Formation of Mono-Organismal and Mixed Staphylococcus aureus and Streptococcus mutans Biofilms in the Presence of NaCl
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Collection of Human Saliva
2.3. Extraction of MVs
2.4. Biofilm Formation Assay
2.5. Observing Live Cells and Glucans During Biofilm Formation
2.6. Statistical Analysis
3. Results
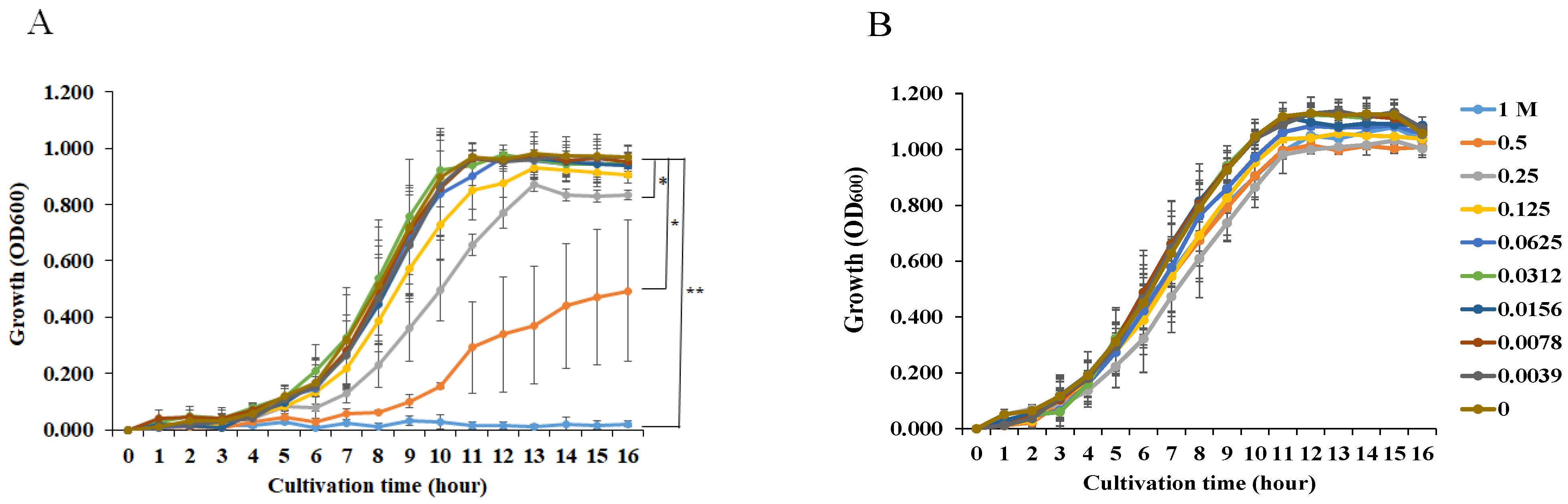
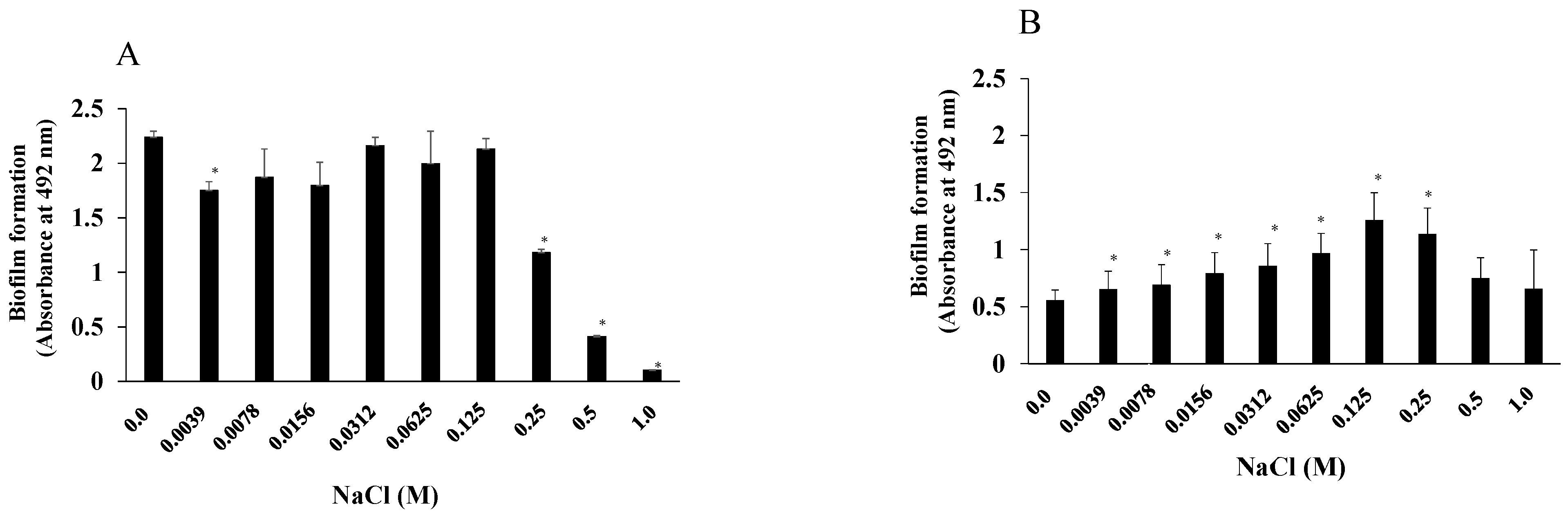
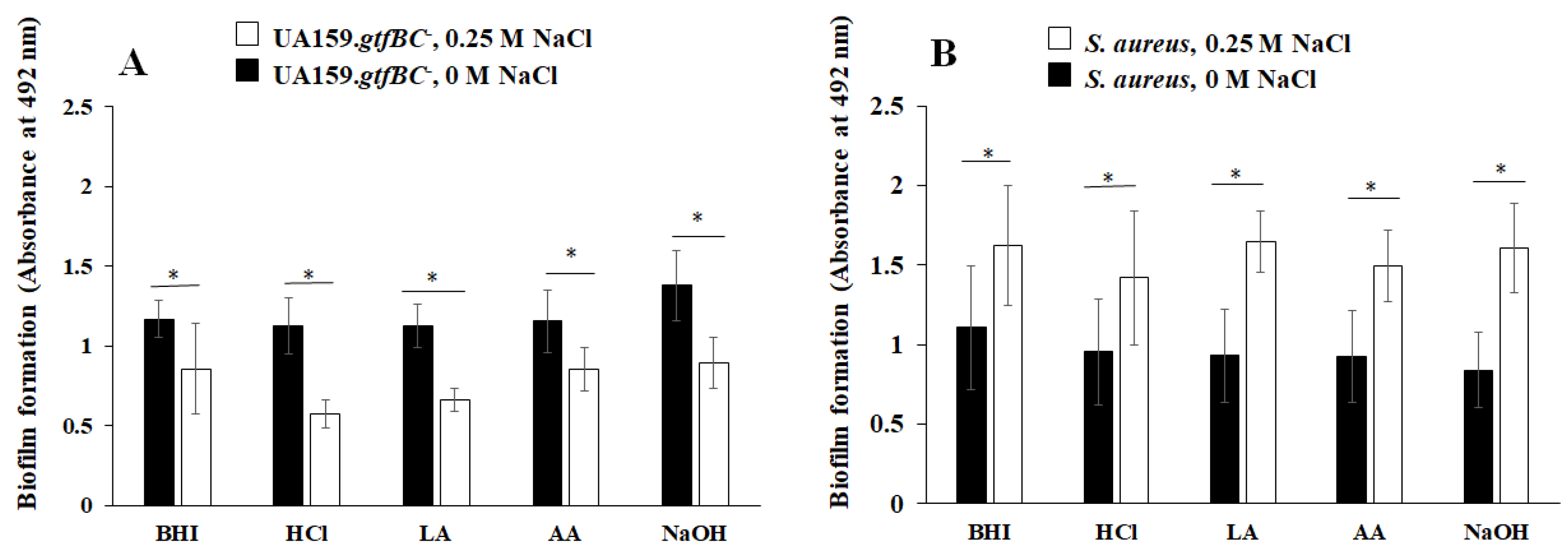
3.1. Effects of NaCl Concentration on S. aureus and S. mutans Growth and Biofilm Formation
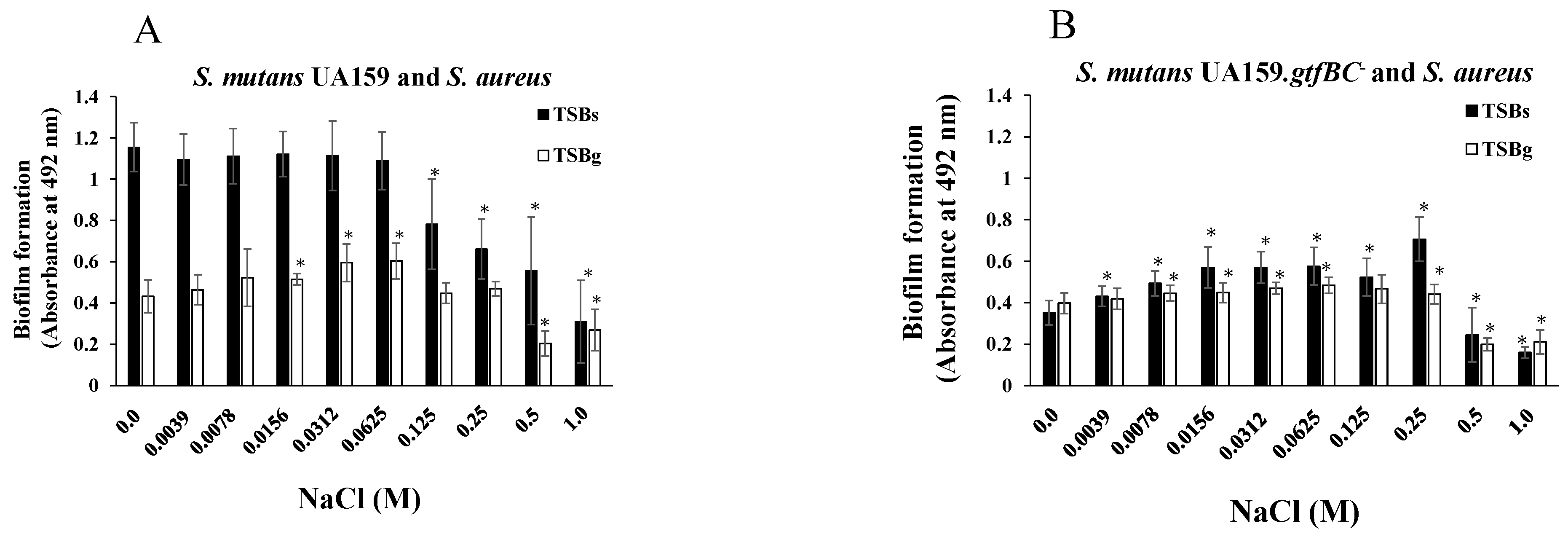
3.2. Effects of NaCl on the Formation of Mixed-Species Biofilms
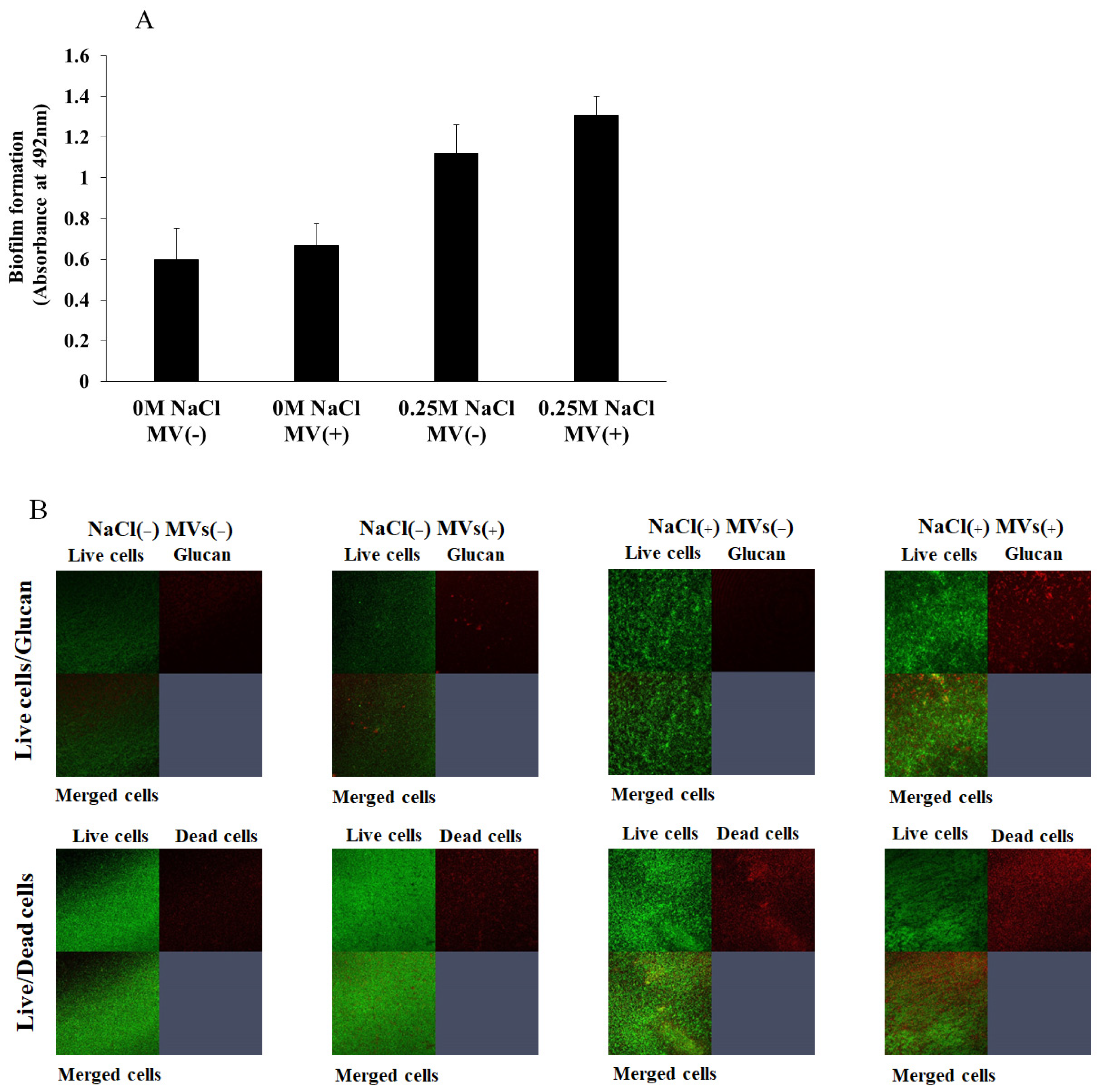
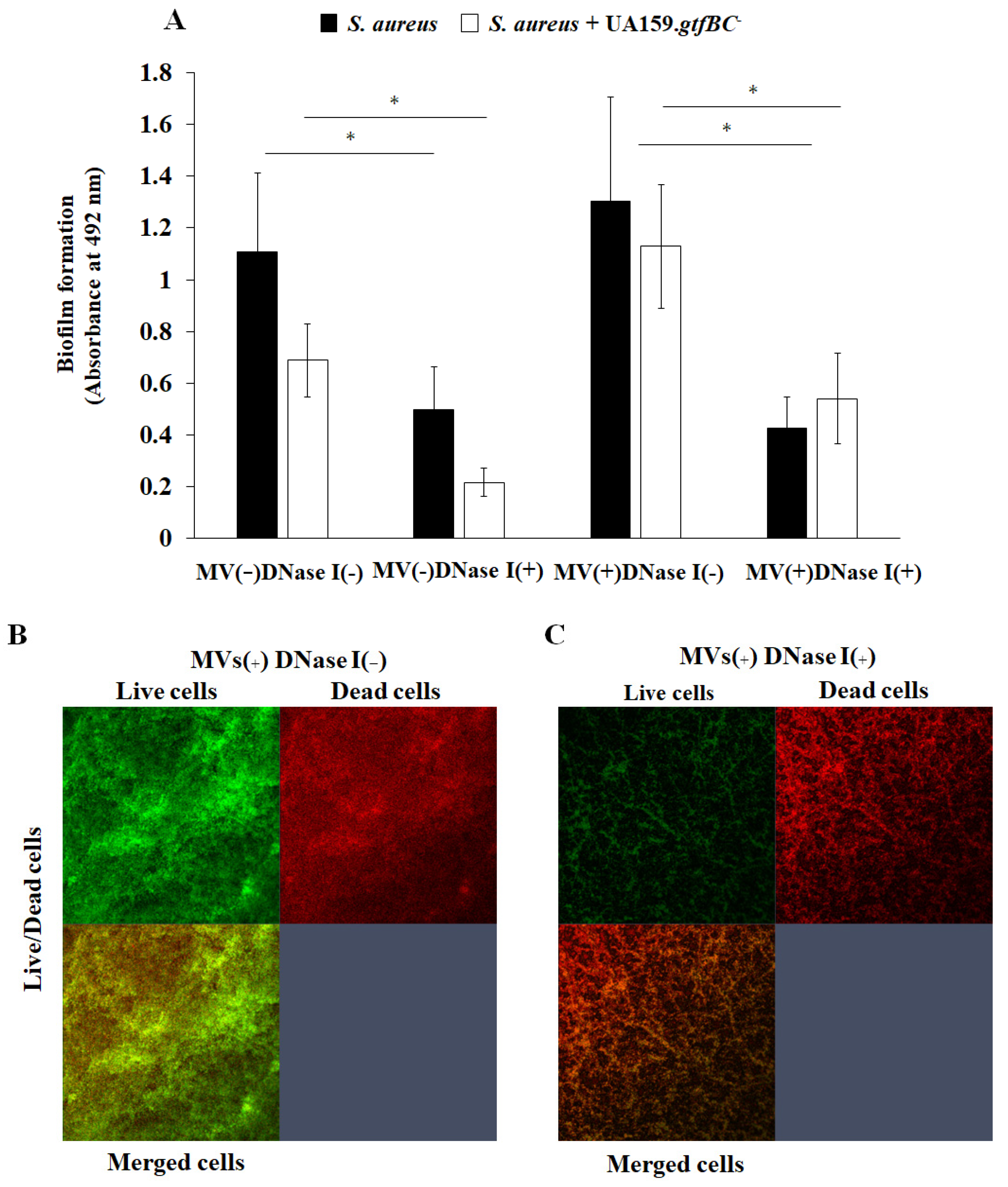
3.3. Effects of MVs on Biofilm Formation
3.4. Effects of eDNA on Biofilm Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Hamada, S.; Slade, H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Mandell, L.A.; Niederman, M.S. Aspiration pneumonia. N. Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef]
- Yoneyama, T.; Yoshida, M.; Matsui, T.; Sasaki, H. Oral care and pneumonia. Oral Care Working Group. Lancet 1999, 354, 515. [Google Scholar] [CrossRef]
- Senpuku, H.; Sogame, A.; Inoshita, E.; Tsuha, Y.; Miyazaki, H.; Hanada, N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology 2003, 49, 301–309. [Google Scholar] [CrossRef]
- Tada, A.; Hanada, N. Opportunistic respiratory pathogens in the oral cavity of the elderly. FEMS Immunol. Med. Microbiol. 2010, 60, 1–17. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Pietrantoni, C.; Bhat, A.; Aquilina, A.T.; Okada, M.; Grover, V.; Gifford, N. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am. J. Respir. Crit. Care Med. 2003, 167, 1650–1654. [Google Scholar] [CrossRef]
- Koukos, G.; Sakellari, D.; Arsenakis, M.; Tsalikis, L.; Slini, T.; Konstantinidis, A. Prevalence of Staphylococcus aureus and methicillin resistant Staphylococcus aureus (MRSA) in the oral cavity. Arch. Oral Biol. 2015, 60, 1410–1415. [Google Scholar] [CrossRef]
- Saeed, K.; Gould, I.; Esposito, S.; Ahmad-Saeed, N.; Ahmed, S.S.; Alp, E.; Bal, A.M.; Bassetti, M.; Bonnet, E.; Chan, M.; et al. Panton-valentine leukocidin-positive Staphylococcus aureus: A position statement from the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2018, 51, 16–25. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the panton valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef]
- Miles, G.; Movileanu, L.; Bayley, H. Subunit composition of a bicomponent toxin: Staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci. 2002, 11, 894–902. [Google Scholar] [CrossRef]
- Tashiro, M.; Ciborowski, P.; Reinacher, M.; Pulverer, G.; Klenk, H.-D.; Rott, R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology 1987, 157, 421–430. [Google Scholar] [CrossRef]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef]
- Scannapieco, F.A. Role of oral bacteria in respiratory infection. J. Periodontol. 1999, 70, 793–802. [Google Scholar] [CrossRef]
- Desai, J.P.; Nair, R.U. Oral health factors related to rapid oral health deterioration among older adults: A narrative review. J. Clin. Med. 2023, 12, 3202. [Google Scholar] [CrossRef]
- Hoover, J.; Tovar, E.; Zlatnik, T.; Karunanayake, C. Efficacy of a rinse containing sea salt and lysozyme on biofilm and gingival health in a group of young adults: A pilot study. Int. J. Dent. 2017, 2017, 4056708. [Google Scholar] [CrossRef]
- Lee, S.; Choi, K.-H.; Yoon, Y. Effect of NaCl on biofilm formation of the isolate from Staphylococcus aureus outbreak linked to ham. Kor. J. Food Sci. Anim. Resour. 2014, 34, 257–261. [Google Scholar] [CrossRef]
- O’Neill, E.; Pozzi, C.; Houston, P.; Smyth, D.; Humphreys, H.; Robinson, D.A.; O’Gara, J.P. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 2017, 45, 1379–1388. [Google Scholar] [CrossRef]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef]
- Senpuku, H.; Nakamura, T.; Iwabuchi, Y.; Hirayama, S.; Nakao, R.; Ohnishi, M. Effects of complex DNA and MVs with GTF extracted from Streptococcus mutans on the oral biofilm. Molecules 2019, 24, 3131. [Google Scholar] [CrossRef]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial vesicles in marine ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef]
- Berleman, J.; Auer, M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ. Microbiol. 2013, 15, 347–354. [Google Scholar] [CrossRef]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef]
- Lai, H.; White, J.; Roe, B.A.; Ferretti, J.J. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 2002, 99, 14434–14439. [Google Scholar]
- Suzuki, Y.; Nagasawa, R.; Senpuku, H. Inhibiting effects of fructanase on competence-stimulating peptide-dependent quorum sensing system in Streptococcus mutans. J. Infect. Chemother. 2017, 23, 634–641. [Google Scholar] [CrossRef]
- Nakamura, T.; Iwabuchi, Y.; Hirayama, S.; Narisawa, N.; Takenaga, F.; Nakao, R.; Senpuku, H. Roles of membrane vesicles from Streptococcus mutans for the induction of antibodies to glucosyltransferase in mucosal immunity. Microb. Pathog. 2020, 149, 104260. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sharma, P.K.; Busscher, H.J.; Van Der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Nagasawa, R.; Sato, T.; Senpuku, H. Raffinose induces biofilm formation by Streptococcus mutans in low concentrations of sucrose by increasing production of extracellular DNA and fructan. Appl. Environ. Microbiol. 2017, 83, e00869-17. [Google Scholar] [CrossRef]
- Motegi, M.; Takagi, Y.; Yonezawa, H.; Kanada, N.; Terajima, J.; Watanabe, H.; Senpuku, H. Assessment of genes associated with Streptococcus mutans biofilm morphology. Appl. Environ. Microbiol. 2006, 72, 6277–6287. [Google Scholar] [CrossRef]
- Liao, S.; Klein, M.I.; Heim, K.P.; Fan, Y.; Bitoun, J.P.; Ahn, S.-J.; Burne, R.A.; Koo, H.; Brady, L.J.; Wen, Z.T. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 2014, 196, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, Y.; Nakamura, T.; Kusumoto, Y.; Nakao, R.; Iwamoto, T.; Shinozuka, O.; Senpuku, H. Effects of pH on the properties of membrane vesicles including glucosyltransferase in Streptococcus mutans. Microorganisms 2021, 9, 2308. [Google Scholar] [CrossRef]
- Toyofuku, M.; Cárcamo-Oyarce, G.; Yamamoto, T.; Eisenstein, F.; Hsiao, C.-C.; Kurosawa, M.; Gademann, K.; Pilhofer, M.; Nomura, N.; Eberl, L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 2017, 8, 481. [Google Scholar] [CrossRef]
- Groessl, M.; Hartinger, C.G.; Polec-Pawlak, K.; Jarosz, M.; Keppler, B.K. Capillary electrophoresis hyphenated to inductively coupled plasma-mass spectrometry: A novel approach for the analysis of anticancer metallodrugs in human serum and plasma. Electrophoresis 2008, 29, 2224–2232. [Google Scholar] [CrossRef]
- Weisbrod, N.; Yechieli, Y.; Shandalov, S.; Lensky, N. On the viscosity of natural hyper-saline solutions and its importance: The dead sea brines. J. Hydrol. 2016, 532, 46–51. [Google Scholar] [CrossRef]
- Dogsa, I.; Brloznik, M.; Stopar, D.; Mandic-Mulec, I. Exopolymer diversity and the role of levan in Bacillus subtilis biofilms. PLoS ONE 2013, 8, e62044. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeter. Biodeg. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Graham, J.E.; Wilkinson, B.J. Staphylococcus aureus osmoregulation: Roles for choline, glycine betaine, proline, and taurine. J. Bacteriol. 1992, 174, 2711–2716. [Google Scholar] [CrossRef]
- Wexler, D.L.; Hudson, M.C.; Burne, R.A. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect. Immun. 1993, 61, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Terao, Y.; Hoshino, T.; Kawabata, S.; Ooshima, T.; Sobue, S.; Kimura, S.; Hamada, S. Molecular analyses of glucosyltransferase genes among strains of Streptococcus mutans. FEMS Microbiol. Lett. 1998, 161, 331–336. [Google Scholar] [CrossRef]
- Smith, D.J.; Akita, H.; King, W.F.; Taubman, M.A. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect. Immunity 1994, 62, 2545–2552. [Google Scholar] [CrossRef]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bavles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef]
- Corrigan, R.M.; Abbott, J.C.; Burhenne, H.; Kaever, V.; Gründling, A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011, 7, e1002217. [Google Scholar] [CrossRef]
- Corrigan, R.M.; Gründling, A. Cyclic di-AMP: Another second messenger enters the fray. Nat. Rev. Microbiol. 2013, 11, 513–524. [Google Scholar] [CrossRef]
- Römling, U. Great times for small molecules: c-di-AMP, a second messenger candidate in bacteria and archaea. Sci. Signal. 2008, 1, 39. [Google Scholar] [CrossRef]
- Witte, G.; Hartung, S.; Büttner, K.; Hopfner, K.P. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 2008, 30, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Zeden, M.S.; Schuster, C.F.; Bowman, L.; Zhong, Q.; Williams, H.D.; Gründling, A. Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J. Biolog. Chem. 2018, 293, 3180–3200. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Luo, S.; Pensinger, D.; Sauer, J.D.; Tong, L.; Woodward, J.J. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc. Natl. Acad. Sci. USA 2015, 112, E747–E756. [Google Scholar] [CrossRef]
- Luo, Y.; Helmann, J.D. Analysis of the role of Bacillus subtilis σ(M) in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol. Microbiol. 2012, 83, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, J.; Eisele, L.; Underwood, A.J.; Koestler, B.J.; Waters, C.M.; Metzger, D.W.; Bai, G. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J. Bacteriol. 2013, 195, 5123–5132. [Google Scholar] [CrossRef]
- Jackson-Litteken, C.D.; Ratliff, C.T.; Kneubehl, A.R.; Siletti, C.; Pack, L.; RLan, R.; Huynh, T.N.; Lopez, J.E.; Blevins, J.S. The diadenylate cyclase CdaA is critical for Borrelia turicatae virulence and physiology. Infect. Immun. 2021, 89, e00787-2089. [Google Scholar] [CrossRef]
- Du, B.; Ji, W.; An, H.; Shi, Y.; Huang, Q.; Cheng, Y.; Fu, Q.; Wang, H.; Yan, Y.; Sun, J. Functional analysis of c-di-AMP phosphodiesterase, GdpP, in Streptococcus suis serotype 2. Microbiol. Res. 2014, 169, 749–758. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Bai, G.; Zhou, X.; Wu, H. Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 2016, 99, 945–959. [Google Scholar] [CrossRef]
- Tada, A.; Senpuku, H.; Motozawa, Y.; Yoshihara, A.; Hanada, N.; Tanzawa, H. Association between commensal bacteria and opportunistic pathogens in the dental plaque of elderly individuals. Clin. Microbiol. Infect. 2006, 12, 776–781. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwabuchi, Y.; Yoshida, H.; Kamei, S.; Uematsu, T.; Saito, M.; Senpuku, H. Formation of Mono-Organismal and Mixed Staphylococcus aureus and Streptococcus mutans Biofilms in the Presence of NaCl. Microorganisms 2025, 13, 1118. https://doi.org/10.3390/microorganisms13051118
Iwabuchi Y, Yoshida H, Kamei S, Uematsu T, Saito M, Senpuku H. Formation of Mono-Organismal and Mixed Staphylococcus aureus and Streptococcus mutans Biofilms in the Presence of NaCl. Microorganisms. 2025; 13(5):1118. https://doi.org/10.3390/microorganisms13051118
Chicago/Turabian StyleIwabuchi, Yusuke, Hiroko Yoshida, Shuichiro Kamei, Toshiki Uematsu, Masanori Saito, and Hidenobu Senpuku. 2025. "Formation of Mono-Organismal and Mixed Staphylococcus aureus and Streptococcus mutans Biofilms in the Presence of NaCl" Microorganisms 13, no. 5: 1118. https://doi.org/10.3390/microorganisms13051118
APA StyleIwabuchi, Y., Yoshida, H., Kamei, S., Uematsu, T., Saito, M., & Senpuku, H. (2025). Formation of Mono-Organismal and Mixed Staphylococcus aureus and Streptococcus mutans Biofilms in the Presence of NaCl. Microorganisms, 13(5), 1118. https://doi.org/10.3390/microorganisms13051118





