Comparison of In Vitro Methods for Assaying the Antibacterial Activity of a Mix of Natural Essential Oils Against Zoonotic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of the EO Mix
2.2. Bacterial Strains
2.3. MIC by the Dilution Tube Method
2.4. MIC by the Automated Turbidimeter Measurements
2.5. Statistical Processing
3. Results
3.1. Composition of the EO Mix
3.2. Comparison of the Microdilution and the Macrodilution Methods
3.3. Growth Parameters of the Microdilution Method
3.3.1. Growth Rate (µmax)
3.3.2. Lag Phase (LagT)
3.3.3. ODmax
3.4. Technical and Statistical Comparison of the Two MIC Methods
3.4.1. Statistical Descriptive Parameters of the Results of the Microdilution Method
Comparison of the Technical Replicates for Lag T, µmax, and ODmax Parameters
Comparison of the Biological Replicates for Lag T, µmax, and ODmax Parameters
3.4.2. MIC Results of the Macrodilution Method
3.4.3. Comparison of the MIC Results of the Macrodilution Method vs. the Microdilution Method
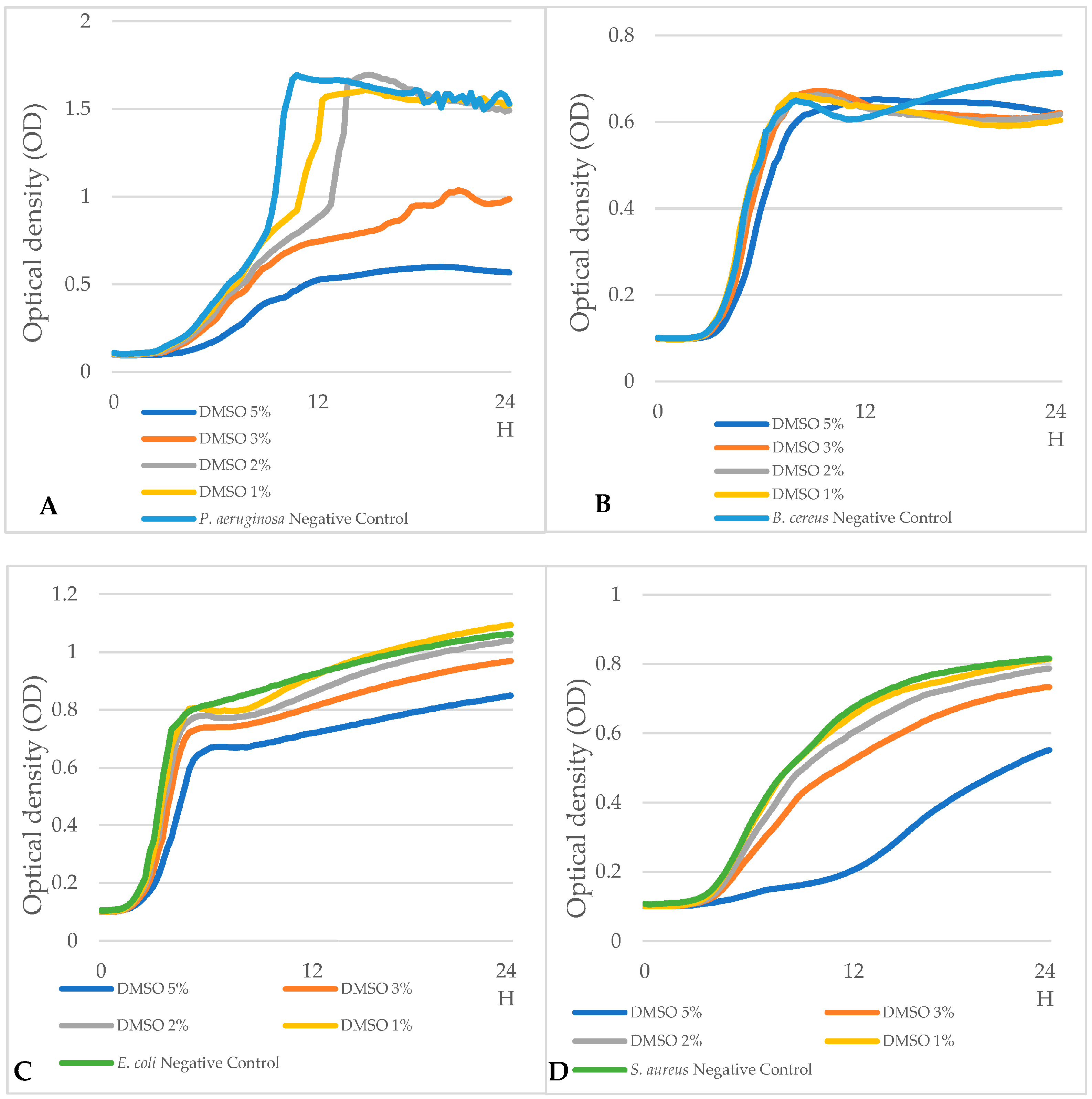
3.5. Evaluation of the Effect of DMSO on Bacterial Growth
4. Discussion
4.1. Statistical and Technical Points
4.2. Antibacterial Activity of the EO Mix
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.L. The Mode of Antibacterial Action of Essential Oils; Scientific Research Publishing Inc.: Irvine, CA, USA, 2011; Volume 2. [Google Scholar]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential Oils and Aromatic Plants in Animal Feeding—A European Perspective. A Review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial Activity and Mechanism of Clove Essential Oil against Foodborne Pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Ojha, S.; Pandey, A.K.; Singh, P. Citrus aurantiifolia Essential Oil Composition, Bioactivity, and Antibacterial Mode of Action on Salmonella Enterica, a Foodborne Pathogen. Food Sci. Eng. 2023, 4, 75–88. [Google Scholar] [CrossRef]
- De Milagres Almeida, J.; Crippa, B.L.; De Martins Alencar Souza, V.V.; Perez Alonso, V.P.; Da Motta Santos Júnior, E.; Siqueira Franco Picone, C.; Prata, A.S.; Cirone Silva, N.C. Antimicrobial Action of Oregano, Thyme, Clove, Cinnamon and Black Pepper Essential Oils Free and Encapsulated against Foodborne Pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Pseudomonas aeruginosa Infection of Avian Origin: Zoonosis and One Health Implications. Vet World 2021, 14, 2155–2159. [Google Scholar] [CrossRef]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the Natural Habitat of Bacillus Spore-Formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Shtylla Kika, T.; Cocoli, S.; Ljubojević Pelić, D.; Puvača, N.; Lika, E.; Pelić, M. Colibacillosis in Modern Poultry Production. J. Agron. Technol. Eng. Manag. 2023, 6, 975–987. [Google Scholar] [CrossRef]
- Song, M.; Tang, Q.; Ding, Y.; Tan, P.; Zhang, Y.; Wang, T.; Zhou, C.; Xu, S.; Lyu, M.; Bai, Y.; et al. Staphylococcus aureus and Biofilms: Transmission, Threats, and Promising Strategies in Animal Husbandry. J. Anim. Sci. Biotechnol. 2024, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, D.; Sridhar, N.B.; Chandra, U.S.; Manjunatha, S.S.; Borthakur, A.; Vinuta, M.H.; Mohan, B.R. Recent Perspectives of Growth Promoters in Livestock: An Overview. J. Livest. Sci. 2023, 14, 53–64. [Google Scholar] [CrossRef]
- Girard, C.; Fayolle, K.; Kerros, S.; Leriche, F. Flow Cytometric Assessment of the Antimicrobial Properties of an Essential Oil Mixture against Escherichia coli. J. Anim. Feed. Sci. 2019, 28, 187–198. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Kettunen, H.; Peuranen, S.; Schulze, H.; Rautonen, N. In Vitro Effects of Essential Oils on Potential Pathogens and Beneficial Members of the Normal Microbiota. Vet. Med. 2010, 55, 71–78. [Google Scholar] [CrossRef]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X.G. The Effect of Essential Oils on Performance, Immunity and Gut Microbial Population in Weaner Pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In Vitro Degradation and in Vivo Passage Kinetics of Carvacrol, Thymol, Eugenol and Trans -cinnamaldehyde along the Gastrointestinal Tract of Piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening Essential Oils for Their Antimicrobial Activities against the Foodborne Pathogenic Bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Atki, Y.E.; Aouam, I. Antibacterial Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Nosocomial Infection-Bacteria. J. Pharm. Sci. Res. 2019, 11, 306–309. [Google Scholar]
- Approved Standard M7; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003.
- Approved Standard M100; MIC Testing Supplemental Tables. National Committee for Clinical Laboratory Standards: Wayne, PA, USA.
- Li, W.; Chen, H.; He, Z.; Han, C.; Liu, S.; Li, Y. Influence of Surfactant and Oil Composition on the Stability and Antibacterial Activity of Eugenol Nanoemulsions. LWT-Food Sci. Technol. 2015, 62, 39–47. [Google Scholar] [CrossRef]
- Vanegas, D.; Abril-Novillo, A.; Khachatryan, A.; Jerves-Andrade, L.; Peñaherrera, E.; Cuzco, N.; Wilches, I.; Calle, J.; León-Tamariz, F. Validation of a Method of Broth Microdilution for the Determination of Antibacterial Activity of Essential Oils. BMC Res. Notes 2021, 14, 439. [Google Scholar] [CrossRef]
- M100; Performance Standards for Antimicrobial Susceptibility Testing. National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003.
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of Tea Tree (Melaleuca alternifolia) Oil on Staphylococcus aureus in Biofilms and Stationary Growth Phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef]
- Baranyi, J.; McClure, P.J.; Sutherland, J.P.; Roberts, T.A. Modeling Bacterial Growth Responses. J. Ind. Microbiol. 1993, 12, 190–194. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Bouix, M.; Abedrabba, M.; Leveau, J.-Y.; Hamdi, M. Mechanism of Action of Melaleuca Armillaris (Sol. Ex Gaertu) Sm. Essential Oil on Six LAB Strains as Assessed by Multiparametric Flow Cytometry and Automated Microtiter-Based Assay. Food Chem. 2008, 111, 707–718. [Google Scholar] [CrossRef]
- Chung, B.H.; Cannon, R.Y.; Smith, R.C. Influence of Growth Temperature on Glucose Metabolism of a Psychotrophic Strain of Bacillus Cereus. Appl. Environ. Microbiol. 1976, 31, 39–45. [Google Scholar] [CrossRef]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment Techniques of Antimicrobial Properties of Natural Compounds of Plant Origin: Current Methods and Future Trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. BioMed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Gu, Z.; Ban, X.; Hong, Y.; Cheng, L.; Li, C. A New Micro-Agar Dilution Method to Determine the Minimum Inhibitory Concentration of Essential Oils against Microorganisms. J. Microbiol. Methods 2023, 211, 106791. [Google Scholar] [CrossRef] [PubMed]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.R.; Warner, S.L.; Cates, R.G.; Gary Young, D. Assessment of Antimicrobial Activity of Fourteen Essential Oils When Using Dilution and Diffusion Methods. Pharm. Biol. 2005, 43, 687–695. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M.A. Evaluation of Common Antibacterial Screening Methods Utilized in Essential Oil Research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Chen, Q.; You, N.; Liang, C.; Xu, Y.; Wang, F.; Zhang, B.; Zhang, P. Effect of Cellulose Nanocrystals-Loaded Ginger Essential Oil Emulsions on the Physicochemical Properties of Mung Bean Starch Composite Film. Ind. Crops Prod. 2023, 191, 116003. [Google Scholar] [CrossRef]
- Zgoda, J.R.; Porter, J.R. A Convenient Microdilution Method for Screening Natural Products Against Bacteria and Fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian Essential Oil of Ruta Graveolens against Nosocomial Antifungal Resistant Candida Strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef]
- Ghosh, V.; Saranya, S.; Mukherjee, A.; Chandrasekaran, N. Cinnamon Oil Nanoemulsion Formulation by Ultrasonic Emulsification: Investigation of Its Bactericidal Activity. J. Nanosci. Nanotechnol. 2013, 13, 114–122. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The Agar Microdilution Method—A New Method for Antimicrobial Susceptibility Testing for Essential Oils and Plant Extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential Oils: Antimicrobial Activities, Extraction Methods, and Their Modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Hulankova, R. The Influence of Liquid Medium Choice in Determination of Minimum Inhibitory Concentration of Essential Oils against Pathogenic Bacteria. Antibiotics 2022, 11, 150. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Sofos, J.N. Effect of Inoculum Size on the Combined Temperature, pH and Aw Limits for Growth of Listeria Monocytogenes. Int. J. Food Microbiol. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Guillier, L.; Nazer, A.I.; Dubois-Brissonnet, F. Growth Response of Salmonella Typhimurium in the Presence of Natural and Synthetic Antimicrobials: Estimation of MICs from Three Different Models. J. Food Prot. 2007, 70, 2243–2250. [Google Scholar] [CrossRef]
- Pascual, C.; Robinson, T.P.; Ocio, M.J.; Aboaba, O.O.; Mackey, B.M. The Effect of Inoculum Size and Sublethal Injury on the Ability of Listeria Monocytogenes to Initiate Growth under Suboptimal Conditions. Lett. Appl. Microbiol. 2001, 33, 357–361. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus aureus and Staphylococcus epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum Vulgare Extract and Its Major Constituents. Oxidative Med. Cell. Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef]
- Pedreira, A.; Martínez-López, N.; Vázquez, J.A.; García, M.R. Modelling the Antimicrobial Effect of Food Preservatives in Bacteria: Application to Escherichia coli and Bacillus cereus Inhibition with Carvacrol. J. Food Eng. 2024, 361, 111734. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as Potential Quorum Sensing Inhibitor of Pseudomonas aeruginosa and Biofilm Production on Stainless Steel Surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- El, A.S.; Ibnsouda, K.S.; Latrache, H.; Zineb, G.; Mouradi, H.; Remmal, A. Carvacrol and Thymol Components Inhibiting Pseudomonas aeruginosa Adherence and Biofilm Formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar] [CrossRef]
- Maggini, V.; Pesavento, G.; Maida, I.; Nostro, A.L.; Calonico, C.; Sassoli, C.; Perrin, E.; Fondi, M.; Mengoni, A.; Chiellini, C.; et al. Exploring the Effect of the Composition of Three Different Oregano Essential Oils on the Growth of Multidrug-Resistant Cystic Fibrosis Pseudomonas aeruginosa Strains. Nat. Prod. Commun. 2017, 12, 1934578X1701201. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial Activity, Cytotoxicity and Chemical Analysis of Lemongrass Essential Oil (Cymbopogon flexuosus) and Pure Citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Saadony, M.T.; Elnesr, S.S.; Farahat, M.; Attia, G.; Madkour, M.; Reda, F.M. Use of Lemongrass Essential Oil as a Feed Additive in Quail’s Nutrition: Its Effect on Growth, Carcass, Blood Biochemistry, Antioxidant and Immunological Indices, Digestive Enzymes and Intestinal Microbiota. Poult. Sci. 2021, 100, 101172. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2020, 17, 2–12. [Google Scholar] [CrossRef]
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapong, S.; Khunkitti, W. The Effect of Lemongrass Oil and Its Major Components on Clinical Isolate Mastitis Pathogens and Their Mechanisms of Action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011, 91, e31–e37. [Google Scholar] [CrossRef]
- Zulfa, Z.; Chia, C.T.; Rukayadi, Y. In Vitro Antimicrobial Activity of Cymbopogon citratus (Lemongrass) Extracts against Selected Foodborne Pathogens. Int. Food Res. J. 2016, 23, 1262. [Google Scholar]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial Activity of Lemongrass (Cymbopogon citratus) Oil against Some Selected Pathogenic Bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; Da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial Activity of Essential Oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Cox, S.D.; Markham, J.L. Susceptibility and Intrinsic Tolerance of Pseudomonas aeruginosa to Selected Plant Volatile Compounds. J. Appl. Microbiol. 2007, 103, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Chevalier, J.; Hassani, L.; Mezrioui, N.-E.; Pagès, J.-M. Natural Extracts Stimulate Membrane-Associated Mechanisms of Resistance in Gram-Negative Bacteria. Lett. Appl. Microbiol. 2014, 58, 472–477. [Google Scholar] [CrossRef]
- Papadopoulos, C.J.; Carson, C.F.; Chang, B.J.; Riley, T.V. Role of the MexAB-OprM Efflux Pump of Pseudomonas aeruginosa in Tolerance to Tea Tree (Melaleuca alternifolia) Oil and Its Monoterpene Components Terpinen-4-Ol, 1,8-Cineole, and α-Terpineol. Appl. Environ. Microbiol. 2008, 74, 1932–1935. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, L. Thymol as a Critical Component of Thymus vulgaris, L. Essential Oil Combats Pseudomonas aeruginosa by Intercalating DNA and Inactivating Biofilm. LWT 2021, 136, 110354. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; Leite, A.D.L.; De Pontes, L.G.; Dos Santos, L.D.; et al. Proteomic Analysis and Antibacterial Resistance Mechanisms of Salmonella Enteritidis Submitted to the Inhibitory Effect of Origanum Vulgare Essential Oil, Thymol and Carvacrol. J. Proteom. 2020, 214, 103625. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Guo, W.; Yang, S.; Li, H.; Gong, G. Antibacterial Mechanism of Thymol against Enterobacter Sakazakii. Food Control 2021, 123, 107716. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Chen, H.; Song, Z.; Guo, H.; Yuan, Y.; Yue, T. Antibacterial Activity of Essential Oils against Stenotrophomonas Maltophilia and the Effect of Citral on Cell Membrane. LWT 2020, 117, 108667. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, P.; He, Y.; Kang, S.; Shen, C.; Wang, S.; Guo, M.; Wang, L.; Shi, C. Antibacterial Effect of Oregano Essential Oil against Vibrio Vulnificus and Its Mechanism. Foods 2022, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shankar, S.; Fernandez, J.; Juillet, E.; Salmieri, S.; Lacroix, M. A Rapid Way of Formulation Development Revealing Potential Synergic Effects on Numerous Antimicrobial Combinations against Foodborne Pathogens. Microb. Pathog. 2021, 158, 105047. [Google Scholar] [CrossRef] [PubMed]
- Harris, R. Synergism in the Essential Oil World. Int. J. Aromather. 2002, 12, 179–186. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, V.; Singh, R.K.; Ebibeni, N. Antimicrobial Activity of Lemongrass (Cymbopogon citratus) Oil against Microbes of Environmental, Clinical and Food Origin. Int. Res. J. Pharm. Pharmacol. 2011, 1, 228–236. [Google Scholar]
- Vale, L.; De Paula, L.G.F.; Vieira, M.S.; Alves, S.D.G.A.; Junior, N.R.D.M.; Gomes, M.D.F.; Teixeira, W.F.P.; Rizzo, P.V.; Freitas, F.M.C.; Ferreira, L.L.; et al. Binary Combinations of Thymol, Carvacrol and Eugenol for Amblyomma Sculptum Control: Evaluation of In Vitro Synergism and Effectiveness under Semi-Field Conditions. Ticks Tick-Borne Dis. 2021, 12, 101816. [Google Scholar] [CrossRef]
- Swetha, T.K.; Vikraman, A.; Nithya, C.; Hari Prasath, N.; Pandian, S.K. Synergistic Antimicrobial Combination of Carvacrol and Thymol Impairs Single and Mixed-Species Biofilms of Candida albicans and Staphylococcus epidermidis. Biofouling 2021, 36, 1256–1271. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Pei, R.; Zhou, F.; Ji, B.; Xu, J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against E. coli with an Improved Method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol toward Bacillus cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef]
- Ayari, S.; Shankar, S.; Follett, P.; Hossain, F.; Lacroix, M. Potential Synergistic Antimicrobial Efficiency of Binary Combinations of Essential Oils against Bacillus cereus and Paenibacillus amylolyticus-Part A. Microb. Pathog. 2020, 141, 104008. [Google Scholar] [CrossRef]

| Compound Name | Rate (%) |
|---|---|
| α-pinene | 0.54 |
| Camphene | 0.23 |
| p-cymene | 4.39 |
| Limonene | 0.40 |
| γ-terpinene | 2.15 |
| Diallyl disulfide | 0.42 |
| Linalol | 0.30 |
| Isopulegol | 2.10 |
| Citronellal | 0.70 |
| Neoisopulegol | 0.31 |
| Terpinene-4-ol | 0.43 |
| α-terpineol | 0.20 |
| Citronellol | 0.80 |
| β-citral | 5.83 |
| Geraniol | 2.63 |
| α-citral | 8.01 |
| Thymol | 37.72 |
| Carvacrol | 26.76 |
| Dially trisulfide | 0.69 |
| Caryophyllene | 0.97 |
| Bacterial Strain | Macrodilution Method EO Mix MIC (µg/mL) | Microdilution Method EO Mix MIC (µg/mL) |
|---|---|---|
| Bacillus cereus | 6.08 × 102 ± 2.06 × 102 a | 2.17 × 102 ± 6.53 × 101 b |
| Pseudomonas aeruginosa | N.D. | N.D. |
| Staphylococcus aureus | 3.92 × 102 ± 2.41 × 102 a | 3.69 × 102 ± 6.53 × 101 a |
| Escherichia coli | 1.39 × 103 ± 7.59 × 102 a | 5.21 × 102 ± 1.95 × 102 a |
| Bacterial Strains Growth Conditions (With or Without EO Mix) | EO Mix MIC/2 (µg/mL) | Lag Phase (LagT) (h) | Growth Rate (µmax) (h-1) | OD Max (uOD) | R2 | Bacterial Strains Growth Conditions (With or Without EO Mix) | EO Mix MIC/2 (µg/mL) | Lag Phase (LagT) (h) | Growth Rate (µmax) (h-1) | OD Max (uOD) |
|---|---|---|---|---|---|---|---|---|---|---|
| mt * | mb ** | mt | mb | mt | mb | mt | mb | mt | mb | |
| Bacillus cereus negative control | mt1 | - | 0.09 ± 0.01 | 0.09a ± 0.03 | 3.73 ± 0.34 | 3.46a ± 0.90 | 0.74 ± 0.00 | 0.73 ± 0.04 | 81.72% | 92.50% |
| mt2 | 0.11 ± 0.00 | 4.27 ± 0.02 | 0.68 ± 0.01 | 99.50% | ||||||
| mt3 | 0.06 ± 0.02 | 2.37 ± 0.31 | 0.77 ± 0.01 | 96.31% | ||||||
| Bacillus cereus with EO mix | mt1 | 108.39 ± 32.67 | 0.67 ± 0.52 | 0.63b ± 0.41 | 0.05 ± 0.04 | 0.39b ± 0.56 | 0.11 ± 0.00 | 0.30b ± 0.14 | 91.59% | 87.90% |
| mt2 | 0.25 ± 0.07 | 1.11 ± 0.29 | 0.39 ± 0.03 | 77.75% | ||||||
| mt3 | 0.97 ± 0.00 | 0.02 ± 0.01 | 0.11 ± 0.00 | 93.49% | ||||||
| mt1 | - | 0.08 ± 0.00 | 0.07a ± 0.01 | 5.36 ± 0.26 | 4.66a ± 0.63 | 0.86 ± 0.01 | 0.86a ± 0.02 | 98.04% | 97.50% | |
| Escherichia coli negative control | mt2 | 0.06 ± 0.00 | 4.42 ± 0.41 | 0.87 ± 0.02 | 96.46% | |||||
| mt3 | 0.06 ± 0.01 | 4.19 ± 0.47 | 0.86 ± 0.04 | 97.00% | ||||||
| Escherichia coli with EO mix | mt1 | 260.50 ± 97.50 | 0.27 ± 0.25 | 0.55b ± 0.08 | 2.02 ± 3.31 | 0.66b ± 1.12 | 0.33 ± 0.27 | 0.18b ± 0.09 | 89.90% | 82.67% |
| mt2 | 0.97 ± 0.00 | (-)0.01 ± 0.04 | 0.10 ± 0.00 | 70.18% | ||||||
| mt3 | 0.40 ± 0.00 | (-)0.03 ± 0.03 | 0.10 ± 0.00 | 87.93% | ||||||
| Pseudomonas aeruginosa negative control | mt1 | - | 0.23 ± 0.02 | 0.03 ± 0.02 | 7.22 ± 0.56 | 7.51 ± 2.51 | 1.55 ± 0.01 | 1.62 ± 0.07 | 98.00% | 98.00% |
| mt2 | 0.24 ± 0.04 | 9.41 ± 4.27 | 1.63 ± 0.07 | 98.00% | ||||||
| mt3 | 0.21 ± 0.01 | 5.92 ± 0.75 | 1.68 ± 0.05 | 98.00% | ||||||
| Pseudomonas aeruginosa with EO mix | mt1 | ind | ind | ind | ind | ind | ind | ind | ind | ind |
| mt2 | ind | ind | ind | ind | ||||||
| mt3 | ind | ind | ind | ind | ||||||
| Staphylococcus aureus negative control | mt1 | - | 0.17 ± 0.05 | 0.15a ± 0.03 | 2.37 ± 0.61 | 2.15a ± 0.32 | 0.89 ± 0.13 | 0.94a ± 0.07 | 99.53% | 99.20% |
| mt2 | 0.14 ± 0.01 | 2.04 ± 0.00 | 0.96 ± 0.01 | 99.02% | ||||||
| mt3 | 0.14 ± 0.01 | 2.04 ± 0.05 | 0.97 ± 0.02 | 99.06% | ||||||
| Staphylococcus aureus with EO mix | mt1 | 184.61 ± 32.67 | 0.18 ± 0.11 | 0.63b ± 0.41 | 1.51 ± 1.29 | 0.58b ± 0.975 | 0.50 ± 0.34 | 0.25b ± 0.26 | 99.22% | 57.44% |
| mt2 | 0.74 ± 0.39 | 0.23 ± 0.40 | 0.16 ± 0.09 | 41.63% | ||||||
| mt3 | 0.97 ± 0.00 | (-)0.00 ± 0.01 | 0.11 ± 0.00 | 31.48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayolle, K.; Girard, C.; Lasfargues, P.; Koteich, S.; Kerros, S. Comparison of In Vitro Methods for Assaying the Antibacterial Activity of a Mix of Natural Essential Oils Against Zoonotic Bacteria. Microorganisms 2025, 13, 1125. https://doi.org/10.3390/microorganisms13051125
Fayolle K, Girard C, Lasfargues P, Koteich S, Kerros S. Comparison of In Vitro Methods for Assaying the Antibacterial Activity of a Mix of Natural Essential Oils Against Zoonotic Bacteria. Microorganisms. 2025; 13(5):1125. https://doi.org/10.3390/microorganisms13051125
Chicago/Turabian StyleFayolle, Karine, Claire Girard, Pauline Lasfargues, Sahar Koteich, and Sylvain Kerros. 2025. "Comparison of In Vitro Methods for Assaying the Antibacterial Activity of a Mix of Natural Essential Oils Against Zoonotic Bacteria" Microorganisms 13, no. 5: 1125. https://doi.org/10.3390/microorganisms13051125
APA StyleFayolle, K., Girard, C., Lasfargues, P., Koteich, S., & Kerros, S. (2025). Comparison of In Vitro Methods for Assaying the Antibacterial Activity of a Mix of Natural Essential Oils Against Zoonotic Bacteria. Microorganisms, 13(5), 1125. https://doi.org/10.3390/microorganisms13051125






