Increased Expression of AbcA Efflux Pump Accelerated Resistance Development from Tolerance to Resistance Against Oxacillin in Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Methods
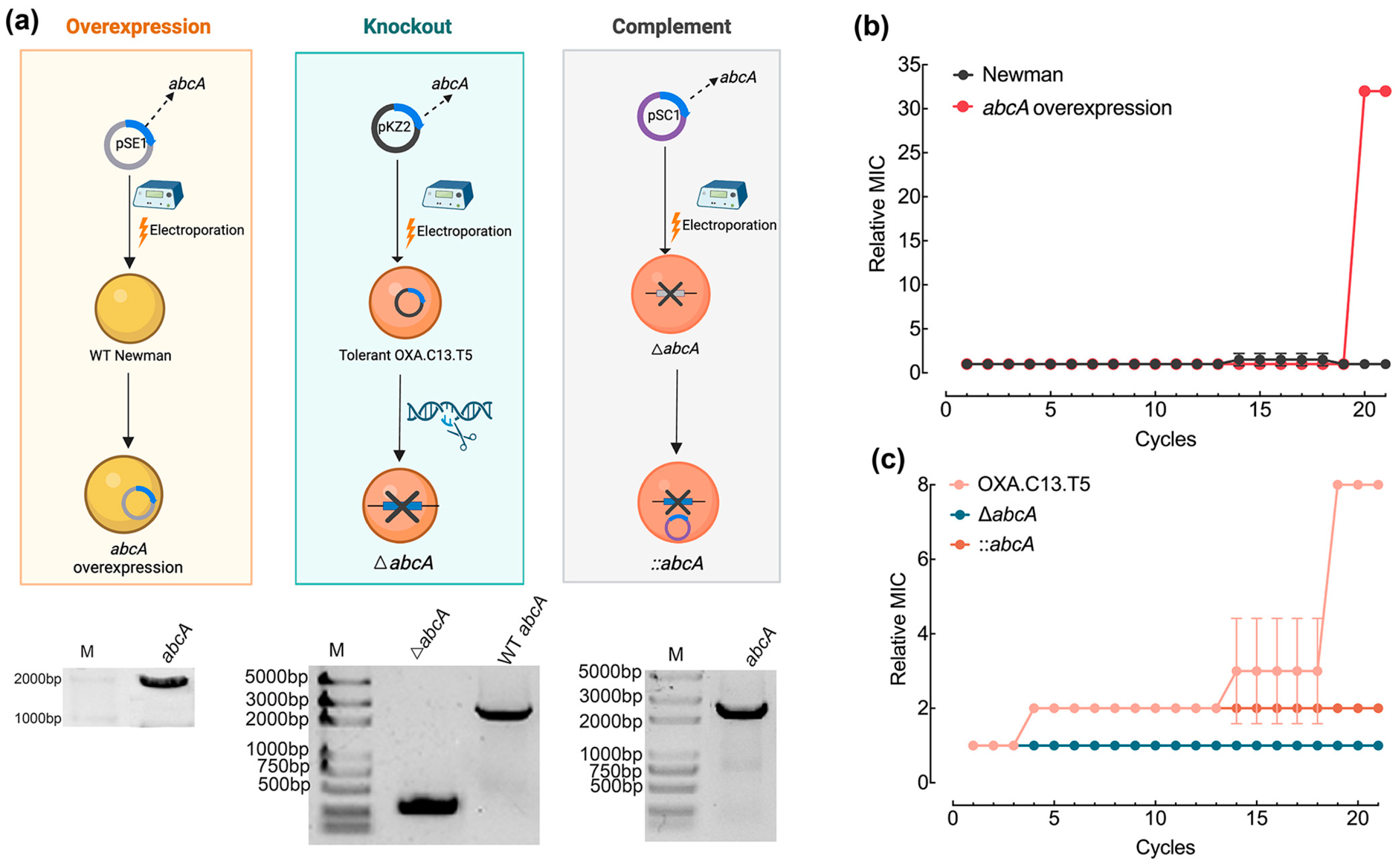
2.2. Construction of AbcA Mutants
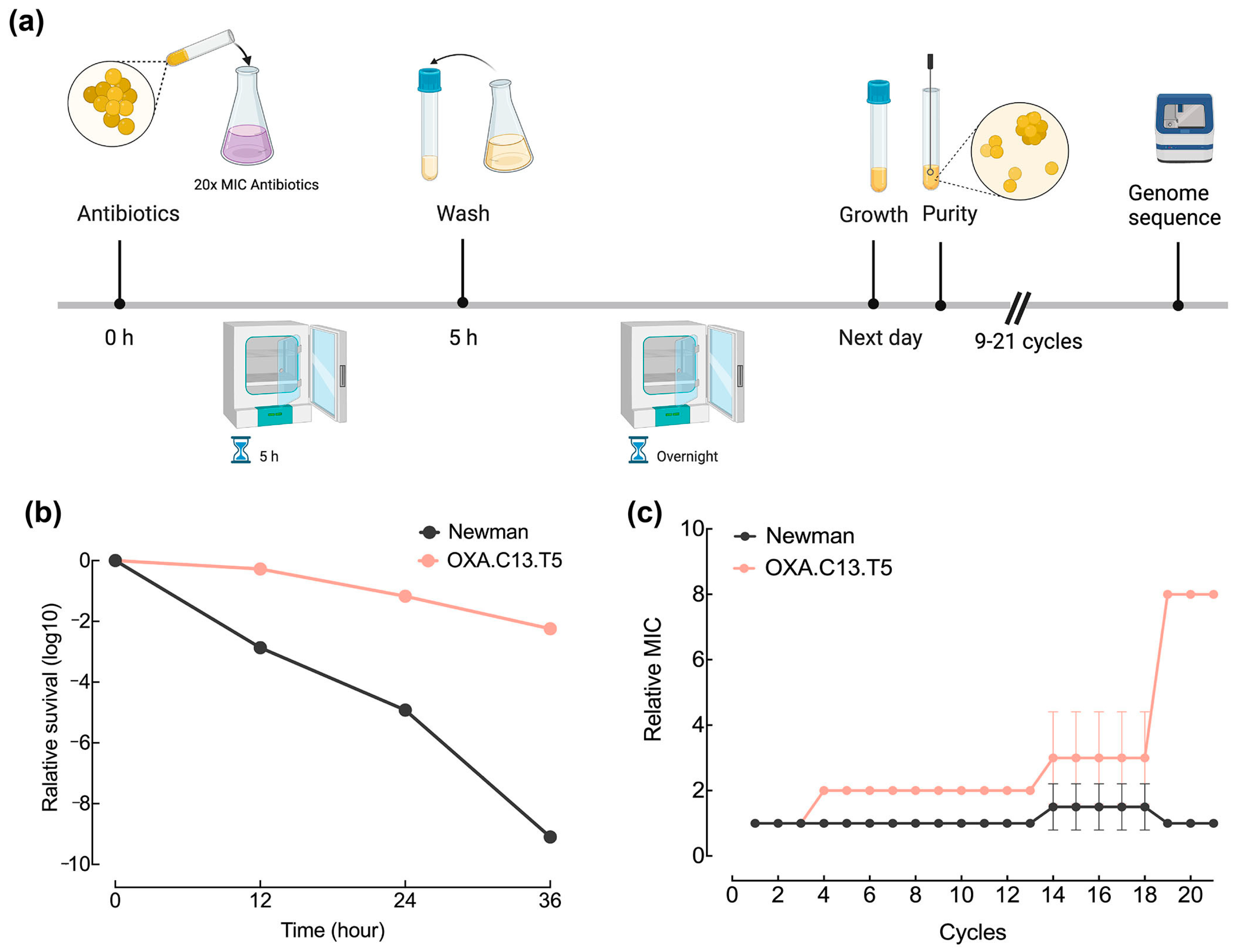
2.3. In Vitro Adaptive Evolution Experiment
2.4. Mutation Rate Estimation
2.5. Survival Ability Assessment Under Antibiotic Pressure
2.6. Statistical Analysis
3. Results
3.1. Tolerant Strain OXA.C13.T5 Developed Resistance Faster Than the Wildtype Strain Newman
3.2. Increased Expression of Efflux Pump AbcA Across Oxacillin Tolerant Strains
3.3. Increased Expression of AbcA Accelerated Resistance Development Against Oxacillin
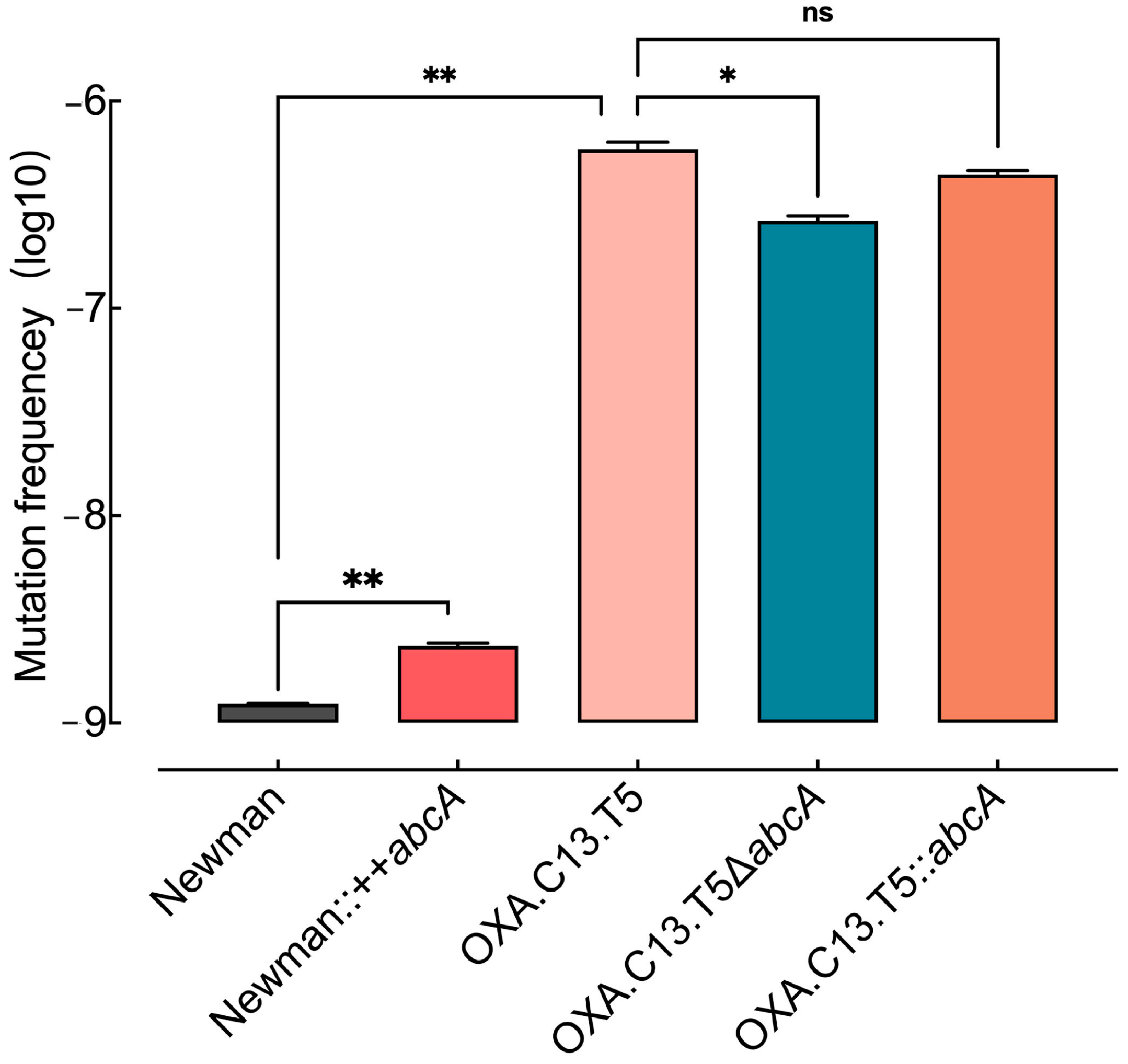
3.4. Elevated AbcA Expression Increased the Emergence of Resistance Mutations
3.5. Elevated AbcA Expression Increased Bacterial Survival Ability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| MIC | Minimum Inhibitory Concentration |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- WHO. WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; p. 655. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Laumen, J.G.E.; Van Dijck, C.; Manoharan-Basil, S.S.; Abdellati, S.; De Baetselier, I.; Cuylaerts, V.; De Block, T.; Van den Bossche, D.; Xavier, B.B.; Malhotra-Kumar, S.; et al. Sub-Inhibitory Concentrations of Chlorhexidine Induce Resistance to Chlorhexidine and Decrease Antibiotic Susceptibility in Neisseria Gonorrhoeae. Front. Microbiol. 2021, 12, 776909. [Google Scholar] [CrossRef] [PubMed]
- Trampari, E.; Holden, E.R.; Wickham, G.J.; Ravi, A.; Martins, L.d.O.; Savva, G.M.; Webber, M.A. Exposure of Salmonella Biofilms to Antibiotic Concentrations Rapidly Selects Resistance with Collateral Tradeoffs. Npj Biofilms Microbiomes 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Cai, Y.; Wang, Z.; Li, G.; An, T. Sub-Lethal Photocatalysis Promotes Horizontal Transfer of Antibiotic Resistance Genes by Conjugation and Transformability. Water Res. 2022, 221, 118808. [Google Scholar] [CrossRef]
- Li, X.; Gu, A.Z.; Zhang, Y.; Xie, B.; Li, D.; Chen, J. Sub-Lethal Concentrations of Heavy Metals Induce Antibiotic Resistance via Mutagenesis. J. Hazard. Mater. 2019, 369, 9–16. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of Lag Time Underlies Antibiotic Tolerance in Evolved Bacterial Populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A.B.; et al. Enhanced Efflux Activity Facilitates Drug Tolerance in Dormant Bacterial Cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef]
- Helaine, S.; Conlon, B.P.; Davis, K.M.; Russell, D.G. Host Stress Drives Tolerance and Persistence: The Bane of Anti-Microbial Therapeutics. Cell Host Microbe 2024, 32, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Deventer, A.T.; Stevens, C.E.; Stewart, A.; Hobbs, J.K. Antibiotic Tolerance among Clinical Isolates: Mechanisms, Detection, Prevalence, and Significance. Clin. Microbiol. Rev. 2024, 37, e0010624. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Hao, Z.H.; Liu, P.L.; Liu, M.M.; Zhao, L.L.; Zhao, X. Increased Expression of Efflux Pump norA Drives the Rapid Evolutionary Trajectory from Tolerance to Resistance against Ciprofloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2022, 66, e00594-22. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; Fauvart, M.; Wenseleers, T.; Van den Bergh, B.; Michiels, J. Bacterial Persistence Promotes the Evolution of Antibiotic Resistance by Increasing Survival and Mutation Rates. ISME J. 2019, 13, 1239–1251. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic Tolerance Facilitates the Evolution of Resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Michiels, J.E.; Wenseleers, T.; Windels, E.M.; Boer, P.V.; Kestemont, D.; De Meester, L.; Verstrepen, K.J.; Verstraeten, N.; Fauvart, M.; et al. Frequency of Antibiotic Application Drives Rapid Evolutionary Adaptation of Escherichia Coli Persistence. Nat. Microbiol. 2016, 1, 16020. [Google Scholar] [CrossRef]
- Barrett, T.C.; Mok, W.W.K.; Murawski, A.M.; Brynildsen, M.P. Enhanced Antibiotic Resistance Development from Fluoroquinolone Persisters After a Single Exposure to Antibiotic. Nat. Commun. 2019, 10, 1177. [Google Scholar] [CrossRef]
- McGowen, K.; Funck, T.; Wang, X.; Zinga, S.; Wolf, I.D.; Akusobi, C.; Denkinger, C.M.; Rubin, E.J.; Sullivan, M.R. Efflux Pumps and Membrane Permeability Contribute to Intrinsic Antibiotic Resistance in Mycobacterium abscessus. PLoS Pathog. 2025, 21, e1013027. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Domanski, T.L.; Bayles, K.W. Analysis of Staphylococcus aureus Genes Encoding Penicillin-Binding Protein 4 and an ABC-Type Transporter. Gene 1995, 167, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Jang, S. Multidrug Efflux Pumps in Staphylococcus aureus and Their Clinical Implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schrader-Fischer, G.; Berger-Bächi, B. The AbcA Transporter of Staphylococcus aureus Affects Cell Autolysis. Antimicrob. Agents Chemother. 2001, 45, 407–412. [Google Scholar] [CrossRef]
- Yoshikai, H.; Kizaki, H.; Saito, Y.; Omae, Y.; Sekimizu, K.; Kaito, C. Multidrug-Resistance Transporter AbcA Secretes Staphylococcus aureus Cytolytic Toxins. J. Infect. Dis. 2016, 213, 295–304. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Wang, Y.; Ferrer-Espada, R.; Reedy, J.L.; Martens, A.T.; Goulev, Y.; Paulsson, J.; Vyas, J.M.; Hooper, D.C. Staphylococcus aureus AbcA Transporter Enhances Persister Formation under β-Lactam Exposure. Antimicrob. Agents Chemother. 2024, 68, e0134023. [Google Scholar] [CrossRef]
- Li, F.; Zhai, D.; Wu, Z.; Zhao, Y.; Qiao, D.; Zhao, X. Impairment of the Cell Wall Ligase, LytR-CpsA-Psr Protein (LcpC), in Methicillin Resistant Staphylococcus aureus Reduces Its Resistance to Antibiotics and Infection in a Mouse Model of Sepsis. Front. Microbiol. 2020, 11, 557. [Google Scholar] [CrossRef]
- El Meouche, I.; Dunlop, M.J. Heterogeneity in Efflux Pump Expression Predisposes Antibiotic-Resistant Cells to Mutation. Science 2018, 362, 686–690. [Google Scholar] [CrossRef]
- Akhtar, A.A.; Turner, D.P.J. The Role of Bacterial ATP-Binding Cassette (ABC) Transporters in Pathogenesis and Virulence: Therapeutic and Vaccine Potential. Microb. Pathog. 2022, 171, 105734. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between Antibiotic Tolerance, Persistence, and Resistance Mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef]
- Mechler, L.; Herbig, A.; Paprotka, K.; Fraunholz, M.; Nieselt, K.; Bertram, R. A Novel Point Mutation Promotes Growth Phase-Dependent Daptomycin Tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of Tolerance on the Evolution of Antibiotic Resistance Under Drug Combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC Transporters: From Microorganisms to Man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC Subfamily A Transporters: Multifaceted Players with Incipient Potentialities in Cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux Pump Activity Potentiates the Evolution of Antibiotic Resistance across S. aureus Isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef]
- Santi, I.; Manfredi, P.; Maffei, E.; Egli, A.; Jenal, U. Evolution of Antibiotic Tolerance Shapes Resistance Development in Chronic Pseudomonas aeruginosa Infections. mBio 2021, 12, e03482-20. [Google Scholar] [CrossRef]
- White, R.R.; Pitzer, K.D.; Fader, R.C.; Rajab, M.H.; Song, J. Pharmacokinetics of Topical and Intravenous Cefazolin in Patients with Clean Surgical Wounds. Plast. Reconstr. Surg. 2008, 122, 1773–1779. [Google Scholar] [CrossRef]
- McDougall, S.; Clausen, L.M.; Hussein, H.M.; Compton, C.W.R. Therapy of Subclinical Mastitis during Lactation. Antibiotics 2022, 11, 209. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Liu, M.; Liu, P.; Hao, Z.; Zhao, L.; Zhao, X. Increased Expression of AbcA Efflux Pump Accelerated Resistance Development from Tolerance to Resistance Against Oxacillin in Staphylococcus aureus. Microorganisms 2025, 13, 1140. https://doi.org/10.3390/microorganisms13051140
Yu X, Liu M, Liu P, Hao Z, Zhao L, Zhao X. Increased Expression of AbcA Efflux Pump Accelerated Resistance Development from Tolerance to Resistance Against Oxacillin in Staphylococcus aureus. Microorganisms. 2025; 13(5):1140. https://doi.org/10.3390/microorganisms13051140
Chicago/Turabian StyleYu, Xiaohui, Miaomiao Liu, Pilong Liu, Zehua Hao, Lili Zhao, and Xin Zhao. 2025. "Increased Expression of AbcA Efflux Pump Accelerated Resistance Development from Tolerance to Resistance Against Oxacillin in Staphylococcus aureus" Microorganisms 13, no. 5: 1140. https://doi.org/10.3390/microorganisms13051140
APA StyleYu, X., Liu, M., Liu, P., Hao, Z., Zhao, L., & Zhao, X. (2025). Increased Expression of AbcA Efflux Pump Accelerated Resistance Development from Tolerance to Resistance Against Oxacillin in Staphylococcus aureus. Microorganisms, 13(5), 1140. https://doi.org/10.3390/microorganisms13051140








