Irritable Bowel Syndrome with Diarrhea (IBS-D): Effects of Clostridium butyricum CBM588 Probiotic on Gastrointestinal Symptoms, Quality of Life, and Gut Microbiota in a Prospective Real-Life Interventional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Primary Outcomes
2.3. Secondary Outcomes
2.4. Participants and Criteria
2.5. Clostridium butyricum CBM588 Probiotic Treatment
2.6. Sample Size Calculation and Comparative Analysis with a Differently Treated Cohort
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features at Enrollment
3.2. Effect of C. butyricum CBM588 Probiotic Treatment on Primary Clinical IBS-D Outcome
3.3. Treatment Effect on IBS Principal Components
3.4. Effect on Tolerability and Adherence
3.5. Effect of C. butyricum CBM588 on Gut Microbiota Structure
3.6. Correlation Between the Primary Clinical Outcomes and k α-Biodiversity
3.7. Correlation Between Primary Clinical Outcomes and Butyrate-Producing Taxa
3.8. Comparison of the C. butyricum CBM588 Probiotic Treatment with a Retrospective IBS-D Control Group
4. Discussion
4.1. Clinical Efficacy of C. butyricum CBM588 in Treating IBS-D
4.2. Comparison with Retrospective IBS-D Control Group
4.3. Impact on Gut Microbiota
4.4. Reduction in Abdominal Pain and Potential NLRP6 Modulation
4.5. Association Between Butyrate Producers and Clinical Outcomes
4.6. Limitation of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef]
- Shin, A.; Chang, L. The Transition from Rome III to Rome IV Irritable Bowel Syndrome: What We Gain and Lose. Clin. Gastroenterol. Hepatol. 2022, 20, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, C.; Van den Houte, K.; Altomare, A.; Guarino, M.P.L.; Besard, L.; Arts, J.; Caenepeel, P.; Piessevaux, H.; Vandenberghe, A.; Matthys, C.; et al. DOMINO trial post hoc analysis: Evaluation of the diet effects on symptoms in IBS subtypes. Ther. Adv. Gastroenterol. 2024, 17, 17562848241255296. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Hreinsson, J.P.; Törnblom, H.; Tack, J.; Drossman, D.A.; Whitehead, W.E.; Bangdiwala, S.I.; Sperber, A.D.; Palsson, O.S.; Simrén, M. Factor. Analysis of the Rome IV Criteria for Major Disorders of Gut-Brain Interaction (DGBI) Globally and Across Geographical, Sex, and Age Groups. Gastroenterology 2023, 164, 1211–1222. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients with Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Li, M.; Li, Y.Y.; Li, L.X.; Zhai, W.Z.; Wang, P.; Yang, X.X.; Gu, X.; Song, L.J.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Andoh, A.; Takizawa, J.; Takizawa, W.; Fujiyama, Y. Clostridium butyricum, a probiotic derivative, suppresses dextran sulfate sodium-induced experimental colitis in rats. Int. J. Mol. Med. 2004, 13, 577–580. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. Commission Implementing Decision of 11 December 2014 authorizing the placing on the market of Clostridium butyricum (CBM588) as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament of the Council (notified under document C(2014), 9345). OJEU 2014, 57, 153. [Google Scholar]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; DiCesare, J.; Puder, K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig. Dis. Sci. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Aragona, A.E.; Spada, C.; DE Luca, L.; Aragona, E.; Ciprandi, G.; COLONSTUDY Study Group. Probiotics for managing patients after bowel preparation for colonoscopy: An interventional, double-arm, open, randomized, multi-center, and national study (COLONSTUDY). Minerva Gastroenterol. 2024, 70, 187–196. [Google Scholar] [CrossRef]
- Salvioli, B. Trimebutine: A state-of-the-art review. Minerva Gastroenterol. Dietol. 2019, 65, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.X.; Wang, D.D.; Dong, P.J.; Zheng, L.H. Probiotics Combined With Trimebutine for the Treatment of Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2025, 40, 677–691. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Hevia, A.; Foroni, E.; Duranti, S.; Turroni, F.; Lugli, G.A.; Sanchez, B.; Martín, R.; Gueimonde, M.; van Sinderen, D.; et al. Assessing the fecal microbiota: An optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS ONE 2013, 8, e68739. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Hasan, B.S.M.; Abdulazeez, A.M. A review of principal component analysis algorithm for dimensionality reduction. J Soft Comput. Data Min. 2021, 2, 20–30. [Google Scholar] [CrossRef]
- Kyriazos, T.; Poga, M. Dealing with multicollinearity in factor analysis: The problem, detections, and solutions. Open J. Stat. 2023, 13, 404–424. [Google Scholar] [CrossRef]
- Libiger, O.; Schork, N.J. Partial Least Squares Regression Can Aid in Detecting Differential Abundance of Multiple Features in Sets of Metagenomic Samples. Front. Genet. 2015, 6, 350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Kowalska-Duplaga, K.; Gosiewski, T.; Kapusta, P.; Sroka-Oleksiak, A.; Wędrychowicz, A.; Pieczarkowski, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Fyderek, K. Differences in the intestinal microbiome of healthy children and patients with newly diagnosed Crohn’s disease. Sci. Rep. 2019, 9, 18880. [Google Scholar] [CrossRef] [PubMed]
- Kolho, K.L.; Korpela, K.; Jaakkola, T.; Pichai, M.V.; Zoetendal, E.G.; Salonen, A.; de Vos, W.M. Fecal Microbiota in Pediatric Inflammatory Bowel Disease and Its Relation to Inflammation. Am. J. Gastroenterol. 2015, 110, 921–930. [Google Scholar] [CrossRef]
- Kulecka, M.; Zeber-Lubecka, N.; Bałabas, A.; Czarnowski, P.; Bagińska, K.; Głowienka, M.; Kluska, A.; Piątkowska, M.; Dąbrowska, M.; Waker, E.; et al. Diarrheal-associated gut dysbiosis in cancer and inflammatory bowel disease patients is exacerbated by Clostridioides difficile infection. Front. Cell. Infect. Microbiol. 2023, 13, 1190910. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Chaparro, M.; Gisbert, J.P. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Franzin, M.; Stefančič, K.; Lucafò, M.; Decorti, G.; Stocco, G. Microbiota Drug Response Inflammatory Bowel Disease. Pathogens 2021, 10, 211. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Yao, C.; Li, Y.; Luo, L.; Xie, F.; Xiong, Q.; Li, T.; Yang, C.; Feng, P.M. Significant Differences in Gut Microbiota Between Irritable Bowel Syndrome with Diarrhea and Healthy Controls in Southwest China. Dig. Dis. Sci. 2023, 68, 106–127. [Google Scholar] [CrossRef]
- Mei, L.; Zhou, J.; Su, Y.; Mao, K.; Wu, J.; Zhu, C.; He, L.; Cui, Y. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Li, S.; Zhao, H.; Qu, J.; Xia, Y.; Li, Y. Clostridium butyricum relieve the visceral hypersensitivity in mice induced by Citrobacter rodentium infection with chronic stress. PeerJ 2021, 9, e11585. [Google Scholar] [CrossRef]
- Zheng, D.; Kern, L.; Elinav, E. The NLRP6 inflammasome. Immunology 2021, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Zhao, K.J.; Wang, S.S.; Wang, X.; Lu, B. Corticotropin-releasing factor induces inflammatory cytokines via the NLRP6-inflammatory cytokine axis in a murine model of irritable bowel syndrome. J. Dig. Dis. 2019, 20, 143–151. [Google Scholar] [CrossRef]
- Teige, E.S.; Hillestad, E.M.R.; Steinsvik, E.K.; Brønstad, I.; Lundervold, A.; Lundervold, A.J.; Valeur, J.; Hausken, T.; Berentsen, B.; Lied, G.A. Fecal bacteria and short-chain fatty acids in irritable bowel syndrome: Relations to subtype. Neurogastroenterol. Motil. 2024, 36, e14854. [Google Scholar] [CrossRef]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kaser, A.; Kiely, C.J.; Neville, B.A.; Powell, N.; et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut 2022, 71, 1821–1830. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: A meta-analysis. BMC Gastroenterol. 2016, 16, 62. [Google Scholar] [CrossRef]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharmacol. Ther. 2014, 40, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Pélerin, F.; Cayzeele Decherf, A.; Maudet, C.; Housez, B.; Cazaubiel, M.; Jüsten, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016, 4, 353–362, Erratum in United Eur. Gastroenterol. J. 2017, 5, 304. [Google Scholar] [CrossRef] [PubMed]
- Niv, E.; Naftali, T.; Hallak, R.; Vaisman, N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome—a double blind, placebo-controlled, randomized study. Clin. Nutr. 2005, 24, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
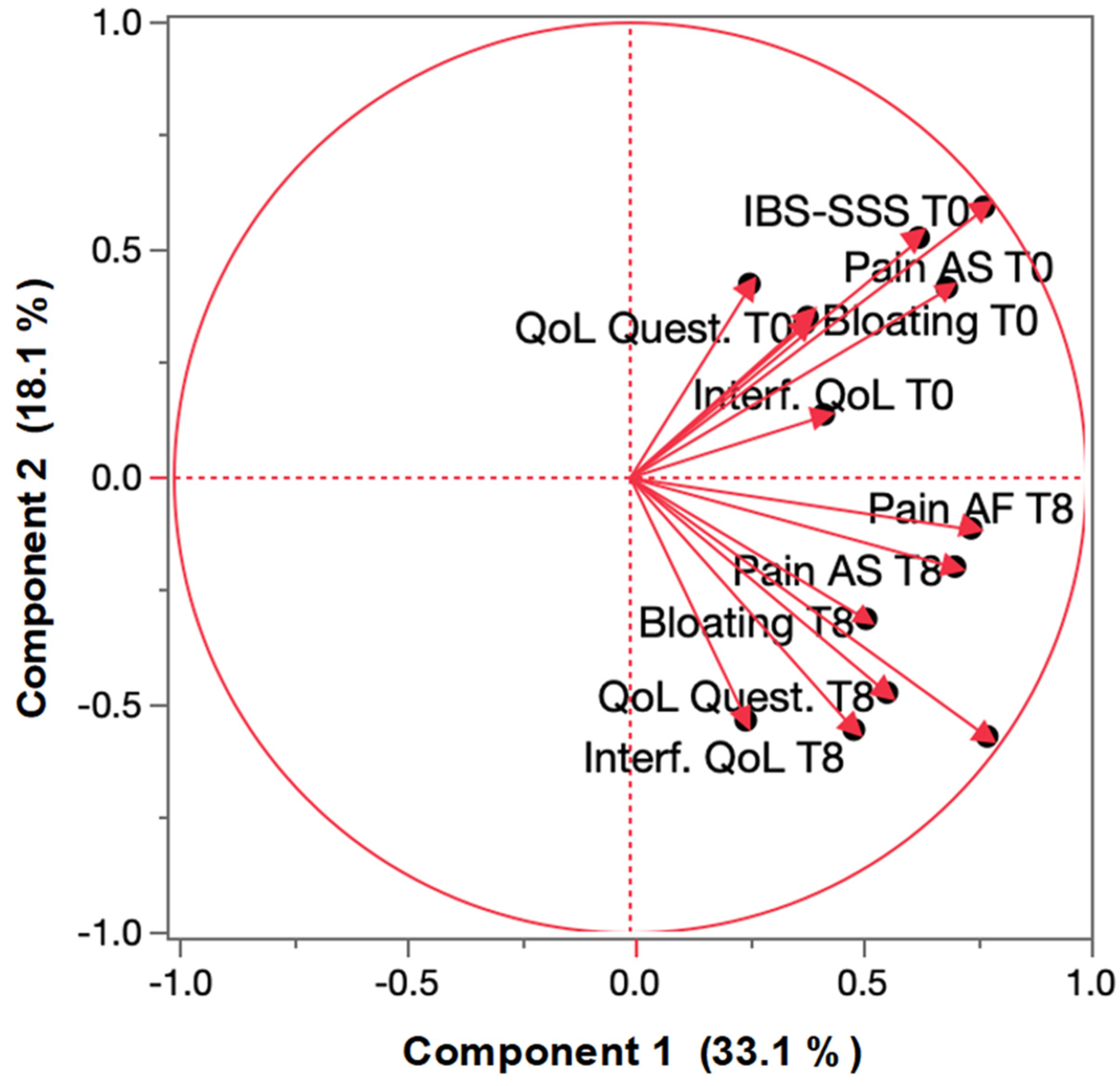

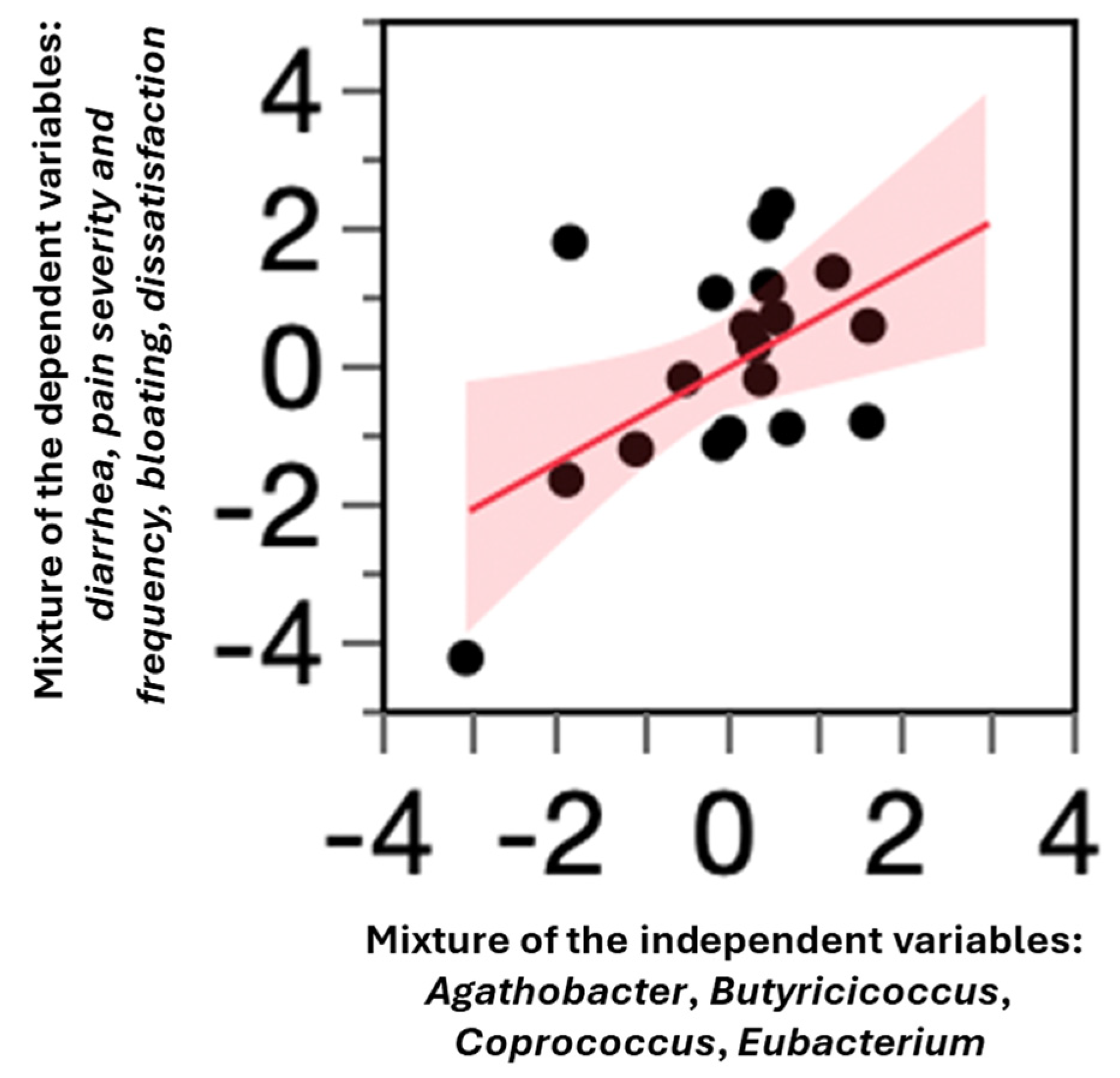

| Clinical Variable | M ± SD | Variation (%) | p |
|---|---|---|---|
| Bristol Stool Score T = 0 | 5.93 ± 1.07 | ||
| Bristol Stool Score T = 8 w | 4.19 ± 0.95 | −29.4 | <0.001 |
| Stool frequency per day T = 0 | 2.93 ± 1.29 | ||
| Stool frequency per day T = 8 w | 1.27 ± 0.63 | −56.7 | <0.001 |
| Episodes of diarrhea per day T = 0 | 2.09 ± 1.50 | ||
| Episodes of diarrhea per day T = 8 w | 0.32 ± 0.68 | −84.7 | <0.0001 |
| Pain (severity) T = 0 | 43.37 ± 27.74 | ||
| Pain (severity) T = 8 w | 17.95 ± 14.68 | −58.6 | 0.001 |
| Pain (frequency; out of 10 days) T = 0 | 38.59 ± 28.59 | ||
| Pain (frequency; out of 10 days) T = 8 w | 13.32 ± 11.70 | −64.5 | <0.0001 |
| Bloating T = 0 | 65.85 ± 27.28 | ||
| Bloating T = 8 w | 30.73 ± 19.35 | −53.4 | <0.0001 |
| Bowel habits dissatisfaction T = 0 | 75.80 ± 18.39 | ||
| Bowel habits dissatisfaction T = 8 w | 40.20 ± 22.84 | −47.9 | <0.0001 |
| Interference with QoL T = 0 | 59.12 ± 19.81 | ||
| Interference with QoL T = 8 w | 23.32 ± 16.80 | −60.6 | <0.0001 |
| IBS-SSS T = 0 | 282.39 ± 79.05 | ||
| IBS-SSS T = 8 w | 125.90 ± 55.83 | −55.5 | <0.0001 |
| QoL T = 0 | 32.91 ± 8.04 | ||
| QoL T = 8 w | 15.87 ± 4.31 | −51.8 | <0.0001 |
| M ± SD | Variation (%) | p | |
|---|---|---|---|
| α-biodiversity T = 0 | 118.74 ± 49.23 | - | - |
| α-biodiversity T = 8 w | 143.37 ± 62.82 | +20.7 | 0.008 |
| Clinical Variable | M ± SD | Variation (%) | p |
|---|---|---|---|
| Bristol Stool Score T = 0 | 6.31 ± 0.95 | ||
| Bristol Stool Score T = 8 w | 3.98 ± 1.50 | −36.9 | <0.001 |
| Stool frequency per day T = 0 | 3.22 ± 0.65 | ||
| Stool frequency per day T = 8 w | 1.77 ± 0.22 | −45.1 | <0.001 |
| Episodes of diarrhea per day T = 0 | 2.42 ± 0.35 | ||
| Episodes of diarrhea per day T = 8 w | 0.95 ± 0.13 | −60.8 | <0.0001 |
| Pain (severity) T = 0 | 47.09 ± 16.80 | ||
| Pain (severity) T = 8 w | 22.15 ± 13.05 | −58.6 | <0.01 |
| Pain (frequency; out of 10 days) T = 0 | 43.24 ± 6.27 | ||
| Pain (frequency; out of 10 days) T = 8 w | 21.18 ± 6.30 | −51.0 | <0.0001 |
| Bloating T = 0 | 60.37 ± 19.43 | ||
| Bloating T = 8 w | 32.12 ± 14.31 | −46.8 | <0.0001 |
| Bowel habits dissatisfaction T = 0 | 66.39 ± 19.79 | ||
| Bowel habits dissatisfaction T = 8 w | 35.63 ± 15.17 | −46.4 | <0.0001 |
| Interference with QoL T = 0 | 65.55 ± 19.87 | ||
| Interference with QoL T = 8 w | 31.53 ± 14.42 | −51.9 | <0.0001 |
| IBS-SSS T = 0 | 281.73 ± 38.35 | ||
| IBS-SSS T = 8 w | 141.28 ± 27.53 | −49.9 | <0.0001 |
| QoL T = 0 | 34.33 ± 5.12 | ||
| QoL T = 8 w | 15.10 ± 2.62 | −56.1 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pierro, F.; Ficuccilli, F.; Tessieri, L.; Menasci, F.; Pasquale, C.; Khan, A.; Rabbani, F.; Memon, N.M.; Cazzaniga, M.; Bertuccioli, A.; et al. Irritable Bowel Syndrome with Diarrhea (IBS-D): Effects of Clostridium butyricum CBM588 Probiotic on Gastrointestinal Symptoms, Quality of Life, and Gut Microbiota in a Prospective Real-Life Interventional Study. Microorganisms 2025, 13, 1139. https://doi.org/10.3390/microorganisms13051139
Di Pierro F, Ficuccilli F, Tessieri L, Menasci F, Pasquale C, Khan A, Rabbani F, Memon NM, Cazzaniga M, Bertuccioli A, et al. Irritable Bowel Syndrome with Diarrhea (IBS-D): Effects of Clostridium butyricum CBM588 Probiotic on Gastrointestinal Symptoms, Quality of Life, and Gut Microbiota in a Prospective Real-Life Interventional Study. Microorganisms. 2025; 13(5):1139. https://doi.org/10.3390/microorganisms13051139
Chicago/Turabian StyleDi Pierro, Francesco, Fabrizio Ficuccilli, Laura Tessieri, Francesca Menasci, Chiara Pasquale, Amjad Khan, Fazle Rabbani, Nazia Mumtaz Memon, Massimiliano Cazzaniga, Alexander Bertuccioli, and et al. 2025. "Irritable Bowel Syndrome with Diarrhea (IBS-D): Effects of Clostridium butyricum CBM588 Probiotic on Gastrointestinal Symptoms, Quality of Life, and Gut Microbiota in a Prospective Real-Life Interventional Study" Microorganisms 13, no. 5: 1139. https://doi.org/10.3390/microorganisms13051139
APA StyleDi Pierro, F., Ficuccilli, F., Tessieri, L., Menasci, F., Pasquale, C., Khan, A., Rabbani, F., Memon, N. M., Cazzaniga, M., Bertuccioli, A., Matera, M., Cavecchia, I., Recchia, M., Palazzi, C. M., Tanda, M. L., & Zerbinati, N. (2025). Irritable Bowel Syndrome with Diarrhea (IBS-D): Effects of Clostridium butyricum CBM588 Probiotic on Gastrointestinal Symptoms, Quality of Life, and Gut Microbiota in a Prospective Real-Life Interventional Study. Microorganisms, 13(5), 1139. https://doi.org/10.3390/microorganisms13051139











