In Silico Characterization of Resistance and Virulence Genes in Aeromonas jandaei Strains Isolated from Oreochromis niloticus in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Genome Sequencing
2.2. Quality Control, Assembly, Completeness, and Annotation
2.3. Data Acquisition
2.4. Species Classification
2.5. Resistance and Virulence Genes
3. Results
3.1. Genomic Metrics
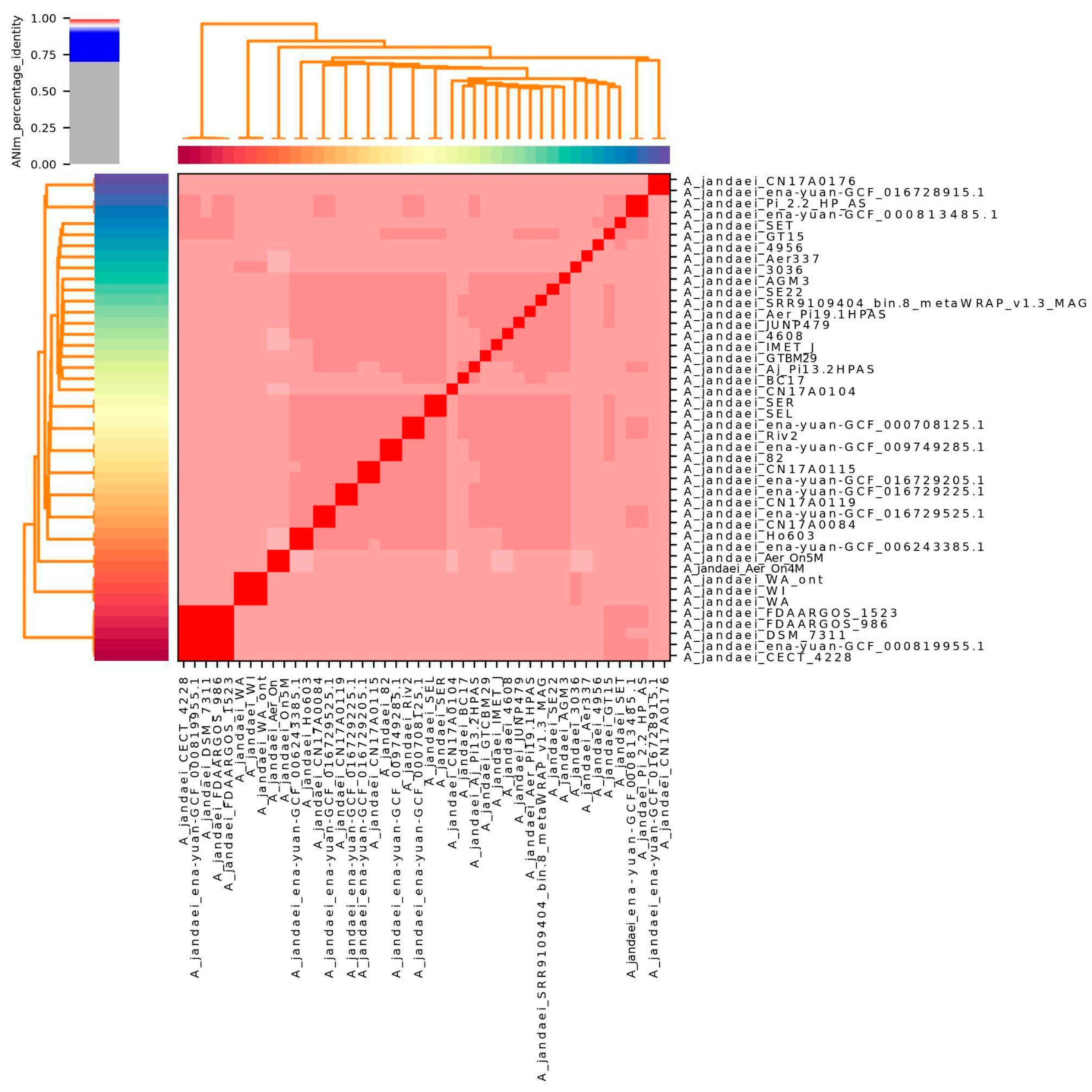
3.2. Genome Nucleotide Similarity
3.3. Phylogenomic Analysis of Orthologous Genes
3.4. Resistance Genes
3.4.1. Antimicrobial Resistance Results
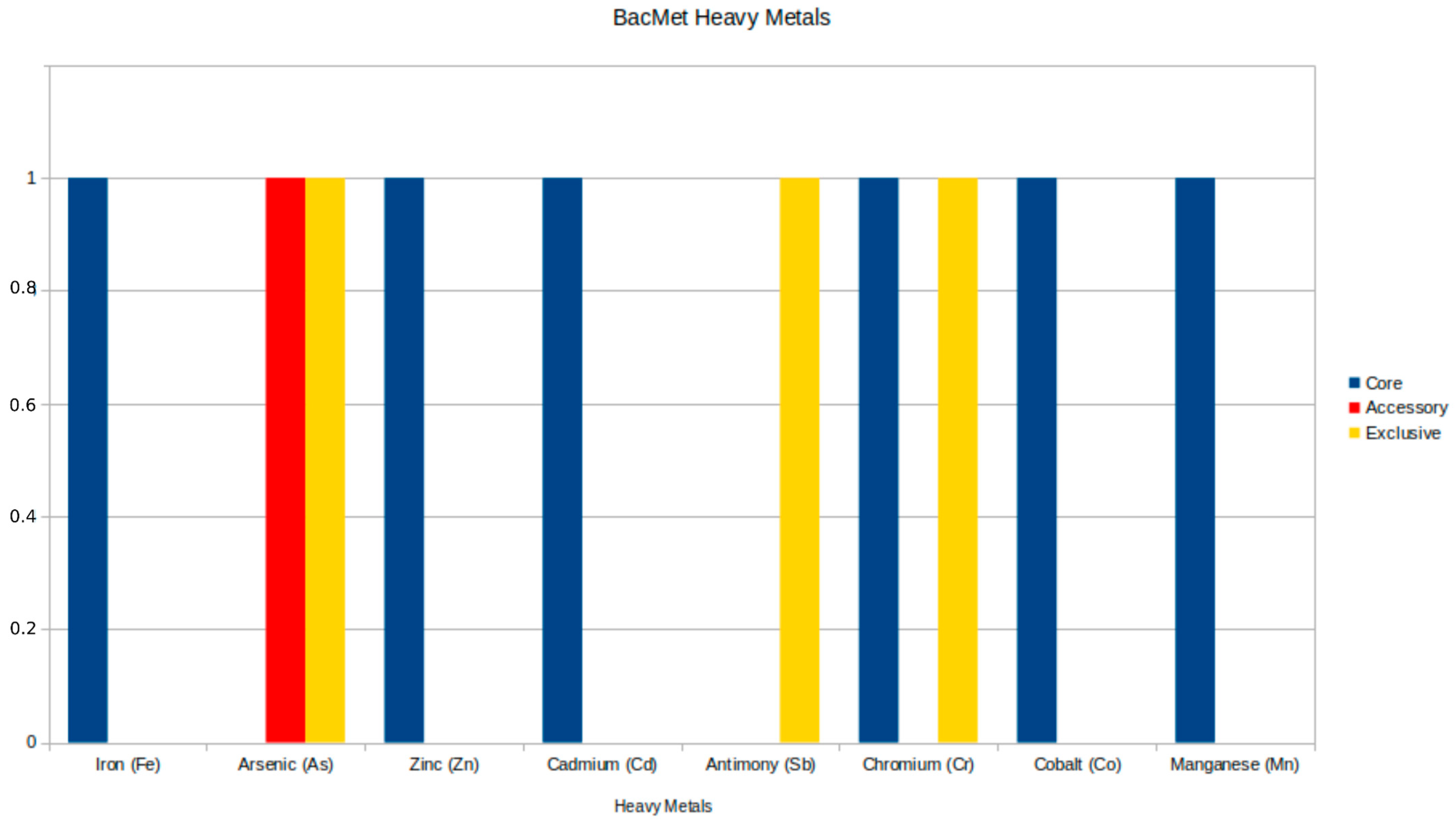
3.4.2. Heavy Metal Resistance Results
3.5. Virulence Genes
4. Discussion
4.1. Resistome Data
4.2. Heavy Metal Resistance
4.3. Virulence Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar]
- FAO (Ed.) The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Tavares-Dias, M.; Martins, M.L. An Overall Estimation of Losses Caused by Diseases in the Brazilian Fish Farms. J. Parasit. Dis. 2017, 41, 913–918. [Google Scholar] [CrossRef]
- Assane, I.M.; de Sousa, E.L.; Valladão, G.M.R.; Tamashiro, G.D.; Criscoulo-Urbinati, E.; Hashimoto, D.T.; Pilarski, F. Phenotypic and Genotypic Characterization of Aeromonas jandaei Involved in Mass Mortalities of Cultured Nile Tilapia, Oreochromis niloticus (L.) in Brazil. Aquaculture 2021, 541, 736848. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Jagoda, S.; Wijewardana, T.; Arulkanthan, A.; Igarashi, Y.; Tan, E.; Kinoshita, S.; Watabe, S.; Asakawa, S. Characterization and Antimicrobial Susceptibility of Motile Aeromonads Isolated from Freshwater Ornamental Fish Showing Signs of Septicaemia. Dis. Aquat. Organ. 2014, 109, 127–137. [Google Scholar] [CrossRef]
- Proietti-Junior, A.A.; Lima, L.S.; Roges, E.M.; Rodrigues, Y.C.; Lima, K.V.B.; Rodrigues, D.P.; Tavares-Dias, M. Experimental Co-infection by Aeromonas hydrophila and Aeromonas jandaei in Pirarucu Arapaima gigas (Pisces: Arapaimidae). Aquac. Res. 2021, 52, 1688–1696. [Google Scholar] [CrossRef]
- Medina-Morillo, M.; Sotil, G.; Arteaga, C.; Cordero, G.; Martins, M.L.; Murrieta-Morey, G.; Yunis-Aguinaga, J. Pathogenic Aeromonas spp. in Amazonian Fish: Virulence Genes and Susceptibility in Piaractus Brachypomus, the Main Native Aquaculture Species in Peru. Aquac. Rep. 2023, 33, 101811. [Google Scholar] [CrossRef]
- Pellin, G.P.; Martins, R.A.; Queiroz, C.A.D.; Sousa, T.F.; Muniz, A.W.; Silva, G.F.D.; Majolo, C. Aeromonas from Farmed Tambaqui from North Brazil: Molecular Identification and Pathogenic Potential. Ciênc. Rural 2023, 53, e20220151. [Google Scholar] [CrossRef]
- Mulia, D.S.; Dwi, N.R.; Suwarsito, S.; Muslimin, B. Molecular Characterization of Pathogenic Aeromonas Jandaei Bacteria Isolated from Cultured Walking Catfish (Clarias sp.). Biodivers. J. Biol. Divers. 2024, 25, 3. [Google Scholar] [CrossRef]
- Laufer, A.S.; Siddall, M.E.; Graf, J. Characterization of the Digestive-Tract Microbiota of Hirudo Orientalis, a European Medicinal Leech. Appl. Environ. Microbiol. 2008, 74, 6151–6154. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Guo, G.; Yang, N.; Li, Q.; Yin, F.; Wang, P.; Zheng, J.; Zeng, J. Three Species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) Isolated from Freshwater Crocodiles (Crocodylus siamensis) with Pneumonia and Septicemia. Lett. Appl. Microbiol. 2019, 68, 212–218. [Google Scholar] [CrossRef]
- Chen, M.; Xue, M.; Chen, J.; Xiao, Z.; Hu, X.; Zhang, C.; Jiang, N.; Fan, Y.; Meng, Y.; Zhou, Y. Isolation, Identification and Characterization of Aeromonas jandaei from Diseased Chinese Soft-Shell Turtles. J. Fish Dis. 2024, 47, e13919. [Google Scholar] [CrossRef]
- Pereira, C.; Duarte, J.; Costa, P.; Braz, M.; Almeida, A. Bacteriophages in the Control of Aeromonas sp. in Aquaculture Systems: An Integrative View. Antibiotics 2022, 11, 163. [Google Scholar] [CrossRef]
- Gazal, L.E.d.S.; Brito, K.C.T.d.; Kobayashi, R.K.T.; Nakazato, G.; Cavalli, L.S.; Otutumi, L.K.; Brito, B.G.d. Antimicrobials and Resistant Bacteria in Global Fish Farming and the Possible Risk for Public Health. Arq. Inst. Biológico 2020, 87, e0362019. [Google Scholar] [CrossRef]
- Gouveia, J.J.d.S.; Regitano, L.C.d.A. Extração de DNA; Embrapa Pecuária Sudeste: São Carlos, Brazil, 2007; pp. 3–8. [Google Scholar]
- Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 30 September 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI Reference Sequences (RefSeq): A Curated Non-Redundant Sequence Database of Genomes, Transcripts and Proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and Taxonomy in Diagnostics for Food Security: Soft-Rotting Enterobacterial Plant Pathogens. Anal. Methods 2015, 8, 12–24. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Rodrigues, D.L.N.; Ariute, J.C.; Rodrigues da Costa, F.M.; Benko-Iseppon, A.M.; Barh, D.; Azevedo, V.; Aburjaile, F. PanViTa: Pan Virulence and resisTance Analysis. Front. Bioinform. 2023, 3, 1070406. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded Curation, Support for Machine Learning, and Resistome Prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Rensing, C.; Kristiansson, E.; Larsson, D.G.J. BacMet: Antibacterial Biocide and Metal Resistance Genes Database. Nucleic Acids Res. 2014, 42, D737–D743. [Google Scholar] [CrossRef]
- Esteve, C.; Alcaide, E.; Giménez, M.J. Multidrug-Resistant (MDR) Aeromonas Recovered from the Metropolitan Area of Valencia (Spain): Diseases Spectrum and Prevalence in the Environment. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 137–145. [Google Scholar] [CrossRef]
- Dhanapala, P.M.; Kalupahana, R.S.; Kalupahana, A.W.; Wijesekera, D.P.H.; Kottawatta, S.A.; Jayasekera, N.K.; Silva-Fletcher, A.; Jagoda, S.S.S.d.S. Characterization and Antimicrobial Resistance of Environmental and Clinical Aeromonas Species Isolated from Fresh Water Ornamental Fish and Associated Farming Environment in Sri Lanka. Microorganisms 2021, 9, 2106. [Google Scholar] [CrossRef]
- Roh, H.; Kannimuthu, D. Comparative Resistome Analysis of Aeromonas Species in Aquaculture Reveals Antibiotic Resistance Patterns and Phylogeographic Distribution. Environ. Res. 2023, 239, 117273. [Google Scholar] [CrossRef]
- Davies, J. Microbes Have the Last Word: A Drastic Re-evaluation of Antimicrobial Treatment Is Needed to Overcome the Threat of Antibiotic-resistant Bacteria. EMBO Rep. 2007, 8, 616–621. [Google Scholar] [CrossRef]
- Quesada, S.P.; Paschoal, J.A.R.; Reyes, F.G.R. Considerations on the Aquaculture Development and on the Use of Veterinary Drugs: Special Issue for Fluoroquinolones—A Review. J. Food Sci. 2013, 78, R1321–R1333. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; Silva, L.H.M.; Gloria, M.B.A. Quinolones and Tetracyclines in Aquaculture Fish by a Simple and Rapid LC-MS/MS Method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef]
- Rosário, A.E.C.D.; Barbanti, A.C.C.; Matos, H.C.; Maia, C.R.M.D.S.; Trindade, J.M.; Nogueira, L.F.F.; Pilarski, F.; Gallani, S.U.; Leal, C.A.G.; Figueiredo, H.C.P.; et al. Antimicrobial Resistance in Lactococcus spp. Isolated from Native Brazilian Fish Species: A Growing Challenge for Aquaculture 2024. Microorganisms 2024, 12, 2327. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Orlando, E.A.; Costa Roque, A.G.; Losekann, M.E.; Colnaghi Simionato, A.V. UPLC–MS/MS Determination of Florfenicol and Florfenicol Amine Antimicrobial Residues in Tilapia Muscle. J. Chromatogr. B 2016, 1035, 8–15. [Google Scholar] [CrossRef]
- Monteiro, S.H.; Francisco, J.G.; Campion, T.F.; Pimpinato, R.F.; Moura Andrade, G.C.R.; Garcia, F.; Tornisielo, V.L. Multiresidue Antimicrobial Determination in Nile Tilapia (Oreochromis niloticus) Cage Farming by Liquid Chromatography Tandem Mass Spectrometry. Aquaculture 2015, 447, 37–43. [Google Scholar] [CrossRef]
- Hooper, D.C. Emerging Mechanisms of Fluoroquinolone Resistance. Emerg. Infect. Dis. 2001, 7, 337–341. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Lakin, S.M.; Dean, C.; Noyes, N.R.; Dettenwanger, A.; Ross, A.S.; Doster, E.; Rovira, P.; Abdo, Z.; Jones, K.L.; Ruiz, J.; et al. MEGARes: An Antimicrobial Resistance Database for High Throughput Sequencing. Nucleic Acids Res. 2017, 45, D574–D580. [Google Scholar] [CrossRef]
- Maia, J.C.d.S.; Silva, G.A.d.A.; Cunha, L.S.d.B.; Gouveia, G.V.; Góes-Neto, A.; Brenig, B.; Araújo, F.A.; Aburjaile, F.; Ramos, R.T.J.; Soares, S.C.; et al. Genomic Characterization of Aeromonas Veronii Provides Insights into Taxonomic Assignment and Reveals Widespread Virulence and Resistance Genes throughout the World. Antibiotics 2023, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Bergum, J.A. Profiling Antimicrobial Resistance Genes in Bacteria Species Isolated from Fish Foods Sold in Retail Outlets. Master’s Thesis, Nord University, Bodø, Norway, 2024. [Google Scholar]
- Das, S.; Sreejith, S.; Babu, J.; Francis, C.; Midhun, J.S.; Aswani, R.; Sebastain, K.S.; Radhakrishnan, E.K.; Mathew, J. Genome Sequencing and Annotation of Multi-Virulent Aeromonas veronii XhG1.2 Isolated from Diseased Xiphophorus hellerii. Genomics 2021, 113, 991–998. [Google Scholar] [CrossRef]
- Alksne, L.E.; Rasmussen, B.A. Expression of the AsbA1, OXA-12, and AsbM1 Beta-Lactamases in Aeromonas Jandaei AER 14 Is Coordinated by a Two-Component Regulon. J. Bacteriol. 1997, 179, 2006–2013. [Google Scholar] [CrossRef]
- Liu, D.; Song, H.; Ke, Y.; Xia, J.; Shen, Y.; Ou, Y.; Hao, Y.; He, J.; Li, X.; Zhou, Y.; et al. Co-Existence of Two Novel Phosphoethanolamine Transferase Gene Variants in Aeromonas jandaei from Retail Fish. Int. J. Antimicrob. Agents 2020, 55, 105856. [Google Scholar] [CrossRef] [PubMed]
- Akinbowale, O.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and Heavy Metal Resistance in Motile Aeromonads and Pseudomonads from Rainbow Trout (Oncorhynchus mykiss) Farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Heo, G.-J. Antibiotic and Heavy Metal Resistance Genes in Aeromonas spp. Isolated from Marketed Manila Clam (Ruditapes philippinarum) in Korea. J. Appl. Microbiol. 2019, 127, 941–952. [Google Scholar] [CrossRef]
- Lee, S.W.; Wendy, W. Antibiotic and Heavy Metal Resistance of Aeromonas hydrophila and Edwardsiella tarda Isolated from Red Hybrid Tilapia (Oreochromis spp.) Coinfected with Motile Aeromonas Septicemia and Edwardsiellosis. Vet. World 2017, 10, 803–807. [Google Scholar] [CrossRef]
- Miranda, C.D.; Castillo, G. Resistance to Antibiotic and Heavy Metals of Motile Aeromonads from Chilean Freshwater. Sci. Total Environ. 1998, 224, 167–176. [Google Scholar] [CrossRef]
- Abdul Raheem Hasan, S.; Sajid Al-Jubori, S.; Abdul Sattar Salman, J. Molecular Analysis of fimA Operon Genes among UPEC Local Isolates in Baghdad City. Arch. Razi Inst. 2021, 76, 829–840. [Google Scholar] [CrossRef]
- Boyd, J.M.; Dacanay, A.; Knickle, L.C.; Touhami, A.; Brown, L.L.; Jericho, M.H.; Johnson, S.C.; Reith, M. Contribution of Type IV Pili to the Virulence of Aeromonas salmonicida subsp. Salmonicida in Atlantic Salmon (Salmo salar L.). Infect. Immun. 2008, 76, 1445–1455. [Google Scholar] [CrossRef]
- Stones, D.H.; Krachler, A.M. Against the Tide: The Role of Bacterial Adhesion in Host Colonization. Biochem. Soc. Trans. 2016, 44, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. npj Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Canals, R.; Ramirez, S.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Polar Flagellum Biogenesis in Aeromonas hydrophila. J. Bacteriol. 2006, 188, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Kazuhiro, K.; Tsutomu, O.; Tatsuki, Y.; Tetsuo, I. Sequence Analysis of the flgA Gene and Its Adjacent Region in Salmonella Typhimurium, and Identification of Another Flagellar Gene, flgN. Gene 1994, 143, 49–54. [Google Scholar] [CrossRef]
- Cambronne, E.D.; Roy, C.R. Recognition and Delivery of Effector Proteins into Eukaryotic Cells by Bacterial Secretion Systems. Traffic 2006, 7, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.M. Extra! Extracellular Effector Delivery into Host Cells via the Type 3 Secretion System. mBio 2017, 8, e00594-17. [Google Scholar] [CrossRef]
- Macion, A.; Wyszyńska, A.; Godlewska, R. Delivery of Toxins and Effectors by Bacterial Membrane Vesicles. Toxins 2021, 13, 845. [Google Scholar] [CrossRef]
- Brooks, T.M.; Unterweger, D.; Bachmann, V.; Kostiuk, B.; Pukatzki, S. Lytic Activity of the Vibrio Cholerae Type VI Secretion Toxin VgrG-3 Is Inhibited by the Antitoxin TsaB. J. Biol. Chem. 2013, 288, 7618–7625. [Google Scholar] [CrossRef]
- Coulthurst, S. The Type VI Secretion System: A Versatile Bacterial Weapon. Microbiology 2019, 165, 503–515. [Google Scholar] [CrossRef]
- Carruthers, M.D.; Nicholson, P.A.; Tracy, E.N.; Munson, R.S. Acinetobacter Baumannii Utilizes a Type VI Secretion System for Bacterial Competition. PLoS ONE 2013, 8, e59388. [Google Scholar] [CrossRef]



| A. jandaei | ||||
|---|---|---|---|---|
| Strain | Size (Mb) | Assembly | Host/ Isolation Source | Location |
| FDAARGOS_986 | 4.6 | GCF_016127195.1 | Unknown | Germany: Braunschweig |
| DSM 7311 | 4.6 | GCF_037890695.1 | Homo sapiens (stool) | USA: Oregon |
| 3036 | 4.6 | GCF_018802365.1 | Chicken | USA: California |
| JUNP479 | 4.5 | GCF_016865345.1 | Homo sapiens | Nepal |
| BC17 | 4.5 | GCF_026651485.1 | River water | Ghana |
| 4608 | 4.5 | GCF_018802325.1 | Poultry | USA: California |
| FDAARGOS_1523 | 4.5 | GCF_020341535.1 | Unknown | Germany: Braunschweig |
| Aer_Pi19.1HPAS | 4.6 | GCF_017310175.1 | Phractocephalus hemioliopterus (kidney) | Brazil: Petrolina |
| AGM3 | 4.4 | GCF_036687345.1 | Heteroapneustes fossilis (lesion) | Bangladesh: Mymensingh |
| CECT 4228 | 4.5 | GCF_000819955.1 | Unknown | Unknown |
| IMET J | 4.7 | GCF_002925765.1 | Marine water | USA |
| CN17A0115 | 4.5 | GCF_016729205.1 | Homo sapiens (stool) | China: Shenzhen, Longgang |
| ena-yuan-GCF_016729205.1 | 4.5 | GCF_963890675.1 | Unknown | Unknown |
| CN17A0084 | 4.5 | GCF_016729525.1 | Homo sapiens (stool) | China: Shenzhen, Longgang |
| ena-yuan-GCF_016729525.1 | 4.5 | GCF_963891405.1 | Unknown | Unknown |
| Pi_2.2_HP_AS | 4.6 | GCF_019348715.1 | Phractocephalus hemioliopterus (kidney) | Brazil: Petrolina |
| ena-yuan-GCF_019348715.1 | 4.6 | GCF_963894795.1 | Unknown | Unknown |
| CN17A0176 | 4.6 | GCF_016728915.1 | Homo sapiens (stool) | China: Shenzhen, Nanshan |
| ena-yuan-GCF_016728915.1 | 4.3 | GCF_963892185.1 | Unknown | Unknown |
| 82 | 4.8 | GCF_009749285.1 | Fish | China: Shenzhen |
| ena-yuan-GCF_009749285.1 | 4.8 | GCF_963886775.1 | Unknown | Unknown |
| CN17A0104 | 4.4 | GCF_016729325.1 | Homo sapiens (stool) | China: Shenzhen, Longgang |
| CN17A0119 | 4.7 | GCF_016729225.1 | Homo sapiens (stool) | China: Shenzhen, Longgang |
| ena-yuan-GCF_016729225.1 | 4.7 | GCF_963890895.1 | Unknown | Unknown |
| Aer337 | 4.7 | GCF_003849685.1 | Homo sapiens (fecal sample) | Brazil: Sao Bento do Una |
| Aj_Pi13.2HPAS | 4.9 | GCF_021735865.1 | Phractocephalus hemioliopterus (kidney) | Brazil: Petrolina |
| WA | 4.6 | GCF_034721275.1 | Unknown | Unknown |
| WI | 4.6 | GCF_034720845.1 | Unknown | Unknown |
| SE22 | 4.5 | GCF_034092435.1 | Unknown | Unknown |
| ena-yuan-GCF_000819955.1 | 4.5 | GCF_963867185.1 | Unknown | Unknown |
| SEL | 4.9 | GCF_034720715.1 | Unknown | Unknown |
| SET | 4.7 | GCF_034092485.1 | Unknown | Unknown |
| SER | 4.9 | GCF_034721165.1 | Unknown | Unknown |
| Ho603 | 4.6 | GCF_006243385.1 | Hirudo orientalis (gut) | Unknown |
| ena-yuan-GCF_006243385.1 | 4.6 | GCF_963883215.1 | Unknown | Unknown |
| Riv2 | 4.5 | GCF_000708125.1 | River water | USA |
| ena-yuan-GCF_000708125.1 | 4.5 | GCF_963865165.1 | Unknown | Unknown |
| 3299 | 4.6 | GCA_014217505.1 | Chicken | USA: California |
| 3348 | 4.6 | GCA_014217485.1 | Chicken | USA: California |
| 4956 | 4.7 | GCA_018802265.1 | Chicken | USA: California |
| Colony25 | 4.6 | GCA_016902935.1 | Rectal swab | Thailand: Pattani |
| Colony119 | 5.1 | GCA_016902955.1 | Rectal swab | Thailand: Udon Thani |
| WA_ont | 4.7 | GCA_036452205.1 | Unknown | Unknown |
| SEL_ont | 5 | GCA_036452235.1 | Unknown | Unknown |
| SRR9109404_bin.8_metaWRAP_v1.3_MAG | 4.4 | GCF_945952205.1 | Fish gut metagenome | Unknown |
| L14h | 4.7 | GCA_000813485.1 | Lake water | Malaysia |
| ena-yuan-GCF_000813485.1 | 4.7 | GCA_963866915.1 | Unknown | Unknown |
| SRR12456170_bin.18_metaWRAP_v1.3_MAG | 4 | GCA_945957145.1 | Fish gut metagenome | Unknown |
| Strain | bp | Contigs | N50 | L50 | GC Content |
|---|---|---|---|---|---|
| GTBM29 | 4681450 | 64 | 417315 | 4 | 58.75 |
| GT15 | 4578060 | 51 | 200574 | 8 | 58.86 |
| On4M | 4487199 | 73 | 197578 | 7 | 59.01 |
| On5M | 4488431 | 74 | 197525 | 6 | 59.01 |
| Strain | BioProject | BioSample |
|---|---|---|
| GTBM29 | PRJNA590577 | SAMN13335576 |
| GT15 | PRJNA590949 | SAMN13343509 |
| On4M | PRJNA592537 | SAMN13428651 |
| On5M | PRJNA607235 | SAMN14124565 |
| Gene | Core | Drug Class | Resistance Mechanism | AMR Gene Family |
|---|---|---|---|---|
| bacA | Yes | peptide antibiotic | antibiotic target alteration | undecaprenyl pyrophosphate related proteins |
| OXA-12 | Yes | penam | antibiotic inactivation | OXA beta-lactamase |
| MCR-7.1 | Yes | peptide antibiotic | antibiotic target alteration | MCR phosphoethanolamine transferase |
| CRP | Yes | penam, fluoroquinolone antibiotic, macrolide antibiotic | antibiotic efflux | resistance-nodulation-cell division (RND) antibiotic efflux pump |
| MCR-3.1 | Yes | peptide antibiotic | antibiotic target alteration | MCR phosphoethanolamine transferase |
| rsmA | Yes | phenicol antibiotic, diaminopyrimidine antibiotic, fluoroquinolone antibiotic | antibiotic efflux | resistance-nodulation-cell division (RND) antibiotic efflux pump |
| arnA | Yes | peptide antibiotic | antibiotic target alteration | pmr phosphoethanolamine transferase |
| cphA7 | Yes | carbapenem | antibiotic inactivation | CphA beta-lactamase |
| FOX-2 * | No | cephamycin, cephalosporin | antibiotic inactivation | FOX beta-lactamase |
| AQU-2 ** | No | cephalosporin | antibiotic inactivation | AQU beta-lactamase |
| Gene | Mechanism | Compound |
|---|---|---|
| ruvB | Holliday junction ATP-dependent DNA helicase RuvB | Chromium (Cr) |
| arsC | Protein ArsC | Arsenic (As) |
| mntH/yfeP | Divalent metal cation transporter MntH | Manganese (Mn), Iron (Fe), Cadmium (Cd), Cobalt (Co), Zinc (Zn) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, M.L.O.; Rodrigues, D.L.N.; de Lima, G.B.V.; Ariute, J.C.; Gouveia, G.V.; de Simoni Gouveia, J.J.; Azevedo, V.; Brenig, B.; Guédon, E.; Tavares, G.C.; et al. In Silico Characterization of Resistance and Virulence Genes in Aeromonas jandaei Strains Isolated from Oreochromis niloticus in Brazil. Microorganisms 2025, 13, 1094. https://doi.org/10.3390/microorganisms13051094
Duarte MLO, Rodrigues DLN, de Lima GBV, Ariute JC, Gouveia GV, de Simoni Gouveia JJ, Azevedo V, Brenig B, Guédon E, Tavares GC, et al. In Silico Characterization of Resistance and Virulence Genes in Aeromonas jandaei Strains Isolated from Oreochromis niloticus in Brazil. Microorganisms. 2025; 13(5):1094. https://doi.org/10.3390/microorganisms13051094
Chicago/Turabian StyleDuarte, Marcela Laryssa Oliveira, Diego Lucas Neres Rodrigues, Gabryel Bernardo Vieira de Lima, Juan Carlos Ariute, Gisele Veneroni Gouveia, João José de Simoni Gouveia, Vasco Azevedo, Bertram Brenig, Eric Guédon, Guilherme Campos Tavares, and et al. 2025. "In Silico Characterization of Resistance and Virulence Genes in Aeromonas jandaei Strains Isolated from Oreochromis niloticus in Brazil" Microorganisms 13, no. 5: 1094. https://doi.org/10.3390/microorganisms13051094
APA StyleDuarte, M. L. O., Rodrigues, D. L. N., de Lima, G. B. V., Ariute, J. C., Gouveia, G. V., de Simoni Gouveia, J. J., Azevedo, V., Brenig, B., Guédon, E., Tavares, G. C., da Costa, M. M., Pereira, U. d. P., & Aburjaile, F. F. (2025). In Silico Characterization of Resistance and Virulence Genes in Aeromonas jandaei Strains Isolated from Oreochromis niloticus in Brazil. Microorganisms, 13(5), 1094. https://doi.org/10.3390/microorganisms13051094









