Heterologous Expression and Enzymatic Properties of β-Glucuronidase from Clostridium perfringens and Its Application in Bilirubin Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid and Strain
2.2. Medium
2.3. Expression and Purification of CpGUS Protein
2.4. Parameter Optimization of CpGUS Fermentation Process
2.5. Enzymatic Properties of CpGUS
2.6. Determination of Enzyme Kinetic Parameters
2.7. Catalytic Mechanism of CpGUS Hydrolysis of pNPG and Conjugated Bilirubin
2.8. E. coli BL21(DE3)/pET28a-CpGUS in a 5 L Fermenter
2.9. Whole-Cell Transformation of Pig Bile to Prepare Unconjugated Bilirubin
3. Results
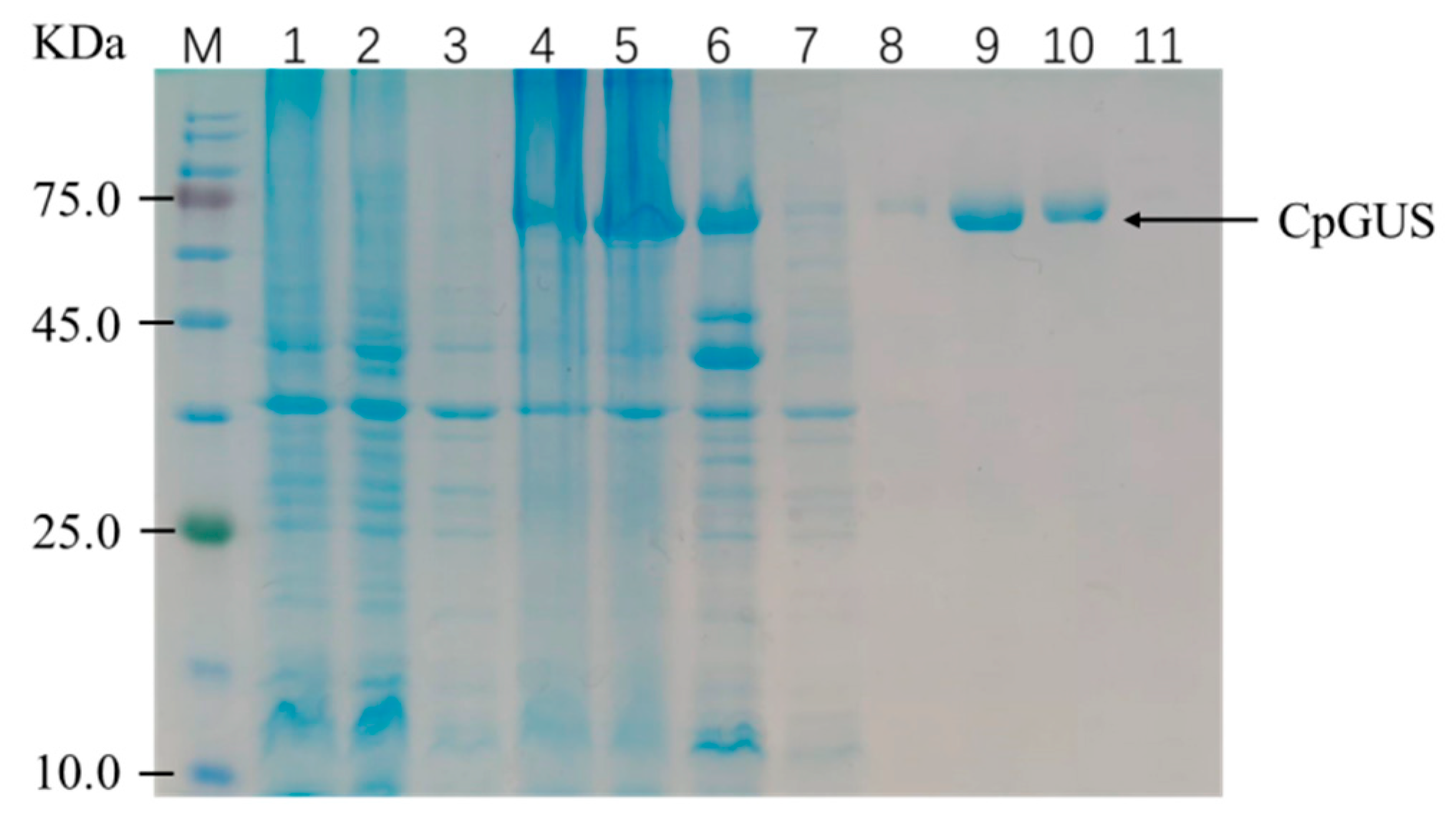
3.1. Construction and Expression of Recombinant Plasmid
3.2. Parameter Optimization of Fermentation Process
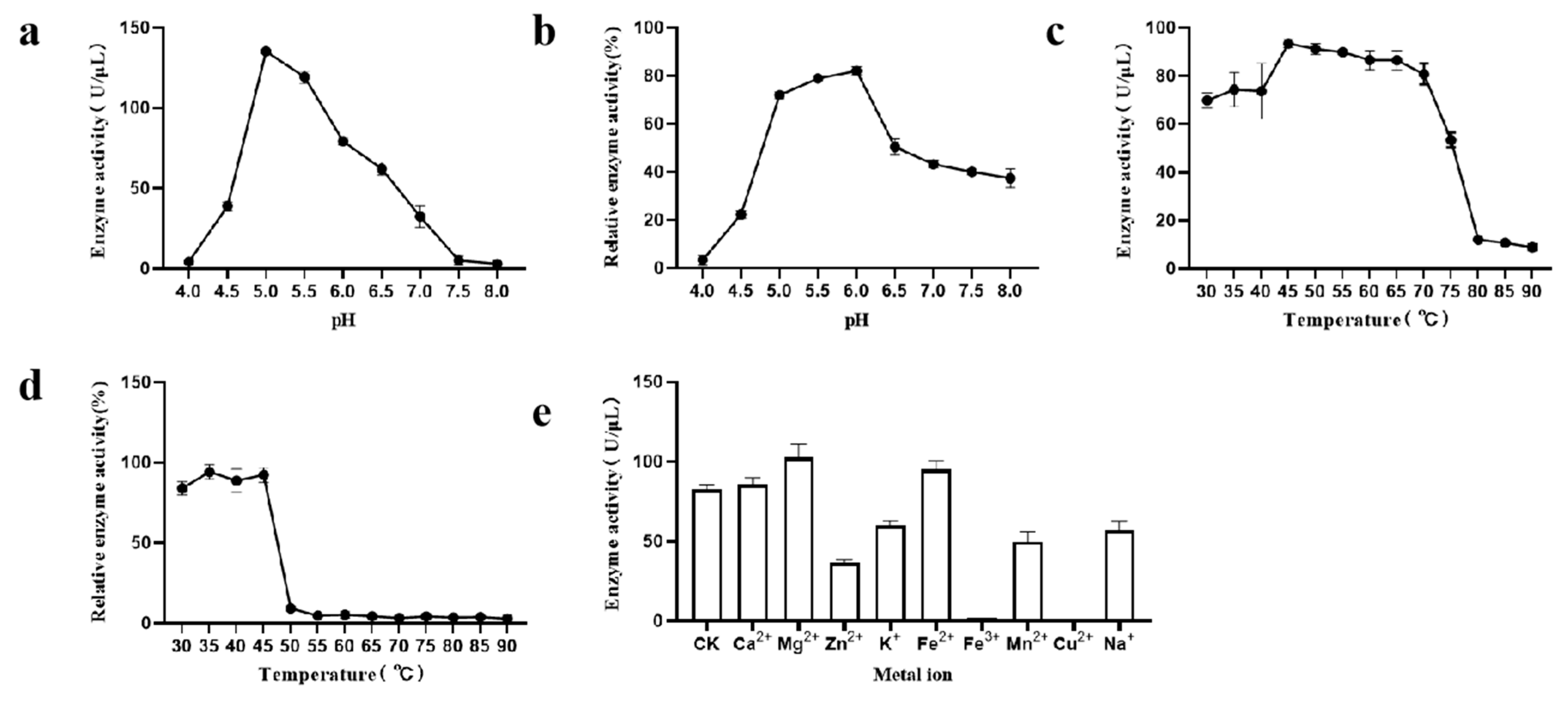
3.3. Characteristics of CpGUS
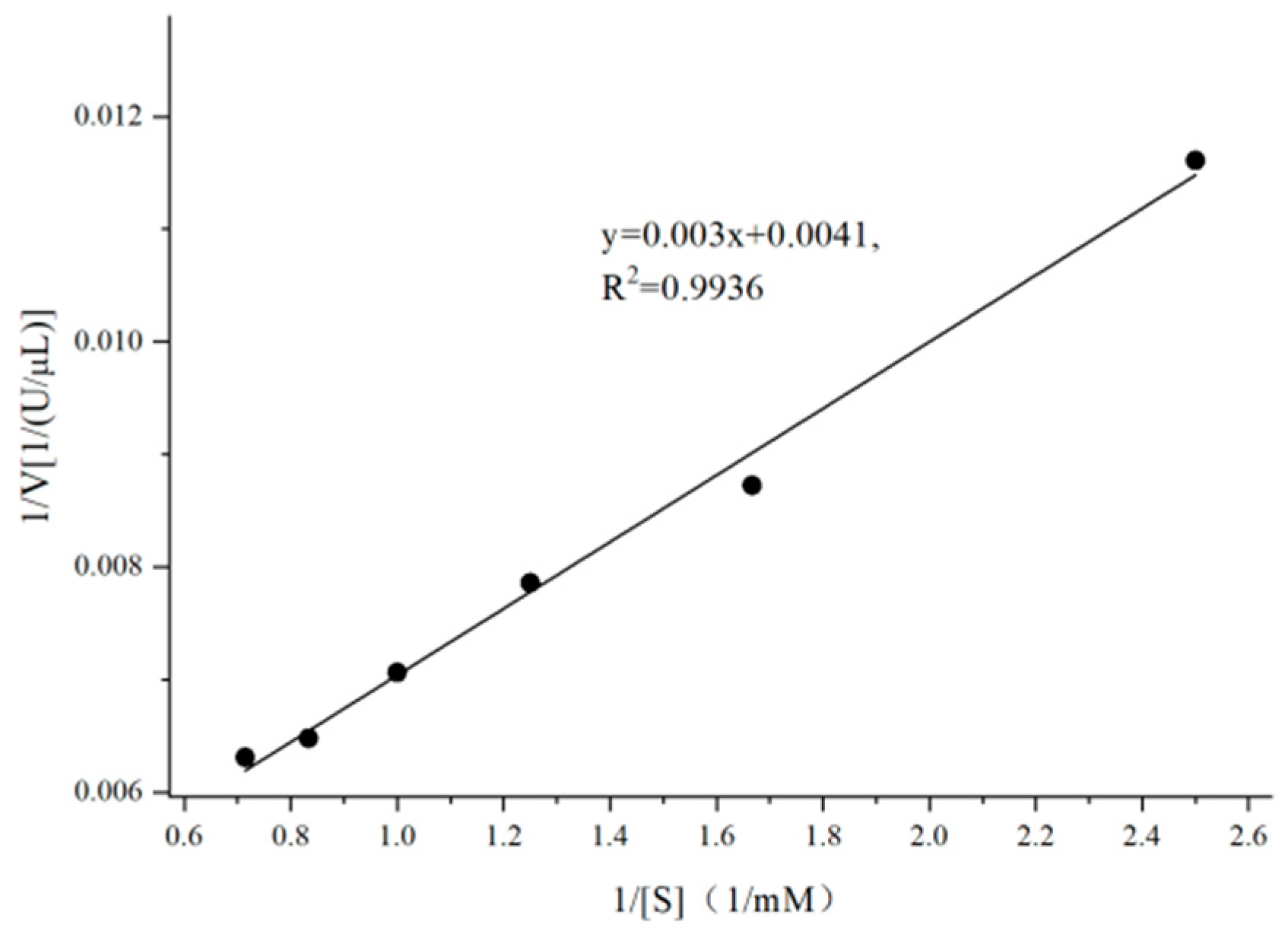
3.4. Analysis of Kinetic Parameters of Enzymes
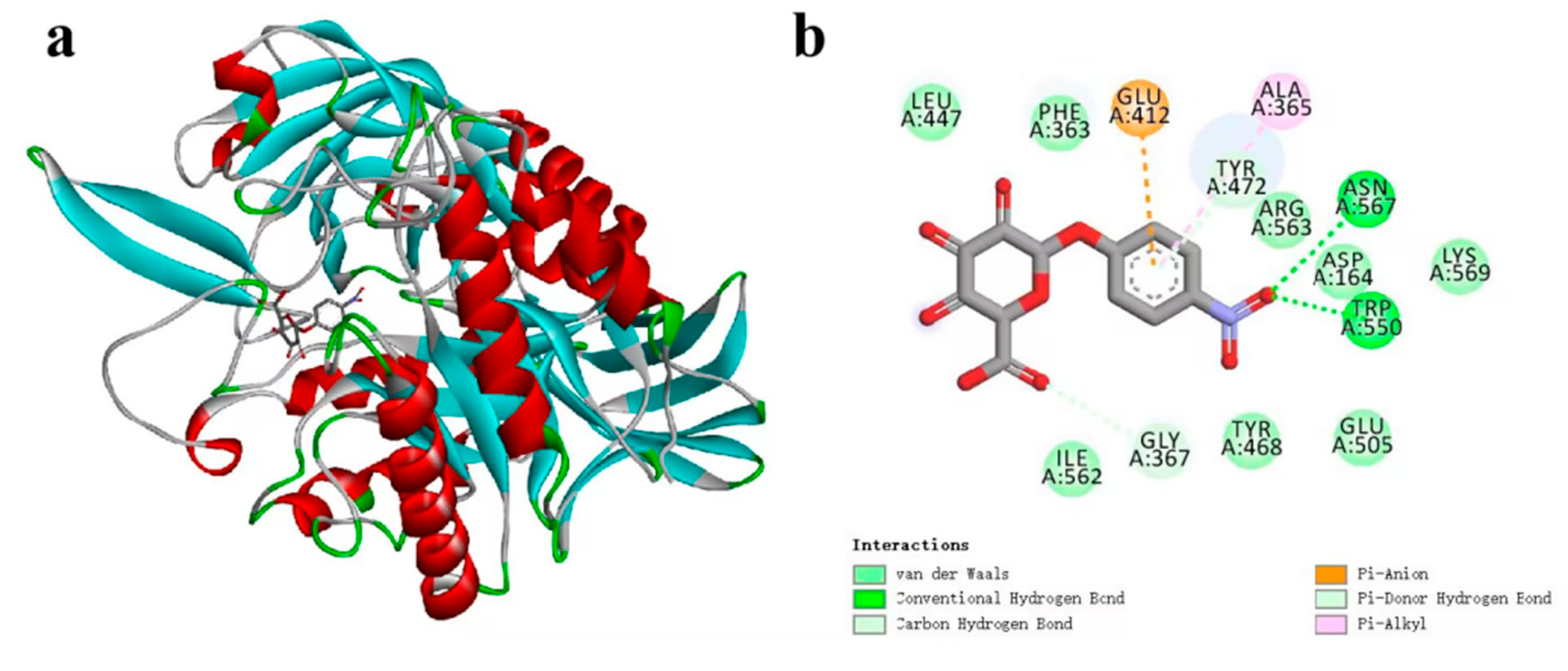
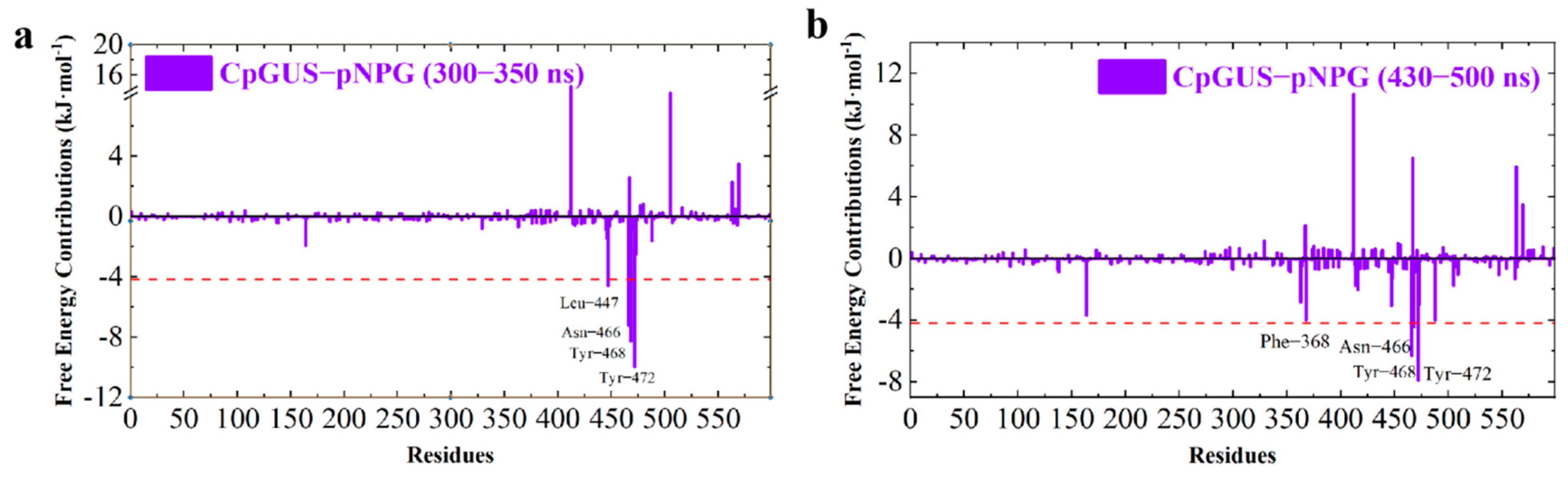
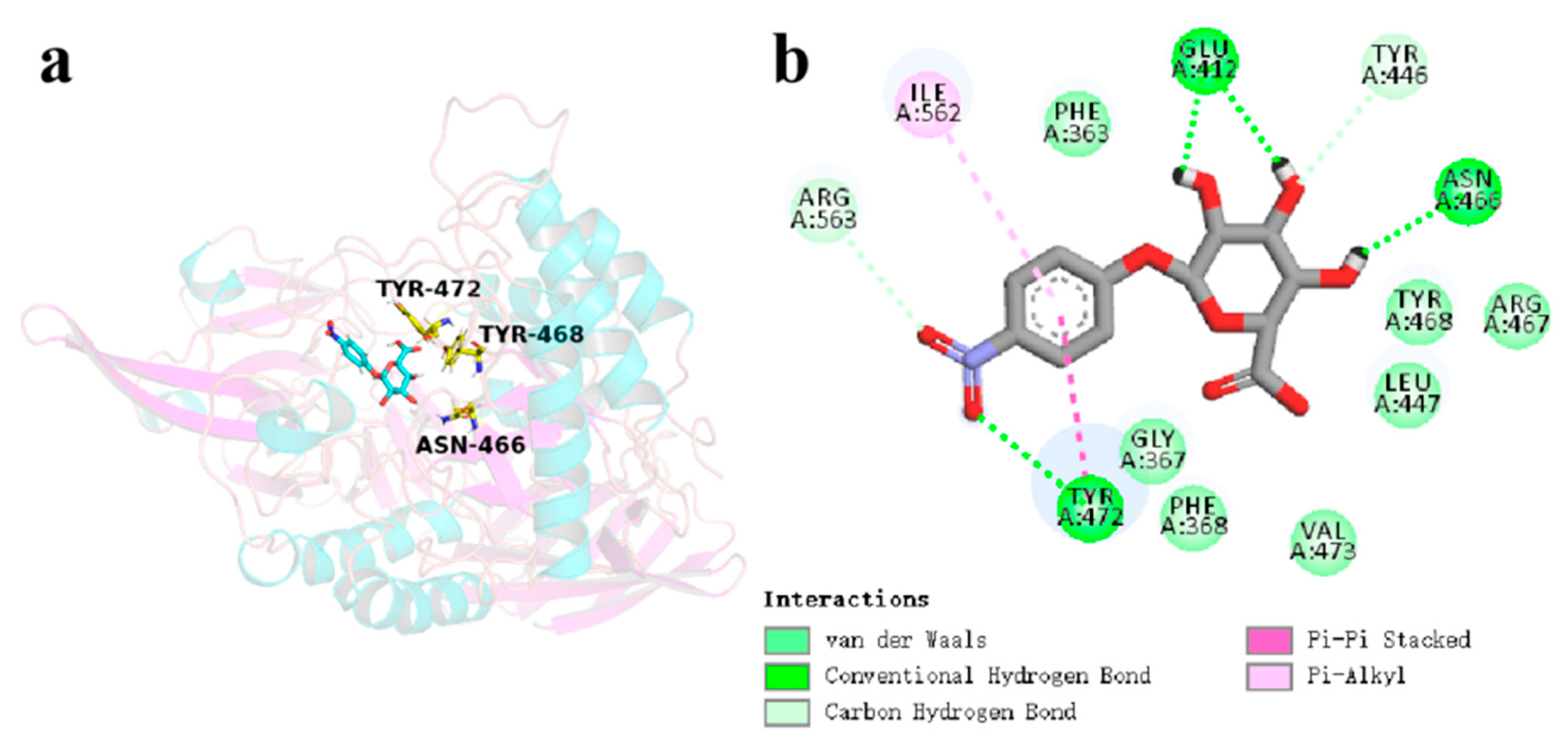
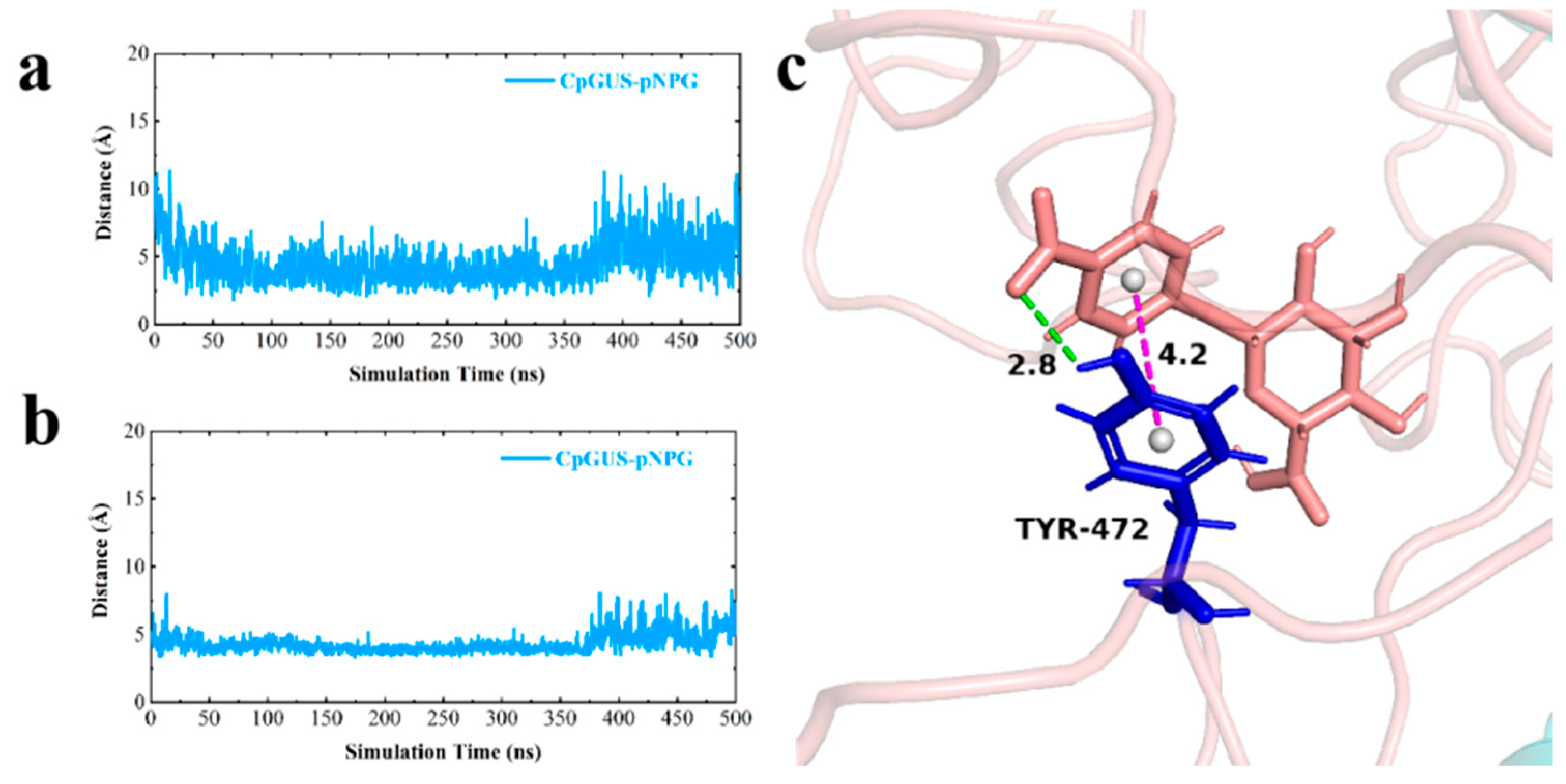
3.5. Catalytic Mechanism of CpGUS Hydrolysis of pNPG
3.6. Preparation of Unconjugated Bilirubin Through High-Density Fermentation and Whole-Cell Transformation of Pig Bile
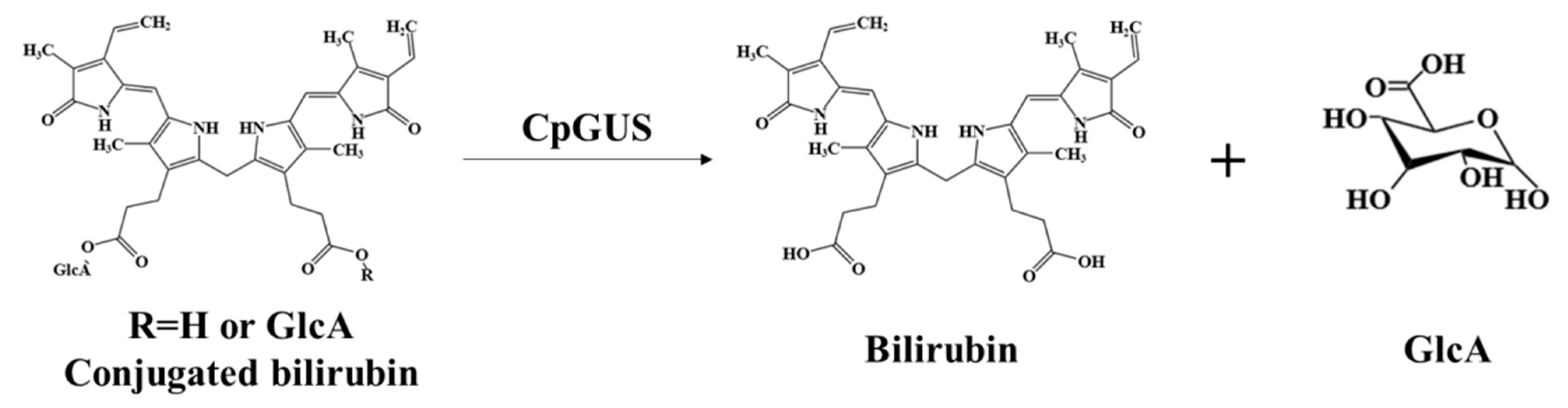
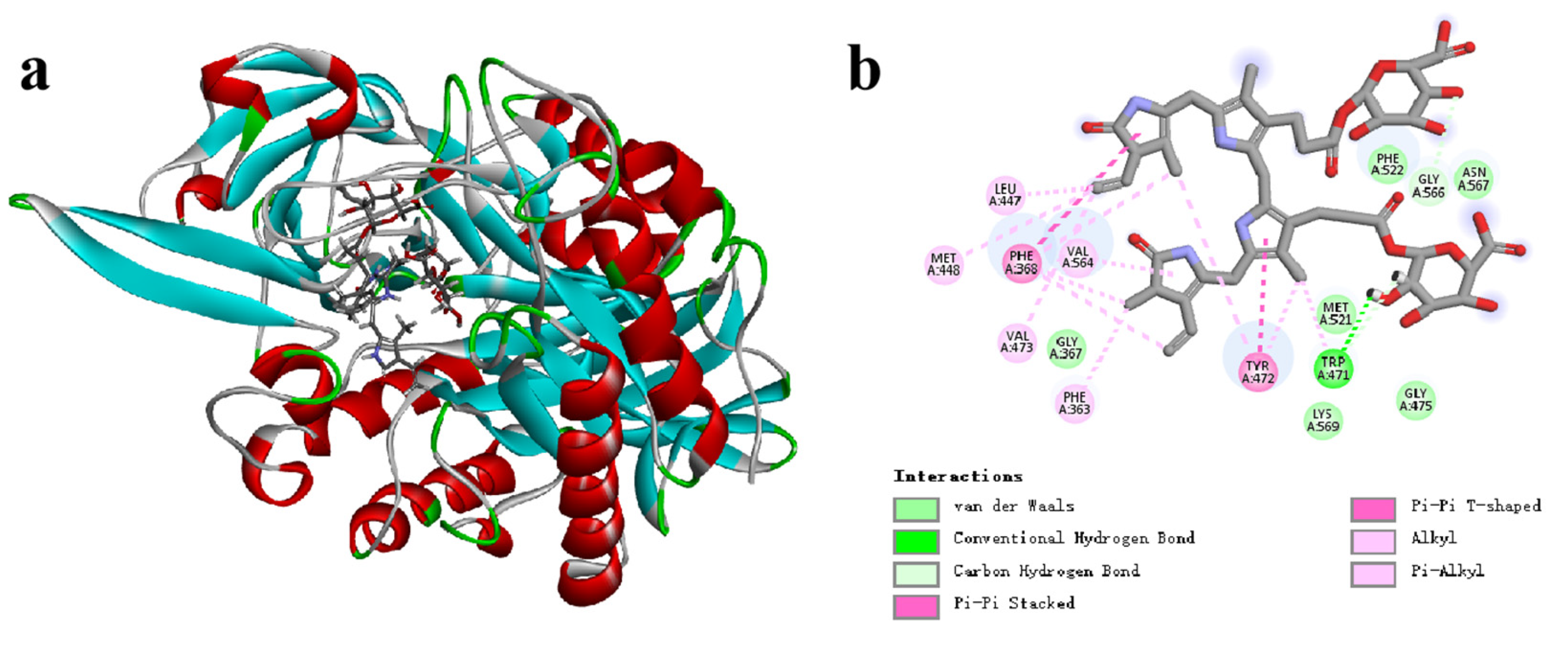
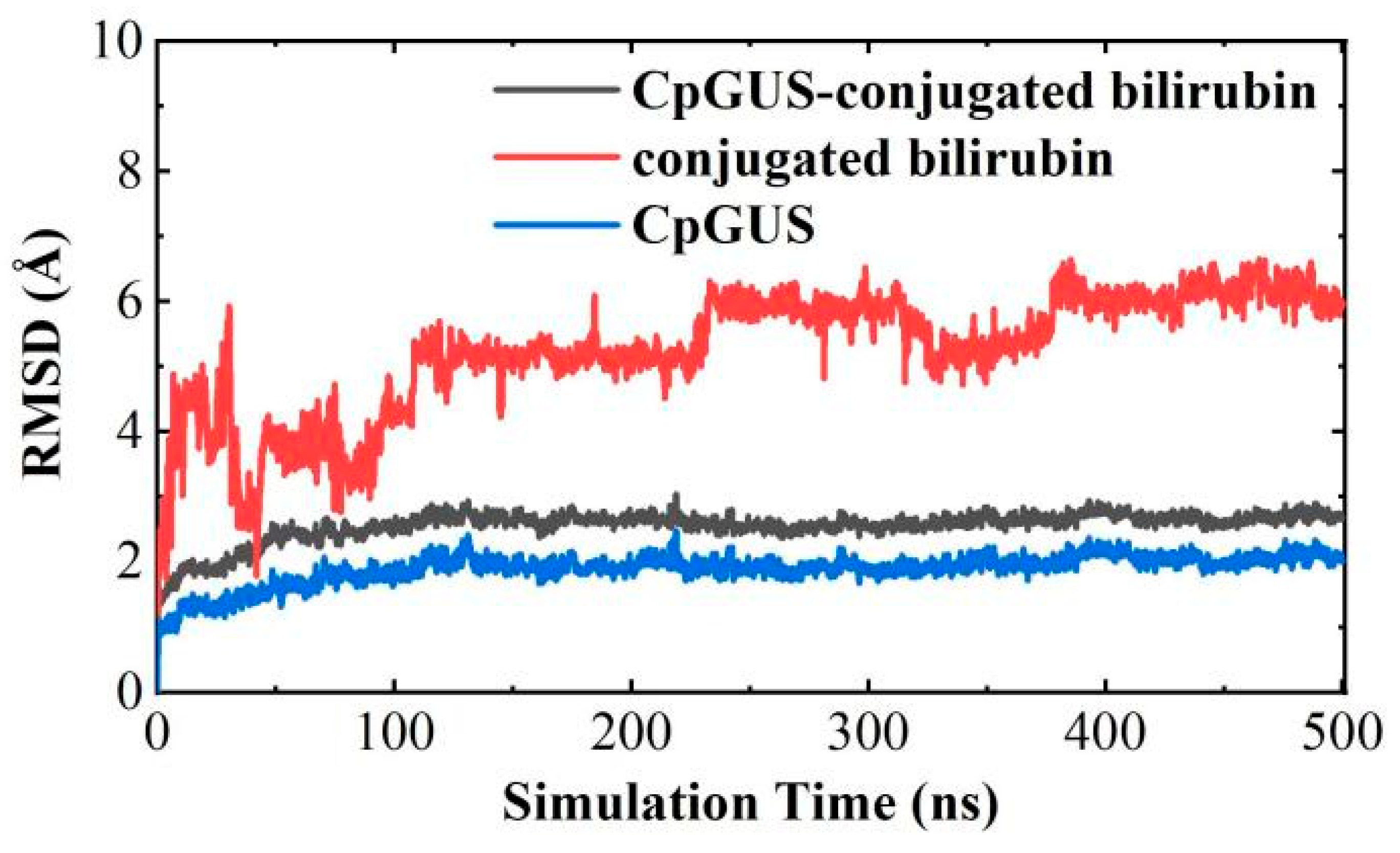
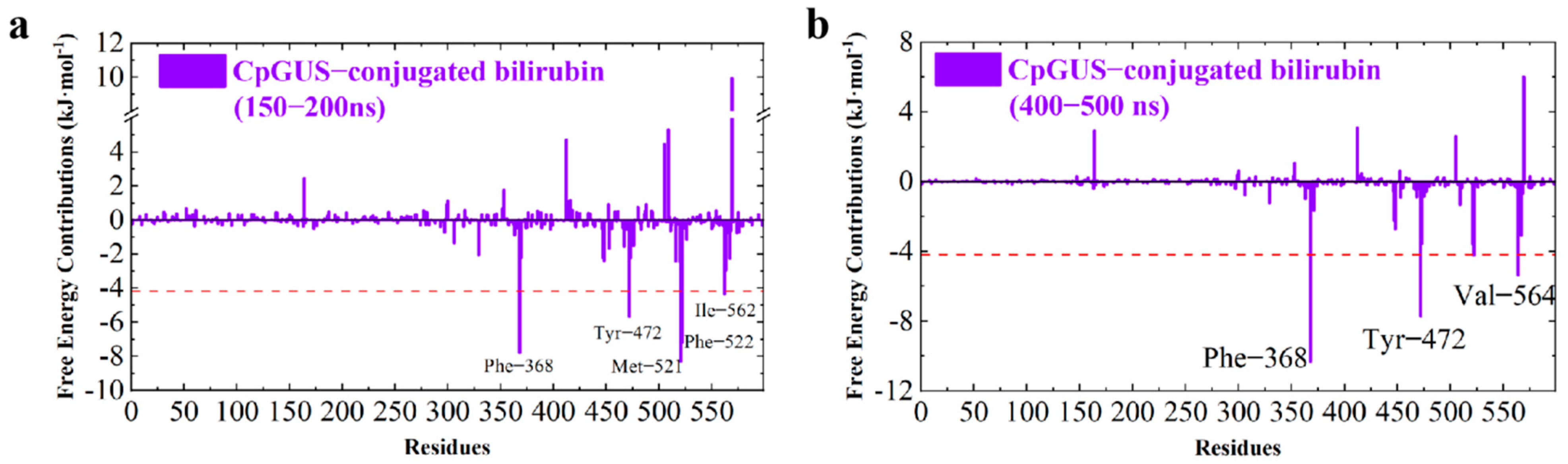
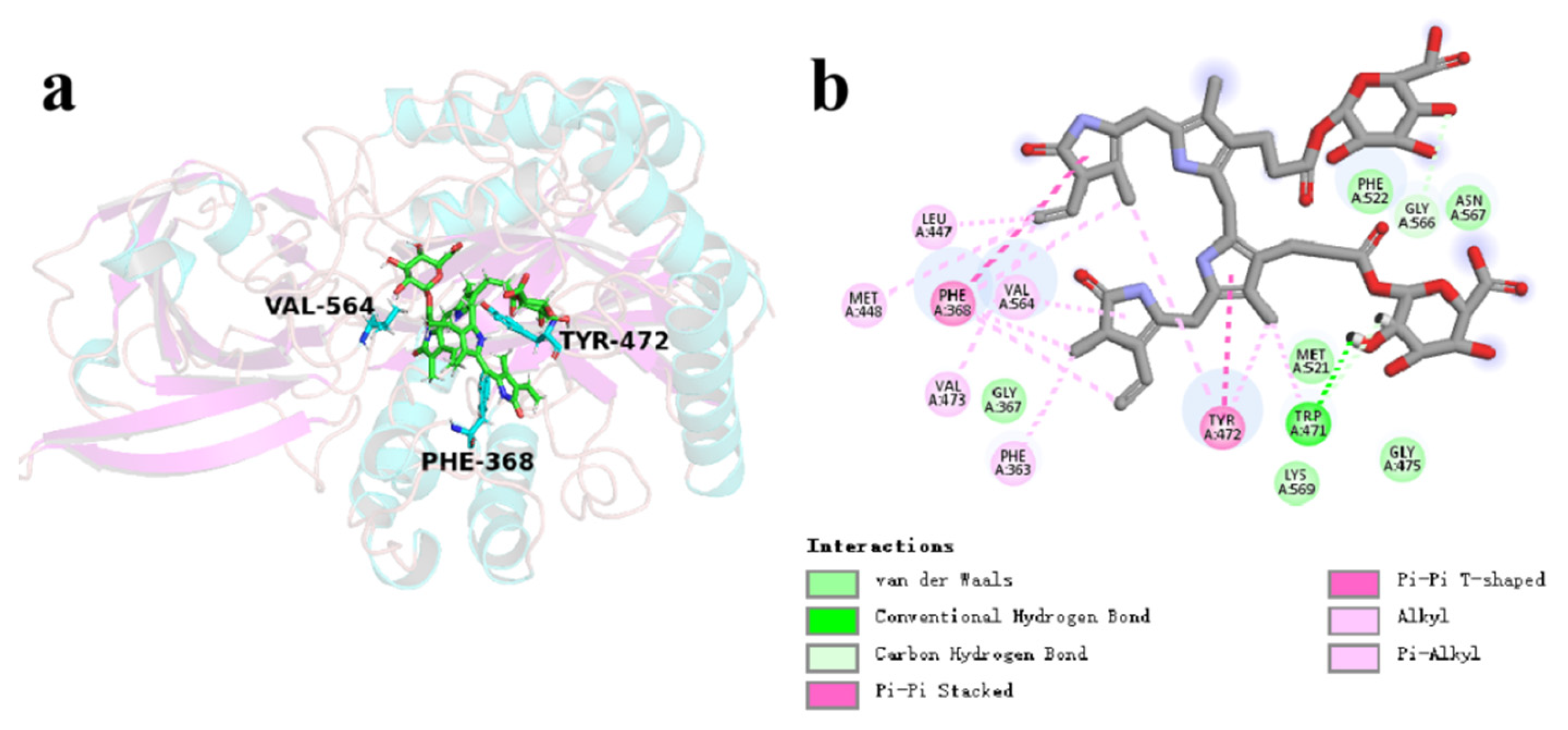
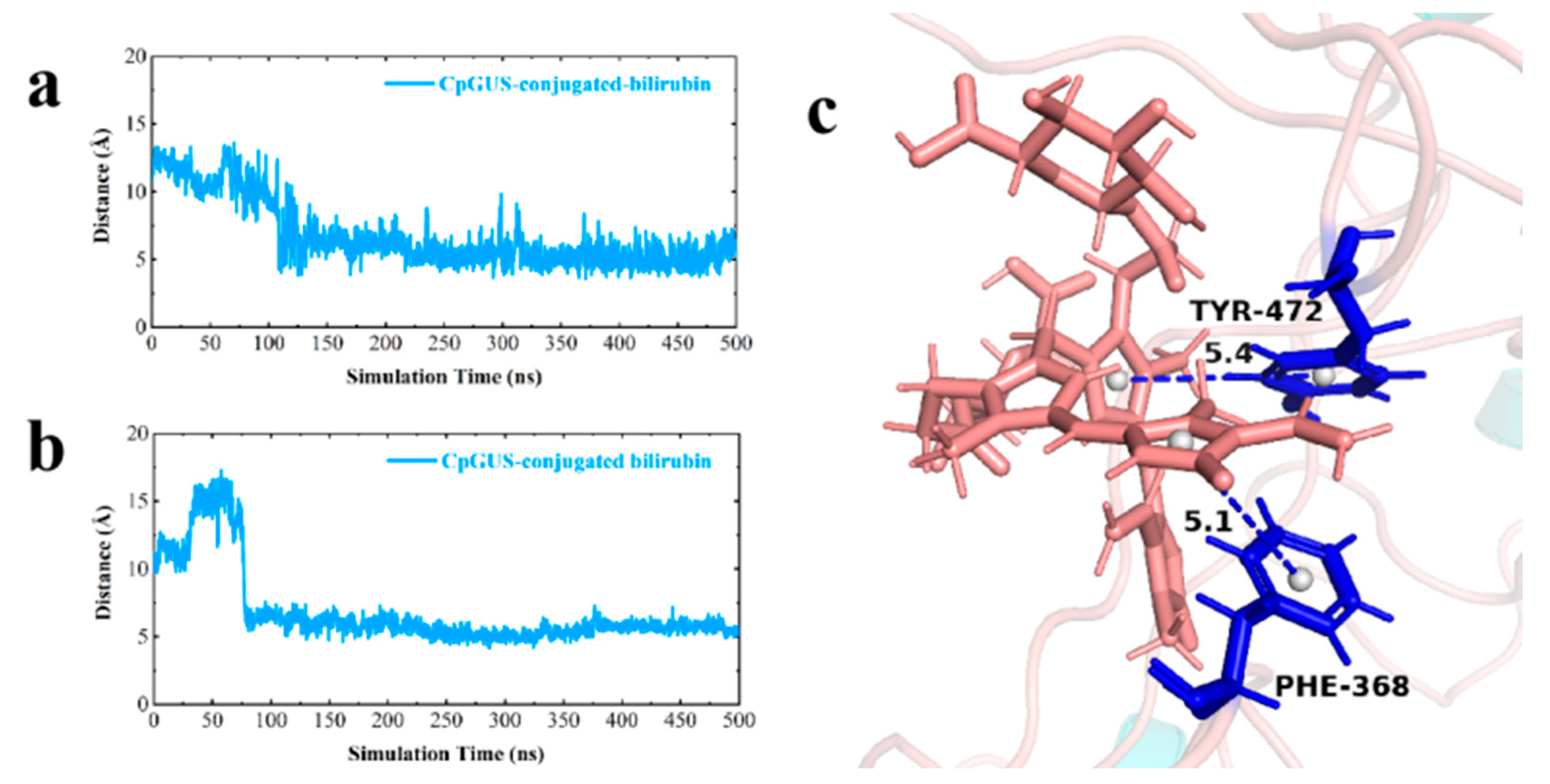
3.7. Catalytic Mechanism of CpGUS Hydrolyzed Conjugated Bilirubin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Drendel, W.B.; Chen, Z.W.; Mathews, F.S.; Sly, W.S.; Grubb, J.H. Structure of human beta-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat. Struct. Biol. 1996, 3, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G.J. Glycoside hydrolases and glycosyltransferases. Families, modules, and implications for genomics. Plant Physiol. 2000, 124, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Bramwell, K.K.; Mock, K.; Ma, Y.; Weis, J.H.; Teuscher, C.; Weis, J.J. β-Glucuronidase, a Regulator of Lyme Arthritis Severity, Modulates Lysosomal Trafficking and MMP-9 Secretion in Response to Inflammatory Stimuli. J. Immunol. 2015, 195, 1647–1656. [Google Scholar] [CrossRef]
- Muñoz, K.; Cramer, B.; Dopstadt, J.; Humpf, H.U.; Degen, G.H. Evidence of ochratoxin A conjugates in urine samples from infants and adults. Mycotoxin Res. 2017, 33, 39–47. [Google Scholar] [CrossRef]
- Honarbakhsh, M.; Villafane, A.A.; Ruhl, I.; Sannino, D.; Bini, E. Development of a thermostable β-glucuronidase-based reporter system for monitoring gene expression in hyperthermophiles. Biotechnol. Bioeng. 2012, 109, 1881–1886. [Google Scholar] [CrossRef]
- Sakurama, H.; Kishino, S.; Uchibori, Y.; Yonejima, Y.; Ashida, H.; Kita, K.; Takahashi, S.; Ogawa, J. β-Glucuronidase from Lactobacillus brevis useful for baicalin hydrolysis belongs to glycoside hydrolase family 30. Appl. Microbiol. Biotechnol. 2014, 98, 4021–4032. [Google Scholar] [CrossRef]
- Liu, Y.; Jie, H.F.; Qi, F.; Kaleem, I.; E, W.; Li, C. Effects of a non-conservative sequence on the properties of β-glucuronidase from Aspergillus terreus Li-20. PLoS ONE 2012, 7, e30998. [Google Scholar] [CrossRef]
- Ku, S.; Zheng, H.; Park, M.S. Optimization of β-glucuronidase activity from Lactobacillus delbrueckii Rh2 and and its use for biotransformation of baicalin and wogonoside. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 275–280. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, H.L.; Wang, X.Q. A β-Glucuronidase and Its Application. Chinese Patent 202210905244.9, 11 June 2021. [Google Scholar]
- Zhou, W.; Cui, Y.; Chen, M.; Gao, Q.; Bao, K.; Wang, Y.; Zhang, M. Production of bilirubin via whole-cell transformation utilizing recombinant Corynebacterium glutamicum expressing a β-glucuronidase from Staphylococcus sp. RLH1. Biotechnol. L. 2024, 46, 223–233. [Google Scholar] [CrossRef]
- Ni, Z.H.; Liu, L.; Chen, K.L.; Li, H.; Yin, Z.C. The Method and Application of Bilirubin Isolation. Chinese Patent 202110652964.4, 29 July 2022. [Google Scholar]
- Chen, Z.; Vong, C.T.; Gao, C.; Chen, S.; Wu, X.; Wang, S.; Wang, Y. Bilirubin Nanomedicines for the Treatment of Reactive Oxygen Species (ROS)-Mediated Diseases. Mol. Pharm. 2020, 17, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Lee, D.Y.; Yu, B.; Miao, W.; Jon, S. Multistimuli-Responsive Bilirubin Nanoparticles for Anticancer Therapy. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 10676–10680. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishimori, H.; Fukutomi, T.; Yamaguchi, T.; Orihashi, K. Dynamics of Oxidative Stress Evoked by Myocardial Ischemia Reperfusion After Off-Pump Coronary Artery Bypass Grafting Elucidated by Bilirubin Oxidation. Circ. J. 2017, 81, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A.; Croker, B.P.; Agarwal, A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am. J. Physiol.-Ren. Physiol. 2005, 288, F778–F784. [Google Scholar] [CrossRef]
- Dong, H.; Huang, H.; Yun, X.; Kim, D.S.; Yue, Y.; Wu, H.; Sutter, A.; Chavin, K.D.; Otterbein, L.E.; Adams, D.B.; et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology 2014, 155, 818–828. [Google Scholar] [CrossRef]
- Bakrania, B.; Du Toit, E.F.; Wagner, K.H.; Headrick, J.P.; Bulmer, A.C. Pre-or post-ischemic bilirubin ditaurate treatment reduces oxidative tissue damage and improves cardiac function. Int. J. Cardiol. 2016, 202, 27–33. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Zhu, Y.; Chen, G.; Yu, F. Low concentrations of bilirubin inhibit activation of hepatic stellate cells in vitro. Mol. Med. Rep. 2017, 15, 1647–1653. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Radivojac, P.; Clark, W.T.; Oron, T.R.; Schnoes, A.M.; Wittkop, T.; Sokolov, A.; Graim, K.; Funk, C.; Verspoor, K.; Ben-Hur, A.; et al. A large-scale evaluation of computational protein function prediction. Nat. Methods 2013, 10, 221–227. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Weng, Z.M. Screening and Structure-Activity Relationship of Flavonoid Inhibitors of β-Glucuronidase and Carboxylesterase 2 in Intestinal Bacteria; Dalian Medical University: Dalian, China, 2019. [Google Scholar]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.W.; Liu, Y.L.; Leung, P.S.; Chan, R.C.; Inciardi, J.F.; Cheng, A.F. Expression of bacterial beta-glucuronidase in human bile: An in vitro study. Gastrointest. Endosc. 2001, 54, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, T.; Liu, R.; Feng, X.; Li, C. Tuning the pH profile of β-glucuronidase by rational site-directed mutagenesis for efficient transformation of glycyrrhizin. Appl. Microbiol. Biotechnol. 2019, 103, 4813–4823. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tang, H.; Han, B.; Zhang, L.; Lv, B.; Li, C. Engineering the thermostability of β-glucuronidase from Penicillium purpurogenum Li-3 by loop transplant. Appl. Microbiol. Biotechnol. 2016, 100, 9955–9966. [Google Scholar] [CrossRef]
- Song, Z.K.; Wang, X.Y.; Chen, G.Q.; Li, C. Cloning and prokaryotic expression of β-glucuronidase gene from Penicillium purpurinogenes. Chin. J. Chem. Eng. 2008, 12, 3101–3106. [Google Scholar]
- Duan, X.; Chen, J.; Wu, J. Optimization of pullulanase production in Escherichia coli by regulation of process conditions and supplement with natural osmolytes. Bioresour. Technol. 2013, 146, 379–385. [Google Scholar] [CrossRef]
- Mergulhão, F.J.; Summers, D.K.; Monteiro, G.A. Recombinant protein secretion in Escherichia coli. Biotechnol. Adv. 2005, 23, 177–202. [Google Scholar] [CrossRef]
- Lu, B. Modification of β-Glucuronidase Substrate Specificity and Immobilization by DNA Aptamers; Beijing Institute of Technology: Beijing, China, 2014. [Google Scholar]
- Qin, B.S.; He, H.Y.; Li, Y.T.; Fu, Y.; Qin, Y.L. Screening and identification of β-glucosidase-producing strain and enzymatic properties of crude enzyme. China Brew. 2024, 43, 128–132. [Google Scholar] [CrossRef]
- Han, B.J. The Regulation Mechanism of Key Loops Toward the Stability and Activity of β-Glucuronidase; Beijing Institute of Technology: Beijing, China, 2018. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lu, H.B.; Li, K.; Guo, Q. Study on extraction technology of bilirubin from pig bile. Liaoning Chem. Ind. 2019, 52, 506–508+512. [Google Scholar]
- Zhu, Y.M.; Yao, G.; Shao, S.; Liu, X.Y.; Xu, J.; Chen, C.; Zhang, X.W.; Huang, Z.R.; Xu, C.Z.; Zhang, L.; et al. Mechanistic Insight into the Enantioselective Degradation of Esterase QeH to (R)/(S)-Quizalofop-Ethyl with Molecular Dynamics Simulation Using a Residue-Specific Force Field. Int. J. Mol. Sci. 2024, 25, 9964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, G.; Mao, Z.; Song, M.; Zhao, R.; Zhang, X.; Chen, C.; Zhang, H.; Liu, Y.; Wang, G.; et al. Experimental and computational approaches to characterize a novel amidase that initiates the biodegradation of the herbicide propanil in Bosea sp. P5. J. Hazard. Mater. 2023, 451, 131155. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Nakayama, F. Bacteria and gallstones. Etiological significance. Dig. Dis. Sci. 1981, 26, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Jacox, R.F. Streptococcal beta-glucuronidase. J. Bacteriol. 1953, 65, 700–705. [Google Scholar] [CrossRef]
- Barber, M.; Brooksbank, B.W.; Haslewood, G.A. Destruction of urinary glucuronide by bacteria. Nature 1948, 162, 701. [Google Scholar] [CrossRef]
- Zou, S.P.; Liu, G.Y.; Kaleem, I.; Li, C. Purification and characterization of a highly selective glycyrrhizin-hydrolyzing β-glucuronidase from Penicillium purpurogenum Li-3. Process Biochem. 2013, 48, 358–363. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liu, Y.L.; Rasool, A.; Li, C. Sequence editing strategy for improving performance of β-glucuronidase from Aspergillus terreus. Chem. Eng. Sci. 2017, 167, 145–153. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.W.; Han, M.J. Biotransformation of glycyrrhizin to 18beta-glycyrrhetinic acid-3-O-beta-D-glucuronide by Streptococcus LJ-22, a human intestinal bacterium. Biol. Pharm. Bull. 1999, 22, 320–322. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Chen, J.P.; Liang, X.M. Purification and characterization of baicalin-β-glucuronidase hydrolyzing baicalin to baicalein from fresh roots of Scutellaria viscidula Bge. Process. Biochem. 2005, 40, 1911–1915. [Google Scholar] [CrossRef]
- Ikegami, F.; Matsunae, K.; Hisamitsu, M.; Kurihara, T.; Yamamoto, T.; Murakoshi, I. Purification and properties of a plant beta-D-glucuronidase form Scutellaria root. Biol. Pharm. Bull. 1995, 18, 1531–1534. [Google Scholar] [CrossRef]
- Kuroyama, H.; Tsutsui, N.; Hashimoto, Y.; Tsumuraya, Y. Purification and characterization of a beta-glucuronidase from Aspergillus niger. Carbohydr. Res. 2001, 333, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, B.; Xiao, Y.; Yang, H.; Wang, Y.; Du, L.; Zhu, D. Purification and characterization of a novel β-glucuronidase precisely converts glycyrrhizin to glycyrrhetinic acid 3-O-mono-β-D-glucuronide from plant endophytic Chaetomium globosum DX-THS3. Int. J. Biol. Macromol. 2020, 159, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.N. Whole Cell Transformation of Recombinant Corynebacterium Glutamate to Produce Bilirubin; Anhui University: Hefei, China, 2024. [Google Scholar]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [PubMed]
- Haugner, J.C., III. In Vitro Evolution of Artificial Enzymes: Method Development and Applications; University of Minnesota Twin Cities: Minneapolis, MN, USA, 2014. [Google Scholar]



| Concentration/mM | OD405 | Enzyme Activity (U/μL) | 1/[S] (1/mM) | 1/V [1/(U/μL)] |
|---|---|---|---|---|
| 0.4 | 0.674 | 86.13 | 2.500 | 0.0116 |
| 0.6 | 0.902 | 114.63 | 1.667 | 0.0087 |
| 0.8 | 1.003 | 127.25 | 1.250 | 0.0079 |
| 1.0 | 1.117 | 141.50 | 1.000 | 0.0071 |
| 1.2 | 1.220 | 154.38 | 0.833 | 0.0065 |
| 1.4 | 1.253 | 158.50 | 0.714 | 0.0063 |
| System | Time Scope/ns | ΔGvdw | ΔGele | ΔGPB | ΔGSA | ∆Gbind |
|---|---|---|---|---|---|---|
| CpGUS–pNPG | 300–350 | −147.17 ± 14.36 | −119.21 ± 9.53 | 216.63 ± 10.94 | −15.30 ± 0.87 | −65.05 ± 12.66 |
| 430–500 | −127.56 ± 14.62 | −113.37 ± 18.78 | 194.37 ± 20.65 | −14.72 ± 0.93 | −61.28 ± 18.01 |
| System | Time Scope/ns | Residue | ΔGMM | ΔGPB | ΔGSA | ∆Gbind |
|---|---|---|---|---|---|---|
| CpGUS–pNPG | 300–350 | Leu447 | −6.83 | 2.90 | −0.66 | −4.60 |
| Asn466 | −3.23 | −3.87 | −0.10 | −7.21 | ||
| Tyr468 | −11.04 | 3.29 | −0.52 | −8.29 | ||
| Tyr472 | −17.33 | 8.93 | −1.60 | −9.99 | ||
| 430–500 | Phe368 | −6.25 | 2.91 | −0.68 | −4.00 | |
| Asn466 | −3.20 | −3.01 | −0.11 | −6.32 | ||
| Tyr468 | −8.30 | 4.29 | −0.47 | −4.47 | ||
| Tyr472 | −13.74 | 7.12 | −1.29 | −7.92 |
| Serial Number | Concentration of Total Bilirubin in Primary Pig Bile (mg/mL) | Concentration of Total Bilirubin in Pig Bile After Hydrolysis Transformation (mg/mL) | Hydrolysis Conversion (%) | Quality of Unconjugated Bilirubin Extracted from 1 L of Pig Bile After Transformation (g) | Unconjugated Bilirubin Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.46 | 0.090 | 80.4 | 0.350 | 76.1 |
| 2 | 0.46 | 0.090 | 80.4 | 0.350 | 76.1 |
| 3 | 0.46 | 0.080 | 82.6 | 0.360 | 78.3 |
| Mean value | 0.46 | 0.087 | 81.1 | 0.353 | 76.8 |
| System | Time Scope/ns | ΔGvdw | ΔGele | ΔGPB | ΔGSA | ∆Gbind |
|---|---|---|---|---|---|---|
| CpGUS– Conjugated bilirubin | 150–200 | −221.44 ± 16.97 | −41.68 ± 27.80 | 220.82 ± 52.65 | −25.67 ± 2.02 | −67.97 ± 36.72 |
| 400–500 | −200.12 ± 26.51 | −35.36 ± 22.78 | 171.31 ± 26.11 | −22.54 ± 2.15 | −86.70 ± 17.18 |
| System | Time Scope/ns | Residue | (ΔGMM) | (ΔGPB) | (ΔGSA) | (∆Gbind) |
|---|---|---|---|---|---|---|
| CpGUS– Conjugated bilirubin | 150–200 | Phe368 | −13.35 | 6.86 | −1.32 | −7.80 |
| Tyr472 | −17.27 | 13.27 | −1.66 | −5.68 | ||
| Met521 | −12.94 | 5.90 | −1.27 | −8.32 | ||
| Phe522 | −9.36 | 3.61 | −1.47 | −7.21 | ||
| Ile562 | −6.03 | 2.34 | −0.65 | −4.37 | ||
| 400–500 | Phe368 | −15.87 | 7.43 | −1.92 | −10.33 | |
| Tyr472 | −23.16 | 17.64 | −2.20 | −7.73 | ||
| Val564 | −6.59 | 2.51 | −1.30 | −5.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Guo, Q.; Yang, F.; Li, M.; Zhu, Y.; Xu, B.; Zhao, L.; Zhang, S.; Xie, Y.; Li, F.; et al. Heterologous Expression and Enzymatic Properties of β-Glucuronidase from Clostridium perfringens and Its Application in Bilirubin Transformation. Microorganisms 2025, 13, 1043. https://doi.org/10.3390/microorganisms13051043
Wu Q, Guo Q, Yang F, Li M, Zhu Y, Xu B, Zhao L, Zhang S, Xie Y, Li F, et al. Heterologous Expression and Enzymatic Properties of β-Glucuronidase from Clostridium perfringens and Its Application in Bilirubin Transformation. Microorganisms. 2025; 13(5):1043. https://doi.org/10.3390/microorganisms13051043
Chicago/Turabian StyleWu, Qianlin, Qing Guo, Fo Yang, Mengru Li, Yumeng Zhu, Binpeng Xu, Lu Zhao, Shanshan Zhang, Youyu Xie, Feng Li, and et al. 2025. "Heterologous Expression and Enzymatic Properties of β-Glucuronidase from Clostridium perfringens and Its Application in Bilirubin Transformation" Microorganisms 13, no. 5: 1043. https://doi.org/10.3390/microorganisms13051043
APA StyleWu, Q., Guo, Q., Yang, F., Li, M., Zhu, Y., Xu, B., Zhao, L., Zhang, S., Xie, Y., Li, F., Wu, X., & Xu, D. (2025). Heterologous Expression and Enzymatic Properties of β-Glucuronidase from Clostridium perfringens and Its Application in Bilirubin Transformation. Microorganisms, 13(5), 1043. https://doi.org/10.3390/microorganisms13051043






