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef]
- Santi, I.; Manfredi, P.; Maffei, E.; Egli, A.; Jenal, U. Evolution of Antibiotic Tolerance Shapes Resistance Development in Chronic Pseudomonas aeruginosa Infections. mBio 2021, 12, e03482-20. [Google Scholar] [CrossRef]
- White, R.R.; Pitzer, K.D.; Fader, R.C.; Rajab, M.H.; Song, J. Pharmacokinetics of Topical and Intravenous Cefazolin in Patients with Clean Surgical Wounds. Plast. Reconstr. Surg. 2008, 122, 1773–1779. [Google Scholar] [CrossRef]
- McDougall, S.; Clausen, L.M.; Hussein, H.M.; Compton, C.W.R. Therapy of Subclinical Mastitis during Lactation. Antibiotics 2022, 11, 209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).