Assessment of the Presence of Free-Living Amoebae in Soil Samples from the Northwest Region of Spain Using Culture and Molecular Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Sampling
2.2. Free-Living Amoebae Isolation
2.3. DNA Extraction
2.4. PCR and Molecular Characterizations
2.5. Multiplex Quantitative Real-Time PCR Assay (q-PCR)
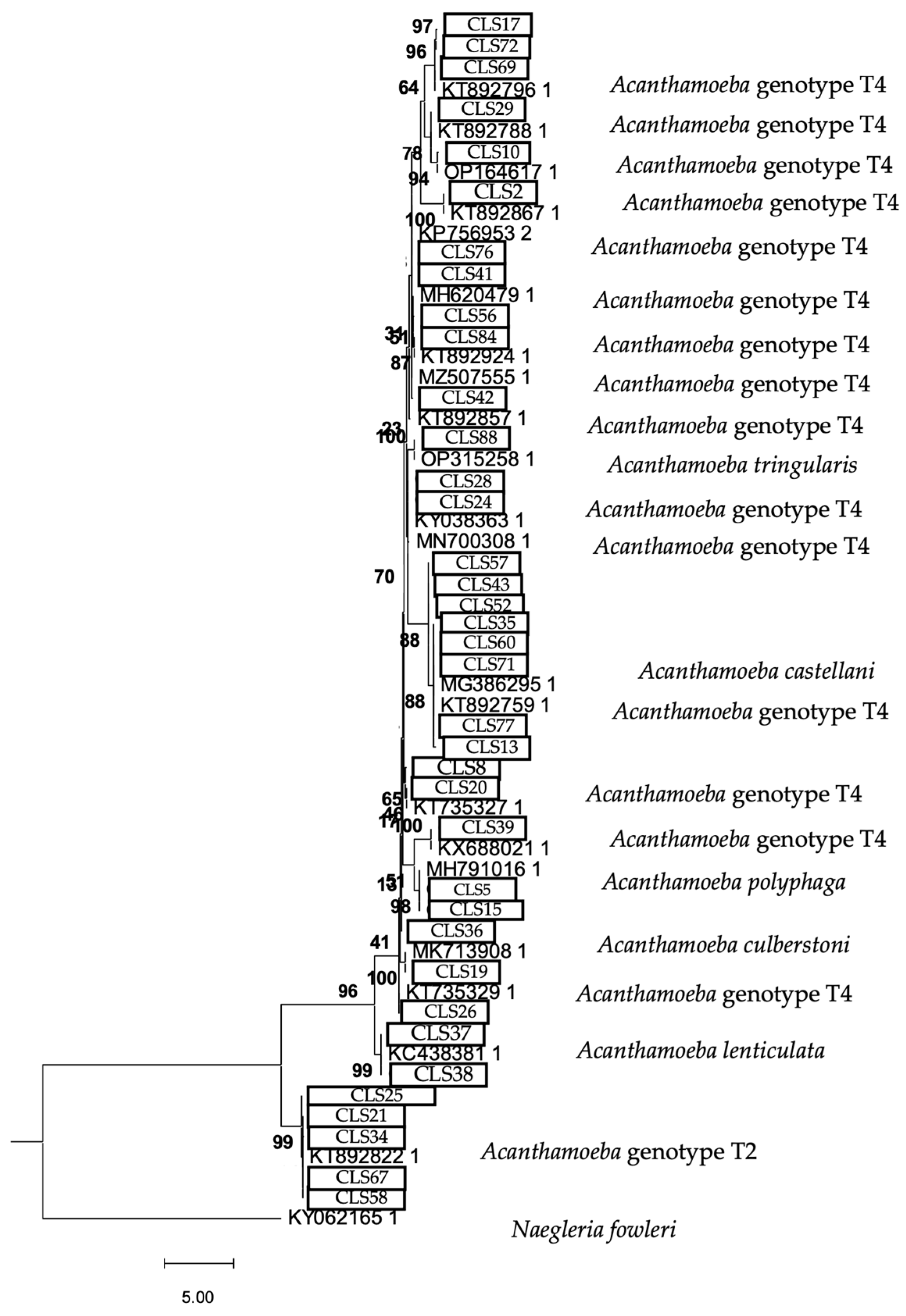
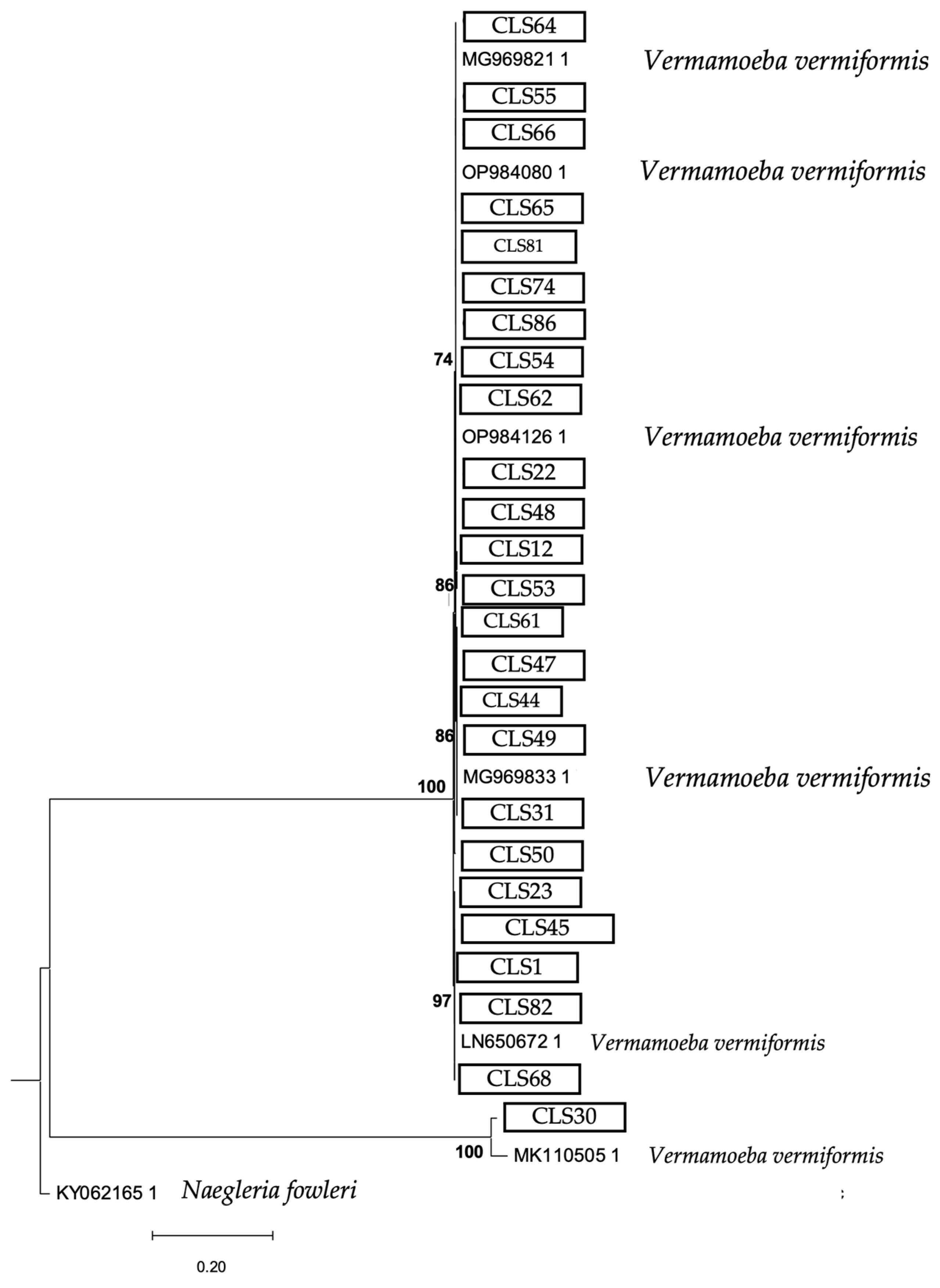
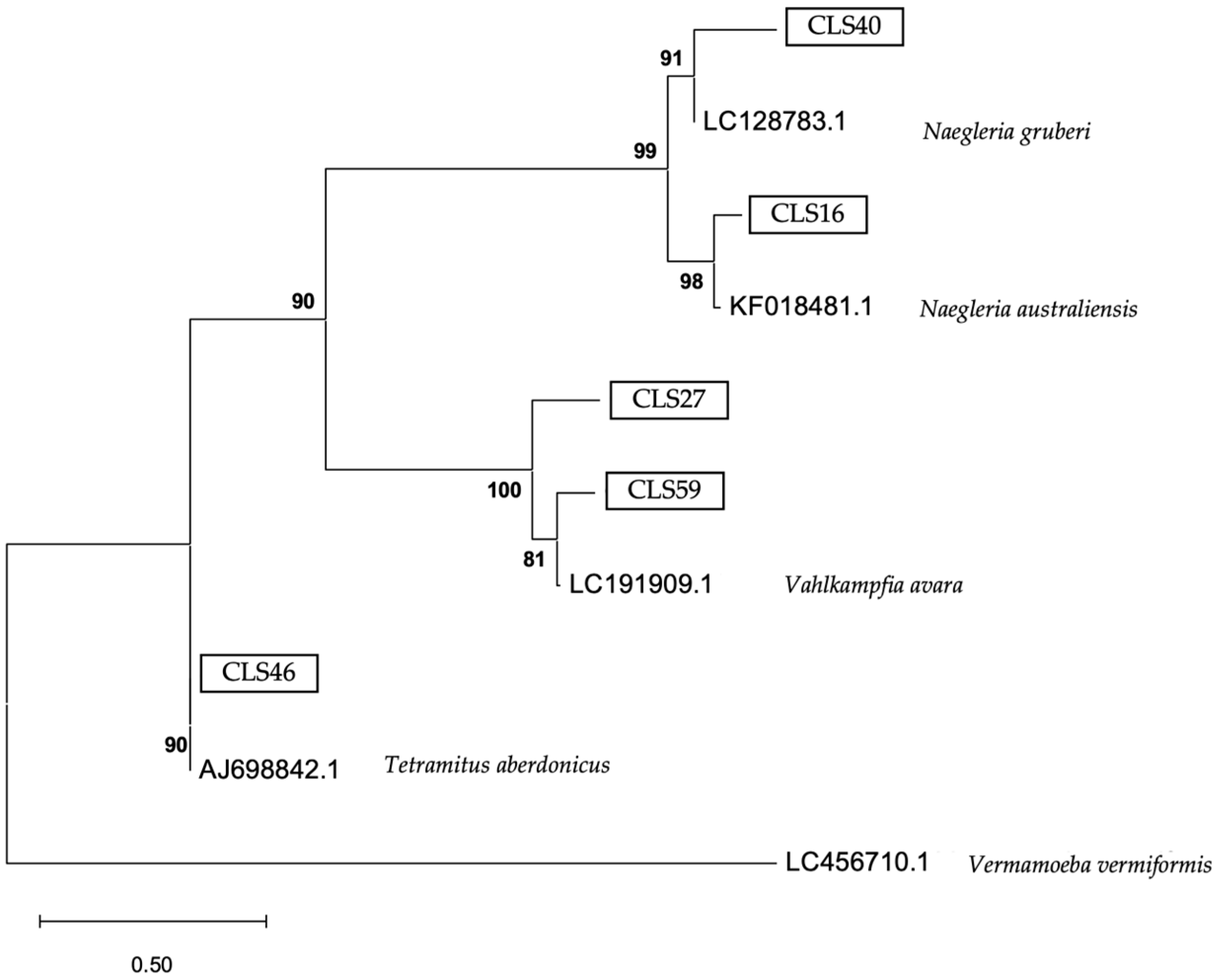
2.6. Phylogenetic Analysis
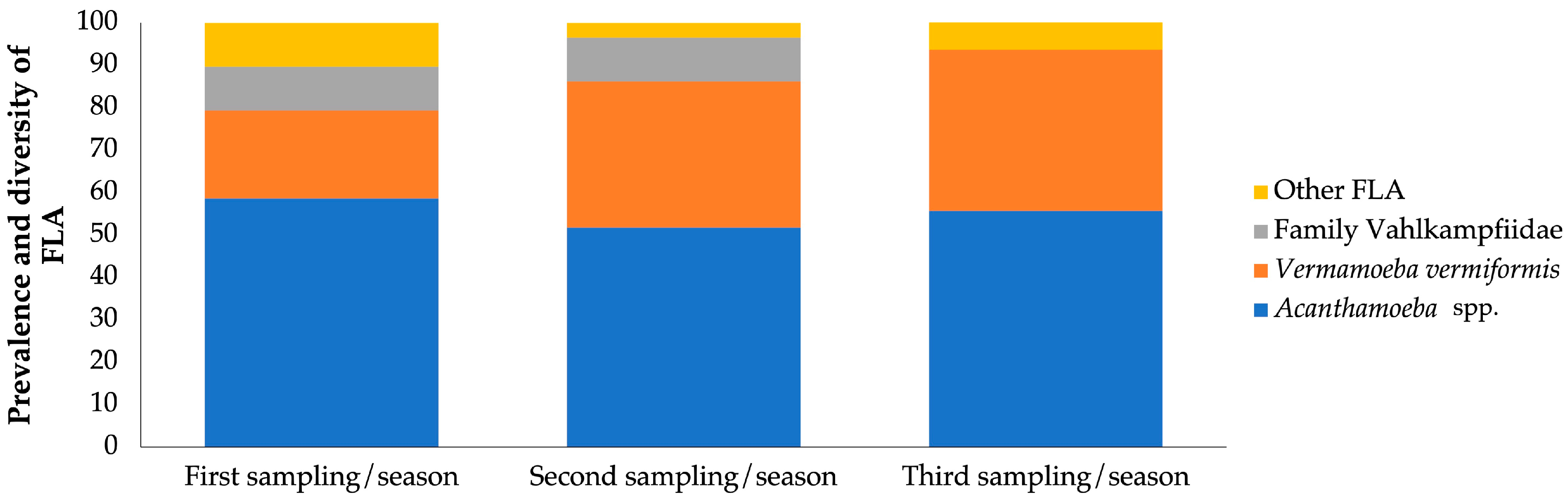
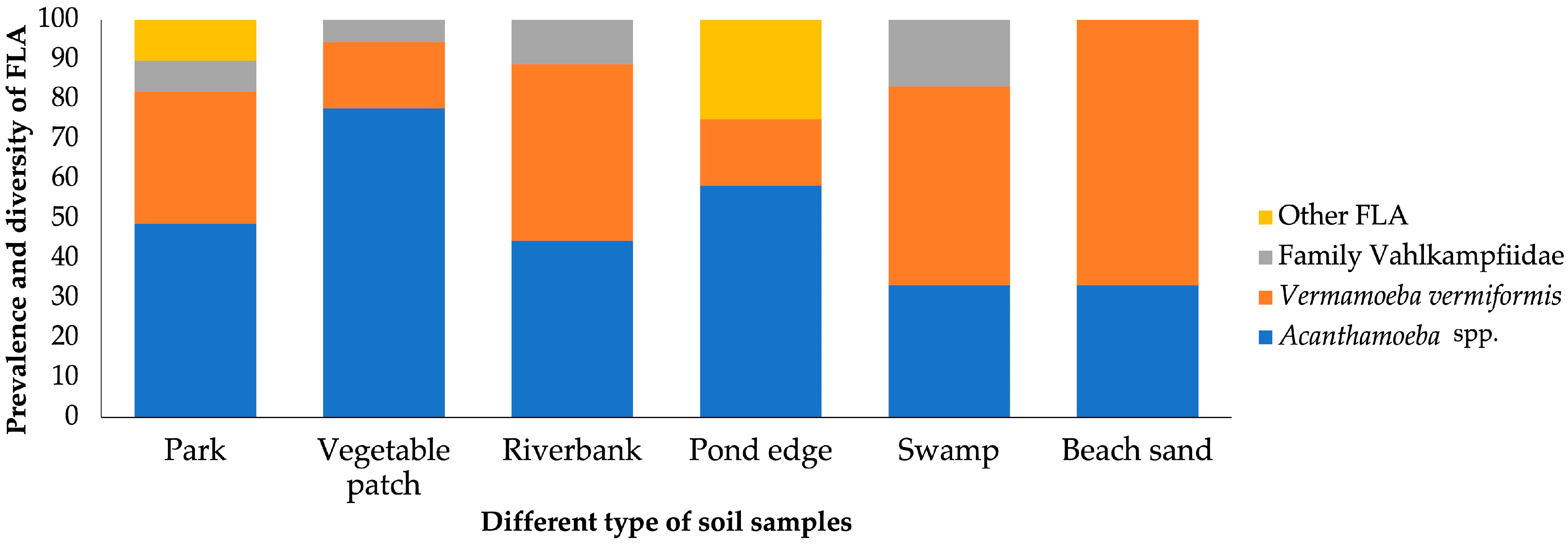
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, N.K.; Parija, S.C. Balamuthia mandrillaris: An opportunistic, free-living ameba—An updated review. Trop. Parasitol. 2021, 11, 78–88. [Google Scholar] [CrossRef]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- Qvarnstrom, Y.; da Silva, A.J.; Schuster, F.L.; Gelman, B.B.; Visvesvara, G.S. Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J. Infect. Dis. 2009, 199, 1139–1142. [Google Scholar] [CrossRef]
- Pinna, A.; Porcu, T.; Boscia, F.; Cano, A.; Erre, G.; Mattana, A. Free-Living Amoebae Keratitis. Cornea 2017, 36, 785–790. [Google Scholar] [CrossRef]
- Aitken, D.A.; Hay, J.; Kinnear, F.B.; Kirkness, C.M.; Lee, W.R.; Seal, D.V. Amebic Keratitis in a Wearer of Disposable Contact Lenses due to a Mixed Vahlkampfia and Hartmannella Infection. Ophthalmology 1996, 103, 485–494. [Google Scholar] [CrossRef]
- Alexandrais, G.; Miller, D.; Huang, A.J. Amebic keratitis due to Vahlkampfia infection following corneal trauma. Arch. Ophthalmol. 1998, 116, 950–951. [Google Scholar]
- Scheid, P.L.; Lâm, T.T.; Sinsch, U.; Balczun, C. Vermamoeba vermiformis as etiological agent of a painful ulcer close to the eye. Parasitol. Res. 2019, 118, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Rodier, M.H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A Free-Living Amoeba of Interest. Microb. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Aykur, M.; Selver, O.B.; Dagci, H.; Palamar, M. Vermamoeba vermiformis as the etiological agent in a patient with suspected non-Acanthamoeba keratitis. Parasitol. Res. 2024, 123, 323. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Delafont, V.; Rodier, M.H.; Cateau, E.; Héchard, Y. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol. Rev. 2019, 43, 415–434. [Google Scholar] [CrossRef]
- Mella, C.; Medina, G.; Flores-Martin, S.; Toledo, Z.; Simaluiza, R.J.; Pérez-Pérez, G.; Fernández, H. Interaction between zoonotic bacteria and free-living amoebas: A new angle of an epidemiological polyhedron of public health importance? Arch. Med. Vet. 2016, 48, 1–10. [Google Scholar] [CrossRef]
- Milanez, G.D.; Carlos, K.B.; Adao, M.E.; Ayson, B.B.; Dicon, A.V.; Gahol, R.A.M.; Lacre, S.K.S.; Marquez, F.P.E.; Perez, A.J.M.; Karanis, P. Epidemiology of free-living amoebae infections in Africa: A review. Pathog. Glob. Health 2023, 117, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Ortega-Rivas, A.; Foronda, P.; Martínez, E.; Valladares, B. Isolation and identification of pathogenic Acanthamoeba strains in Tenerife, Canary Islands, Spain from water sources. Parasitol. Res. 2005, 95, 273–277. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Monteverde-Miranda, C.A.; Jiménez, C.; Tejedor, M.L.; Valladares, B.; Ortega-Rivas, A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann. Agric. Environ. Med. 2005, 12, 233–236. [Google Scholar]
- Reyes-Batlle, M.; Zamora-Herrera, J.; Vargas-Mesa, A.; Valerón-Tejera, M.A.; Wagner, C.; Martín-Navarro, C.M.; López-Arencibia, A.; Sifaoui, I.; Martínez-Carretero, E.; Valladares, B.; et al. Acanthamoeba genotypes T2, T4, and T11 in soil sources from El Hierro island, Canary Islands, Spain. Parasitol. Res. 2016, 115, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Batlle, M.; Todd, C.D.; Martín-Navarro, C.M.; López-Arencibia, A.; Cabello-Vilchez, A.M.; González, A.C.; Córdoba-Lanús, E.; Lindo, J.F.; Valladares, B.; Piñero, J.E.; et al. Isolation and characterization of Acanthamoeba strains from soil samples in Gran Canaria, Canary Islands, Spain. Parasitol. Res. 2014, 113, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Magnet, A.; Galván, A.L.; Fenoy, S.; Izquierdo, F.; Rueda, C.; Vadillo, C.F.; Pérez-Irezábal, J.; Bandyopadhyay, K.; Visvesvara, G.S.; da Silva, A.J.; et al. Molecular characterization of Acanthamoeba isolated in water treatment plants and comparison with clinical isolates. Parasitol. Res. 2012, 111, 383–392. [Google Scholar] [CrossRef]
- Corsaro, D.; Saucedo Pages, G.; Catalan, V.; Loret, J.F.; Greub, G. Biodiversity of amoebae and amoeba-associated bacteria in water treatment plants. Int. J. Hyg. Environ. Health 2010, 213, 158–166. [Google Scholar] [CrossRef]
- Agencia Estatal de Meteorología, Ministerio de Agricultura, Alimentación y Medio Ambiente. Iberian Climate Atlas. Air Temperature and Precipitation (1971–2000); Agencia Estatal de Meteorología, Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2021.
- Page, F.C. Mitosis and pseudopod formation in Vexillifera bacillipedes n. sp.; A majorellid amoeba. Trans. Am. Microsc. Soc. 1969, 88, 394–400. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Wagner, C.; Zamora-Herrera, J.; Vargas-Mesa, A.; Sifaoui, I.; González, A.C.; López-Arencibia, A.; Valladares, B.; Martínez-Carretero, E.; Piñero, J.E.; et al. Isolation of thermotolerant Vermamoeba vermiformis strains from water sources in Lanzarote Island, Canary Islands, Spain. Acta Parasitol. 2016, 61, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, N.; Schild, M.; Panaiotov, S.; Kurdova-Mintcheva, R.; Gottstein, B.; Walochnik, J.; Aspöck, H.; Lucas, M.S.; Müller, N. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol. Res. 2004, 92, 405–413. [Google Scholar] [CrossRef]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef]
- Qvarnstrom, Y.; Visvesvara, G.S.; Sriram, R.; da Silva, A.J. Multiplex Real-Time PCR Assay for Simultaneous Detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 2006, 44, 3589–3595. [Google Scholar] [CrossRef]
- Córdoba-Lanús, E.; Reyes-Batlle, M.; Domínguez-de-Barros, A.; Pérez-Pérez, P.; Rodríguez-Expósito, R.L.; García-Ramos, A.; Sifaoui, I.; García-Pérez, O.; Aneiros-Giraldez, G.; Piñero, J.E.; et al. Multiplex Real-Time Polymerase Chain Reaction Assay to Detect Acanthamoeba spp., Vermamoeba vermiformis, Naegleria fowleri, and Balamuthia mandrillaris in Different Water Sources. Am. J. Trop. Med. Hyg. 2024, 111, 785–790. [Google Scholar] [CrossRef]
- De Jonckheere, J.F.; Brown, S. The identification of vahlkampfiid amoebae by ITS sequencing. Protist 2005, 156, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.L.N.; Paes, J.; Rott, M.B. Genotype distribution of Acanthamoeba in keratitis: A systematic review. Parasitol. Res. 2021, 120, 3051–3063. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Kofman, A.; Guarner, J. Infections Caused by Free-Living Amoebae. J. Clin. Microbiol. 2022, 60, e00228-21. [Google Scholar] [CrossRef]
- Stahl, L.M.; Olson, J.B. Environmental abiotic and biotic factors affecting the distribution and abundance of Naegleria fowleri. FEMS Microbiol. Ecol. 2020, 97, fiaa238. [Google Scholar] [CrossRef] [PubMed]
- Resumen Climático de Noviembre de 2022. Available online: https://www.aemet.es/es/noticias/2022/12/Resumen_clima_noviembre_2022 (accessed on 15 January 2025).
- Bass, P.; Bischoff, P.J. Seasonal variability in abundance and diversity of soil gymnamoebae along a short transect in southeastern USA. J. Eukaryot. Microbiol. 2001, 48, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zaragoza, S.; Marín-Morales, B.; Torres-Guzmán, J.C.; Lagunas-Martínez, A.; Rodríguez-García, A.; González-Vázquez, M.C. Primer registro de amibas de vida libre en el balneario natural “Hierve el Agua” en el municipio de San Lorenzo Albarradas, estado de Oaxaca. Rev. Mex. Biodivers. 2018, 89, 672–681. [Google Scholar]
- Rodríguez-Zaragoza, S.; Mayzlish, E.; Steinberger, Y. Vertical distribution of the free-living amoeba population in soil under desert shrubs in the Negev desert. Appl. Environ. Microbiol. 2005, 71, 2053–2060. [Google Scholar] [CrossRef]
- Bonilla-Lemus, P.; Ramírez-Bautista, G.; Zamora-Muñoz, C.; Ibarra-Montes, R.; Ramírez-Flores, E.; Hernández-Martínez, M. Acanthamoeba spp. in domestic tap water in houses of contact lens wearers in the metropolitan area of Mexico City. Exp. Parasitol. 2020, 126, 54–58. [Google Scholar] [CrossRef]
- Maciver, S.K.; Asif, M.; Simmen, M.W.; Lorenzo-Morales, J. A systematic analysis of Acanthamoeba genotype frequency correlated with source and pathogenicity: T4 is confirmed as a pathogen-rich genotype. Eur. J. Protistol. 2013, 49, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Maghsood, A.H.; Sissons, J.; Rezaian, M.; Nolder, D.; Warhurst, D.; Khan, N.A. Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J. Med. Microbiol. 2005, 54, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Behera, H.S.; Panda, A.; Satpathy, G.; Bandivadekar, P.; Vanathi, M.; Agarwal, T.; Nayak, N.; Tandon, R. Genotyping of Acanthamoeba spp. and characterization of the prevalent T4 type along with T10 and unassigned genotypes from amoebic keratitis patients in India. J. Med. Microbiol. 2016, 65, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Koller, R.; Hünninghaus, M.; Dumack, K.; Urich, T.; Bonkowski, M. The soil food web revisited: Diverse and widespread mycophagous soil protists. Soil Biol. Biochem. 2016, 94, 10–18. [Google Scholar] [CrossRef]
- Otero-Ruiz, A.; Gonzalez-Zuñiga, L.D.; Rodriguez-Anaya, L.Z.; Lares-Jiménez, L.F.; Gonzalez-Galaviz, J.R.; Lares-Villa, F. Distribution and Current State of Molecular Genetic Characterization in Pathogenic Free-Living Amoebae. Pathogens 2022, 11, 1199. [Google Scholar] [CrossRef]
- Walochnik, J.; Aichelburg, A.; Assadian, O.; Steuer, A.; Visvesvara, G.; Vetter, N.; Aspöck, H. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 2008, 46, 338–340. [Google Scholar] [CrossRef]
- Mascaró, C.; Fluviá, C.; Osuna, A.; Guevara, D. Virulent Naegleria sp. Isolated from a River in Cadiz (Spain). J. Parasitol. 1981, 67, 599. [Google Scholar] [CrossRef]
- King, K.M.; Van Doorslaer, K. Building (Viral) Phylogenetic Trees Using a Maximum Likelihood Approach. Curr. Protoc. Microbiol. 2018, 51, e63. [Google Scholar] [CrossRef]
- Pazoki, H.; Niyyati, M.; Javanmard, E.; Lasjerdi, Z.; Spotin, A.; Mirjalali, H.; Behravan, M.R. Isolation and Phylogenetic Analysis of Free-Living Amoebae (Acanthamoeba, Naegleria, and Vermamoeba) in the Farmland Soils and Recreational Places in Iran. Acta Parasitol. 2020, 65, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, P.; Artigas, P.; Reyes-Batlle, M.; Córdoba-Lanús, E.; Rodríguez-Expósito, R.L.; Cuervo, P.F.; Domínguez-de-Barros, A.; García-Pérez, O.; Valero, M.A.; De Elías, A.; et al. Potentially pathogenic free-living amoebae at very high altitude: Detection by multiplex qPCR in the Northern Altiplano fascioliasis hyperendemic area in Bolivia. One Health 2025, 20, 100985. [Google Scholar] [CrossRef]
- Reynaud, Y.; Ducat, C.; Talarmin, A.; Marcelino, I. Cartography of Free-Living Amoebae in Soil in Guadeloupe (French West Indies) Using DNA Metabarcoding. Pathogens 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Batlle, M.; Wagner, C.; López-Arencibia, A.; Sifaoui, I.; Martínez-Carretero, E.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Isolation and molecular characterization of a Naegleria strain from a recreational water fountain in Tenerife, Canary Islands, Spain. Acta Parasitol. 2017, 62, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Batlle, M.; Rizo-Liendo, A.; Viera-Santana, R.A.; Afonso-Morales, S.; López-Arencibia, A.; Sifaoui, I.; Chiboub, O.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Rodríguez-Expósito, R.L.; et al. Isolation and Molecular Identification of Naegleria australiensis in Irrigation Water of Fuerteventura Island, Spain. Acta Parasitol. 2019, 64, 331–335. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Díaz, F.J.; Sifaoui, I.; Rodríguez-Expósito, R.; Rizo-Liendo, A.; Piñero, J.E.; Lorenzo-Morales, J. Free living amoebae isolation in irrigation waters and soils of an insular arid agroecosystem. Sci. Total Environ. 2021, 753, 141833. [Google Scholar] [CrossRef]
- Aykur, M.; Dagci, H. Molecular identification of Acanthamoeba spp., Balamuthia mandrillaris and Naegleria fowleri in soil samples using quantitative real-time PCR assay in Turkey; Hidden danger in the soil! Acta Trop. 2023, 244, 106956. [Google Scholar] [CrossRef]
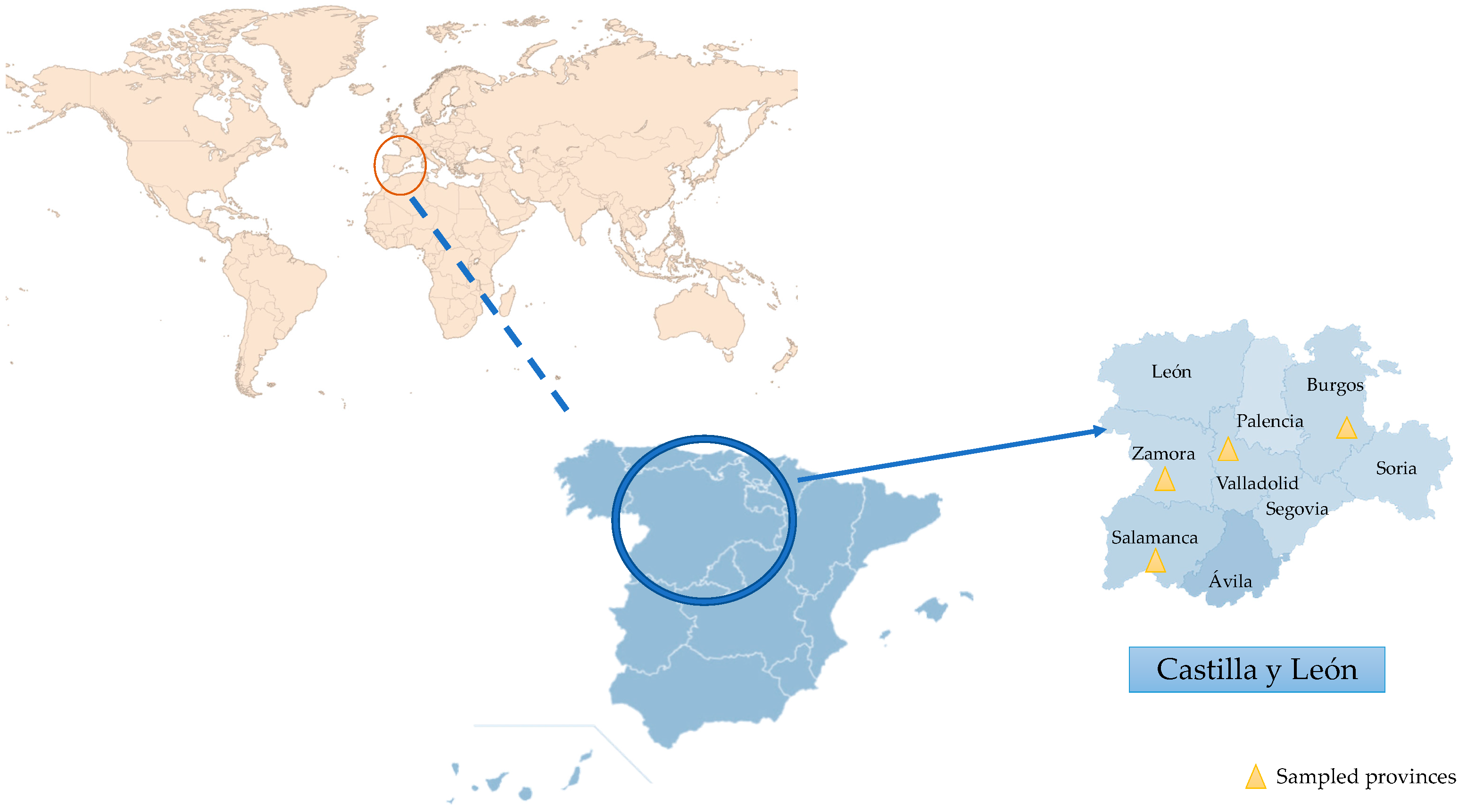

| Sample | Locality | Province | Coordinates | Soil Type | Sampling Period |
|---|---|---|---|---|---|
| SS1 | Salamanca | Salamanca | 40.959703, −5.665465 | Park | T1–T3 |
| SS2 | Salamanca | Salamanca | 40.956167, −5.670507 | Riverbank | T1–T3 |
| SS3 | Salamanca | Salamanca | 40.9612710, −5.6556529 | Park | T1–T3 |
| SS4 | Salamanca | Salamanca | 40.967759, −5.657005 | Park | T1–T3 |
| SS5 | Salamanca | Salamanca | 40.967934, −5.651229 | Park | T1–T3 |
| SS6 | Salamanca | Salamanca | 40.966007, −5.680474 | Park | T1–T3 |
| SS7 | Salamanca | Salamanca | 40.964590, −5.682484 | Park | T1–T3 |
| SS8 | Salamanca | Salamanca | 40.962817, −5.677331 | Park | T1–T3 |
| SS9 | Aldeatejada | Salamanca | 40.946595, −5.681442 | Edge of a pond | T1–T3 |
| SS10 | Galindo y Perahuy | Salamanca | 40.925187, −5.805347 | Vegetable patch | T1–T3 |
| SS11 | Trabanca | Salamanca | 41.231362, −6.386124 | Vegetable patch | T1–T3 |
| SS12 | Trabanca | Salamanca | 41.231895, −6.383861 | Vegetable patch | T1–T3 |
| SS13 | Espadaña | Salamanca | 41.131510, −6.361370 | Edge of a pond | T1–T3 |
| SS14 | Almendra | Salamanca | 41.273762, −6.321856 | Swamp | T1–T3 |
| SS15 | Almendra | Salamanca | 41.270194, −6.334739 | Swamp | T1–T3 |
| SS16 | Miranda de Azán | Salamanca | 40.888489, −5.683101 | Park | T1–T3 |
| SS17 | Miranda de Azán | Salamanca | 40.874163, −5.683229 | Edge of a pond | T1–T3 |
| SS18 | Miranda de Azán | Salamanca | 40.875737, −5.683251 | Vegetable patch | T1–T3 |
| SS19 | Miranda de Azán | Salamanca | 40.875737, −5.683251 | Vegetable patch | T1–T3 |
| VS1 | Valladolid | Valladolid | 41.657199, −4.733434 | Beach sand | T1–T3 |
| VS2 | Valladolid | Valladolid | 41.579598, −4.661287 | Edge of a pond | T1–T3 |
| VS3 | Valladolid | Valladolid | 41.657347, −4.732622 | Park | T1–T3 |
| VS4 | Valladolid | Valladolid | 41.644965, −4.730745 | Park | T1–T3 |
| VS5 | Valladolid | Valladolid | 41.645413, −4.729856 | Park | T1–T3 |
| ZS1 | Benavente | Zamora | 42.003000, −5.677081 | Park | T1–T3 |
| ZS2 | Benavente | Zamora | 41.995946, −5.682736 | Park | T1–T3 |
| ZS3 | Benavente | Zamora | 41.996640, −5.676092 | Vegetable patch | T1–T3 |
| ZS4 | Zamora | Zamora | 41.495446, −5.754557 | Riverbank | T1–T3 |
| BS1 | Burgos | Burgos | 42.337932, −3.705546 | Riverbank | T1–T3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pérez, P.; Rodríguez-Escolar, I.; Córdoba-Lanús, E.; Domínguez-de-Barros, A.; García-Pérez, O.; Piñero, J.E.; Morchón, R.; Lorenzo-Morales, J. Assessment of the Presence of Free-Living Amoebae in Soil Samples from the Northwest Region of Spain Using Culture and Molecular Assays. Microorganisms 2025, 13, 1065. https://doi.org/10.3390/microorganisms13051065
Pérez-Pérez P, Rodríguez-Escolar I, Córdoba-Lanús E, Domínguez-de-Barros A, García-Pérez O, Piñero JE, Morchón R, Lorenzo-Morales J. Assessment of the Presence of Free-Living Amoebae in Soil Samples from the Northwest Region of Spain Using Culture and Molecular Assays. Microorganisms. 2025; 13(5):1065. https://doi.org/10.3390/microorganisms13051065
Chicago/Turabian StylePérez-Pérez, Patricia, Iván Rodríguez-Escolar, Elizabeth Córdoba-Lanús, Angélica Domínguez-de-Barros, Omar García-Pérez, José E. Piñero, Rodrigo Morchón, and Jacob Lorenzo-Morales. 2025. "Assessment of the Presence of Free-Living Amoebae in Soil Samples from the Northwest Region of Spain Using Culture and Molecular Assays" Microorganisms 13, no. 5: 1065. https://doi.org/10.3390/microorganisms13051065
APA StylePérez-Pérez, P., Rodríguez-Escolar, I., Córdoba-Lanús, E., Domínguez-de-Barros, A., García-Pérez, O., Piñero, J. E., Morchón, R., & Lorenzo-Morales, J. (2025). Assessment of the Presence of Free-Living Amoebae in Soil Samples from the Northwest Region of Spain Using Culture and Molecular Assays. Microorganisms, 13(5), 1065. https://doi.org/10.3390/microorganisms13051065










