Physiological, Biochemical, and Genetic Reactions of Winter Wheat to Drought Under the Influence of Plant Growth Promoting Microorganisms and Calcium
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Experimental Design and Drought Conditions
2.3. Sampling
2.4. Morphometrical Measurements
2.5. RWC
2.6. Assessment of Biochemical Parameters
2.6.1. H2O2
2.6.2. Lipid Peroxidation According to MDA
2.6.3. Proline
2.7. Molecular Techniques
2.7.1. RNA Extraction and Reverse Transcription
2.7.2. Real-Time Quantitative PCR
2.7.3. Primers
2.8. Statistical Analysis
3. Results
3.1. Impact of PGPMs and Ca Salts on Morphometric Parameters of Wheat Exposed to Prolonged Drought Stress
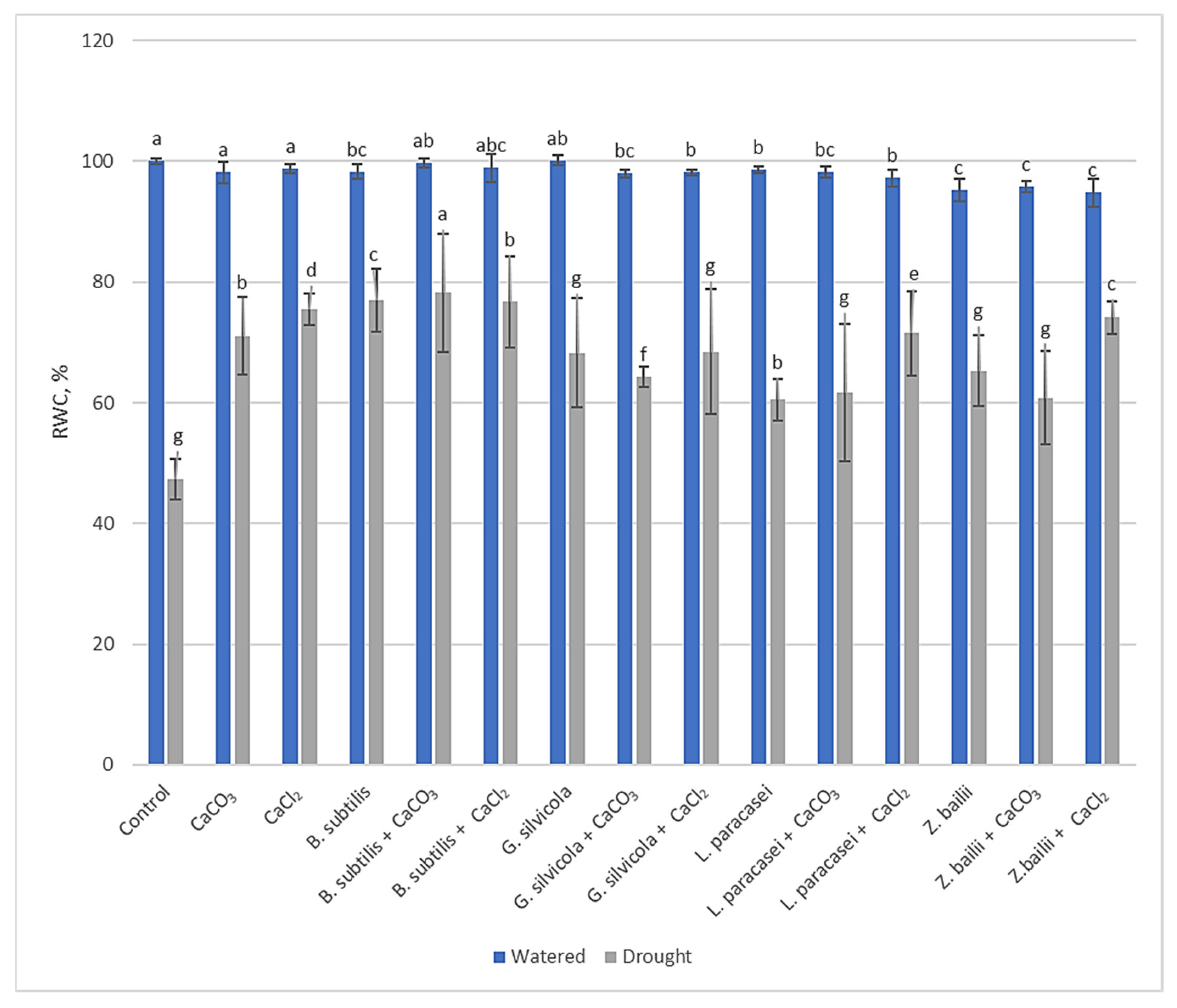
3.2. Effect of Used PGPMs and Ca Salts on RWC of Wheat Seedlings Exposed to Prolonged Drought
3.3. Effect of PGPMs and Ca Salts on Biochemical Responses of Wheat Plants Exposed to Prolonged Drought
3.3.1. Hydrogen Peroxide (H2O2)
3.3.2. Malondialdehyde (MDA)
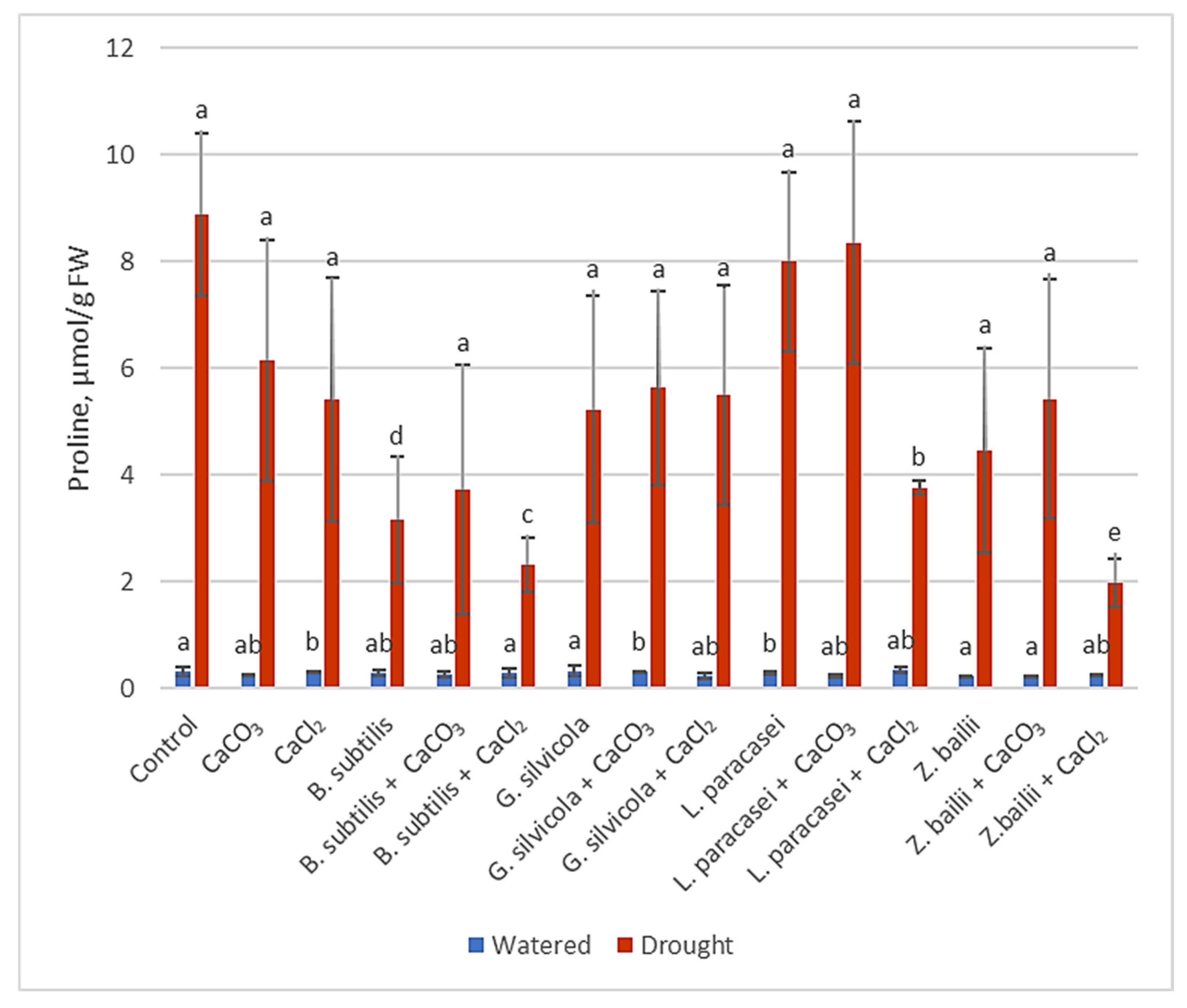
3.3.3. Free Proline
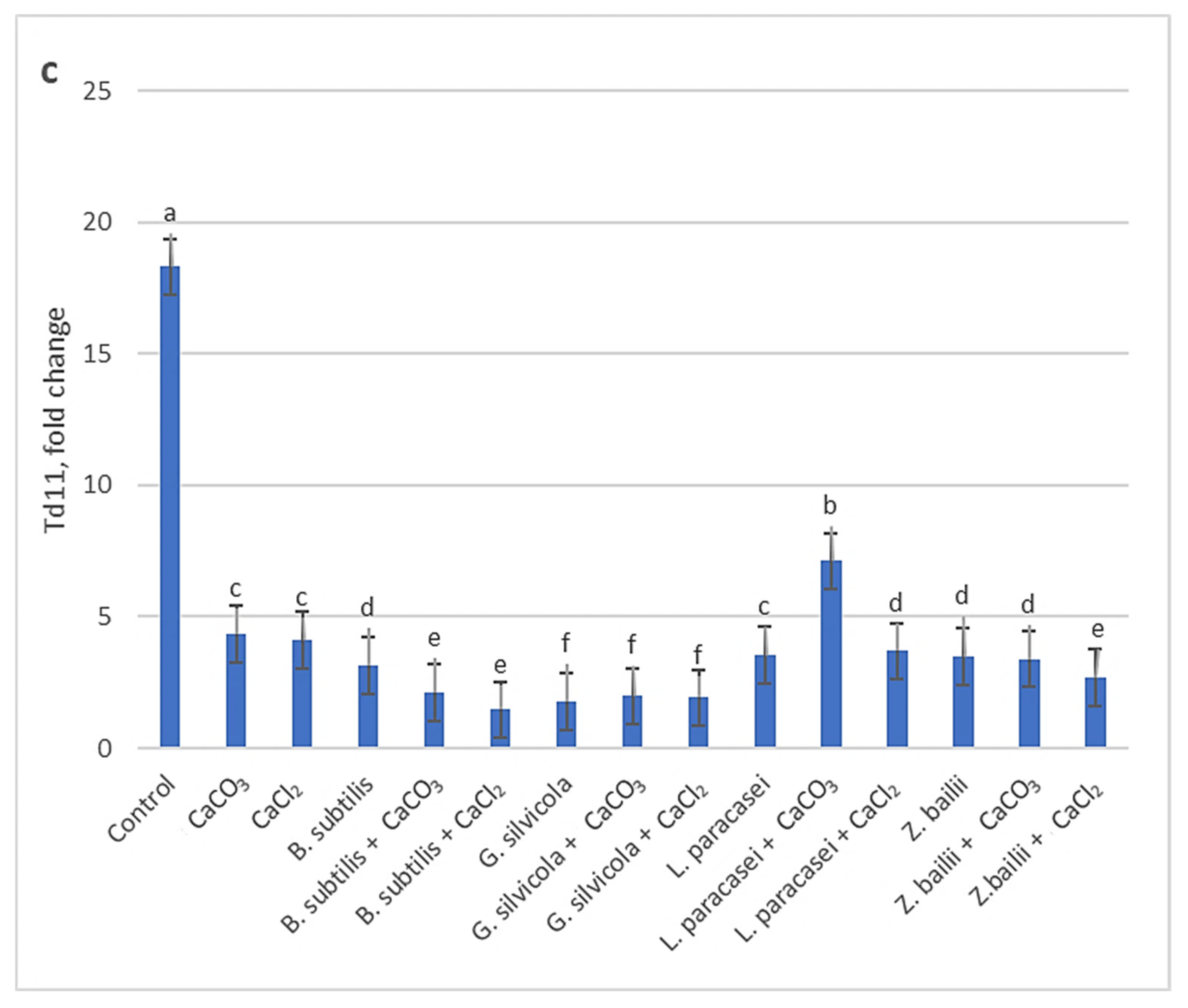
3.4. Effect of PGPMs and Ca Salts Application on Late Embryogenesis Abundant (Lea) Genes Expression Levels in Wheat Plants Exposed to Prolonged Drought
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ca | Calcium |
| MDA | Malondialdehyde |
| LEA | Late embryogenesis abundant |
| PGPM | Plant growth-promoting microorganisms |
| RWC | Relative water content |
| ROS | Reactive oxygen species |
References
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the biochemical responses of wheat seedlings to soil drought after application of selective herbicide. Plants 2021, 10, 733. [Google Scholar] [CrossRef]
- Chattaraj, S.; Samantaray, A.; Ganguly, A.; Thatoi, H. Employing plant growth-promoting rhizobacteria for abiotic stress mitigation in plants: With a focus on drought stress. Discov. Appl. Sci. 2025, 7, 68. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 12, 610721. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jägermeyr, J. Severity of drought and heatwave crop losses tripled over the last five decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A potential cereal for producing healthy and functional foods. Food Qual. Saf. 2022, 6, fyac012. [Google Scholar] [CrossRef]
- Batîr Rusu, D.C.; Murariu, D.; Gheorghita, R.; Graur, M. Some nutritional value aspects of barley and oat and their impact in human nutrition and healthy life. Plants 2024, 13, 2764. [Google Scholar] [CrossRef]
- Sultan, M.A.R.F.; Hui, L.; Yang, L.Y.; Xian, Z.H. Assessment of drought tolerance of some Triticum L. species through physiological indices. Czech J. Genet. Plant Breed. 2012, 48, 178–184. [Google Scholar] [CrossRef]
- US Wheat Associates 2020. Available online: https://www.uswheat.org/wheatletter/2020-global-wheat-harvest-update/ (accessed on 12 January 2025).
- USDA Foreign Agricultural Service. World Agricultural Supply and Demand Estimates (WASDE). 2021. Available online: https://www.usda.gov/about-usda/general-information/staff-offices/office-chief-economist/commodity-markets/wasde-report (accessed on 20 January 2025).
- Eurostat. Available online: https://ec.europa.eu/eurostat/web/agriculture/publications (accessed on 15 February 2025).
- European Drought Observatory. Available online: https://joint-research-centre.ec.europa.eu/european-and-global-drought-observatories/current-drought-situation-europe_en (accessed on 15 February 2025).
- Farooq, S.; Ul-Allah, S.; Hussain, M. Drought sress in crop plants and its management. In Disaster Risk Reduction in Agriculture; Ahmed, M., Ahmad, S., Eds.; Springer: Singapore, 2023; pp. 21–42. [Google Scholar]
- Cousson, A. Involvement of phospholipase C-independent calcium-mediated abscisic acid signaling during Arabidopsis response to drought. Biol. Plant 2009, 53, 53–62. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Réthoré, E.; Pluchon, S.; Ali, N.; Billiot, B.; Yvin, J.C. Calcium application enhances drought stress tolerance in sugar beet and promotes plant biomass and beetroot sucrose concentration. Int. J. Mol. Sci. 2019, 20, 3777. [Google Scholar] [CrossRef]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous calcium: Its mechanisms and research advances involved in plant stress tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Fathi, F.; Moradzadeh-Eskandari, M. The effect of some probiotic bacteria in induction of drought tolerance in cucumber plants. Microbiol. Metabolites Biotechnol. 2018, 2, 113–127. [Google Scholar]
- Kumar, M.; Shamshad Ahmad, P.; Singh, R.P. Plant growth promoting microbes: Diverse roles for sustainable and ecofriendly agriculture. Energy Nexus 2022, 7, 100133. [Google Scholar] [CrossRef]
- Lubyanova, A.R.; Allagulova, C.R.; Lastochkina, O.V. The effects of seed pretreatment with endophytic bacteria Bacillus subtilis on the water balance of spring and winter wheat seedlings under short-time water deficit. Plants 2023, 12, 2684. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses in crops—A review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‚Katahdin‘-derived potato cultivars. Breed Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef]
- González-Espíndola, L.Á.; Pedroza-Sandoval, A.; Trejo-Calzada, R.; Jacobo-Salcedo, M.d.R.; García de los Santos, G.; Quezada-Rivera, J.J. Relative water content, chlorophyll index, and photosynthetic pigments on Lotus corniculatus L. in Response to Water Deficit. Plants 2024, 13, 961. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregon, S.; Cartea, M.E.; Chaibi, W.; Djebali, W. Salicylic acid increases tolerance to oxidative stress induced by hydrogen peroxide accumulation in leaves of cadmium-exposed flax (Linum usitatissimum L.). J. Plant Interact. 2014, 9, 647–654. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Pnueli, L.; Hallak-Herr, E.; Rozenberg, M.; Cohen, M.; Goloubinoff, P.; Kaplan, A.; Mittler, R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. Plant J. 2002, 31, 319–330. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Liu, C.; Zhang, T.; Han, L.; Li, L.; Pei, X.; Long, Y. Genome-wide identification and functional characterization of LEA genes during seed development process in linseed flax (Linum usitatissimum L.). BMC Plant Biol. 2021, 21, 193. [Google Scholar]
- Weber, E.; Bleiholder, H.; Lancashire, P.D.; Langelüddecke, R.; Stauss, R.; Van der Boom, T.; Witzen-Berger, A. Growth stages of mono- and dicotyledonous plants. In BBCH Monograph., 1st ed.; Meier, U., Ed.; Julius Kühn-Institut: Quedlinburg, Germany, 2018; pp. 18–21. [Google Scholar]
- Weng, M.; Cui, L.; Liu, F.; Zhang, M.; Shan, L.; Yang, S.; Deng, X.-P. Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Extraction and Determination of Proline. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_prolineproline (accessed on 10 July 2016).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ali-Benali, M.A.; Alary, R.; Joudrier, P.; Gautier, M.-F. Comparative expression of five LEA genes during wheat seed development and in response to abiotic stresses by real-time quantitative RT-PCR. Biochim. Biophys. Acta Gene Struct. Expression 2005, 1730, 56–65. [Google Scholar] [CrossRef]
- Menendez, E.; Garcia-Fraile, P. Plant probiotic bacteria: Solutions to feed the world. AIMS Microbiol. 2017, 3, 502–524. [Google Scholar] [CrossRef]
- Mockevičiūtė, R.; Jurkonienė, S.; Šveikauskas, V.; Zareyan, M.; Jankovska-Bortkevič, E.; Jankauskienė, J.; Kozeko, L.; Gavelienė, V. Probiotics, proline and calcium induced protective responses of Triticum aestivum under drought stress. Plants 2023, 12, 1301. [Google Scholar] [CrossRef]
- Dikilitas, M.; Şimşek, E.; Roychoudhury, A. Role of proline and glycine betaine in overcoming abiotic stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives, 1st ed.; Roychoudhury, A., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–23. [Google Scholar]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.; Wang, Y. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness of various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef]
- Lalarukh, I.; Al-Dhumri, S.A.; Al-Ani, L.K.T.; Hussain, R.; Al Mutairi, K.A.; Mansoora, N.; Amjad, S.F.; Abbas, M.H.H.; Abdelhafez, A.A.; Poczai, P.; et al. A Combined use of Rhizobacteria and Moringa leaf extract mitigates the adverse effects of drought stress in wheat (Triticum aestivum L.). Front Microbiol. 2022, 13, 813415. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, R.C.; Choudhary, P.; Sharma, S.K.; Bhagat, N. Mitigation of drought stress in wheat (Triticum aestivum L.) by inoculation of drought tolerant Bacillus paramycoides DT-85 and Bacillus paranthracis DT-97. J. Appl. Biol. Biotechnol. 2022, 10, 59–69. [Google Scholar] [CrossRef]
- Xie, J.; Bai, L.; Zhou, W. Research progress of Bacillus subtilis in wheat growth promotion and disease control. Int. J. Biol. Life Sci. 2024, 7, 68–72. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Raveschot, C.; Coutte, F.; Frémont, M.; Vaeremans, M.; Dugersuren, J.; Demberel, S.; Drider, D.; Dhulster, P.; Flahaut, C.; Cudennec, B. Probiotic Lactobacillus strains from Mongolia improve calcium transport and uptake by intestinal cells in vitro. Food Res. Int. 2020, 133, 109201. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J. Probiotics and isoflavones as a promising therapeutic for calcium status and bone health: A narrative review. Foods 2021, 10, 2685. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Ditscheida, B.; Kiehntop, M.; Jahreis, G. A combination of calcium phosphate and probiotics beneficially influences intestinal lactobacilli and cholesterol metabolism in humans. Clin Nutr. 2012, 31, 230–237. [Google Scholar] [CrossRef]
- Bilek, F.N.; Rezki, M.A.; Grondin, C.; Yahia, N.; Bekki, A. Plant growth promoting characteristics and stress tolerance of yeasts isolated from algerian agricultural soils. South Asian J. Exp. Biol. 2020, 10, 413–426. [Google Scholar]
- Pérez-Moncada, U.A.; Santander, C.; Ruiz, A.; Vidal, C.; Santos, C.; Cornejo, P. Design of microbial consortia based on Arbuscular Mycorrhizal fungi, yeasts, and bacteria to improve the biochemical, nutritional, and physiological status of strawberry plants growing under water deficits. Plants 2024, 13, 1556. [Google Scholar] [CrossRef]
- Souza Vandenberghe, L.P.; Garcia, L.M.B.; Rodrigues, C.; Camara, M.C.; de Melo Pereira, G.V.; de Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]



| Treatment | CaCO3 | CaCl2 | Microorganisms |
|---|---|---|---|
| Control | − | − | − |
| CaCO3 | + | − | − |
| CaCl2 | − | + | − |
| Bacillus subtilis | − | − | + |
| B. subtilis + CaCO3 | + | − | + |
| B. subtilis + CaCl2 | − | + | + |
| Geotrichum silvicola | − | − | + |
| G. silvicola + CaCO3 | + | − | + |
| G. silvicola + CaCl2 | − | + | + |
| Lactobacillus paracasei | − | − | + |
| L. paracasei + CaCO3 | + | − | + |
| L. paracasei + CaCl2 | − | + | + |
| Zygosaccharomyces bailii | − | − | + |
| Z. bailii + CaCO3 | + | − | + |
| Z. bailii + CaCl2 | − | + | + |
| Gene | LEA Proteins Group | Primer Pairs | Primer Sequences (5′-3′) |
|---|---|---|---|
| 26S | F/R | CCGGTTGTTATGCCAATAGCA/GCGGCGCAGCAGTTCT | |
| Td11 | 2 | F/R | AGGTGATCGATGACAACGGTG/ACCCTCGGTGTCCTTGTGG |
| Td29b | 4 | F/R | CGCACCCAGCTAGTAAGTTCG/CCCAGCCCAGTAATAACCCAT |
| Td27e | 2 | F/R | CAGCACTGAGCCGACGG/ACGTGGAACTAGAAGGCATTGAC |
| Average Shoot Length, cm | Average Shoot Biomass, g | |||
|---|---|---|---|---|
| Watered | Drought | Watered | Drought | |
| Control | 24.2 d | 15.95 f | 0.288 e | 0.039 f |
| CaCO3 | 26.95 b | 17.05 e | 0.3159 c | 0.036 f |
| CaCl2 | 24.55 d | 17.75 e | 0.267 f | 0.0335 f |
| Bacillus subtilis | 27.95 a | 20.65 b | 0.333 b | 0.1278 b |
| B. subtilis + CaCO3 | 27.55 a | 21.65 a | 0.345 a | 0.1164 c |
| B. subtilis + CaCl2 | 27.85 a | 19.73 c | 0.3197 c | 0.1218 b |
| Geotrichum silvicola | 26.6 b | 20.5 b | 0.29 e | 0.107 d |
| G. silvicola + CaCO3 | 26.9 b | 19.56 c | 0.297 d | 0.109 d |
| G. silvicola + CaCl2 | 27.1 ab | 18.45 d | 0.316 c | 0.112 c |
| Lactobacillus paracasei | 25.3 c | 18.55 d | 0.269 f | 0.068 e |
| L. paracasei + CaCO3 | 26.55 b | 18.85 d | 0.306 d | 0.057 e |
| L. paracasei + CaCl2 | 25.8 c | 20.45 b | 0.253 f | 0.134 a |
| Zygosaccharomyces bailii | 25.1 c | 18.65 d | 0.2846 e | 0.072 e |
| Z. bailii + CaCO3 | 26.45 b | 16.4 f | 0.309 d | 0.035 f |
| Z. bailii + CaCl2 | 27.4 a | 20.5 b | 0.289 e | 0.1454 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zareyan, M.; Mockevičiūtė, R.; Jurkonienė, S.; Gavelienė, V.; Paškevičius, A.; Šveikauskas, V. Physiological, Biochemical, and Genetic Reactions of Winter Wheat to Drought Under the Influence of Plant Growth Promoting Microorganisms and Calcium. Microorganisms 2025, 13, 1042. https://doi.org/10.3390/microorganisms13051042
Zareyan M, Mockevičiūtė R, Jurkonienė S, Gavelienė V, Paškevičius A, Šveikauskas V. Physiological, Biochemical, and Genetic Reactions of Winter Wheat to Drought Under the Influence of Plant Growth Promoting Microorganisms and Calcium. Microorganisms. 2025; 13(5):1042. https://doi.org/10.3390/microorganisms13051042
Chicago/Turabian StyleZareyan, Mariam, Rima Mockevičiūtė, Sigita Jurkonienė, Virgilija Gavelienė, Algimantas Paškevičius, and Vaidevutis Šveikauskas. 2025. "Physiological, Biochemical, and Genetic Reactions of Winter Wheat to Drought Under the Influence of Plant Growth Promoting Microorganisms and Calcium" Microorganisms 13, no. 5: 1042. https://doi.org/10.3390/microorganisms13051042
APA StyleZareyan, M., Mockevičiūtė, R., Jurkonienė, S., Gavelienė, V., Paškevičius, A., & Šveikauskas, V. (2025). Physiological, Biochemical, and Genetic Reactions of Winter Wheat to Drought Under the Influence of Plant Growth Promoting Microorganisms and Calcium. Microorganisms, 13(5), 1042. https://doi.org/10.3390/microorganisms13051042








