Probiotic Fermentation of Defatted Cottonseed Meal for Sustainable Foods and Non-Food Applications
Abstract
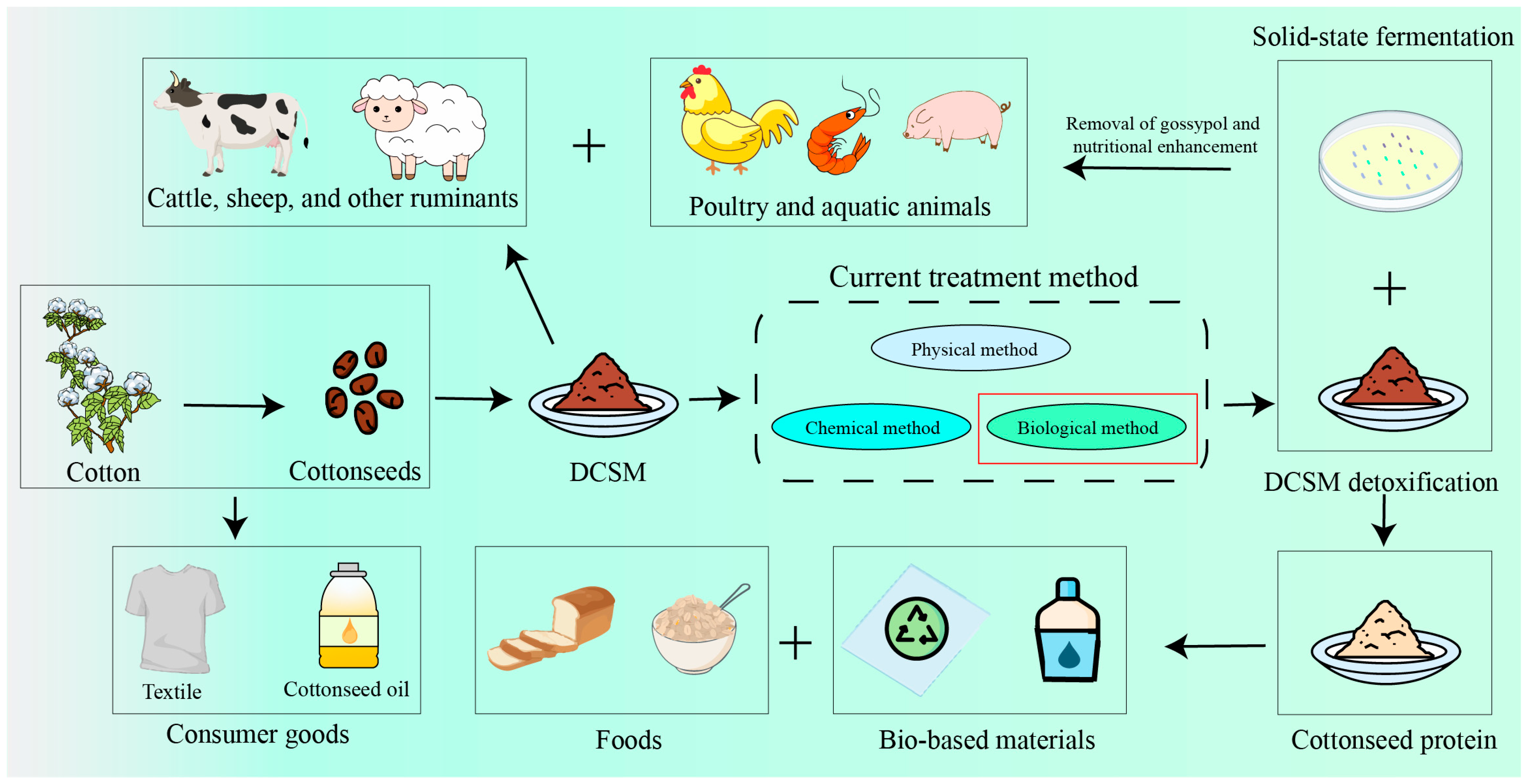
1. Introduction
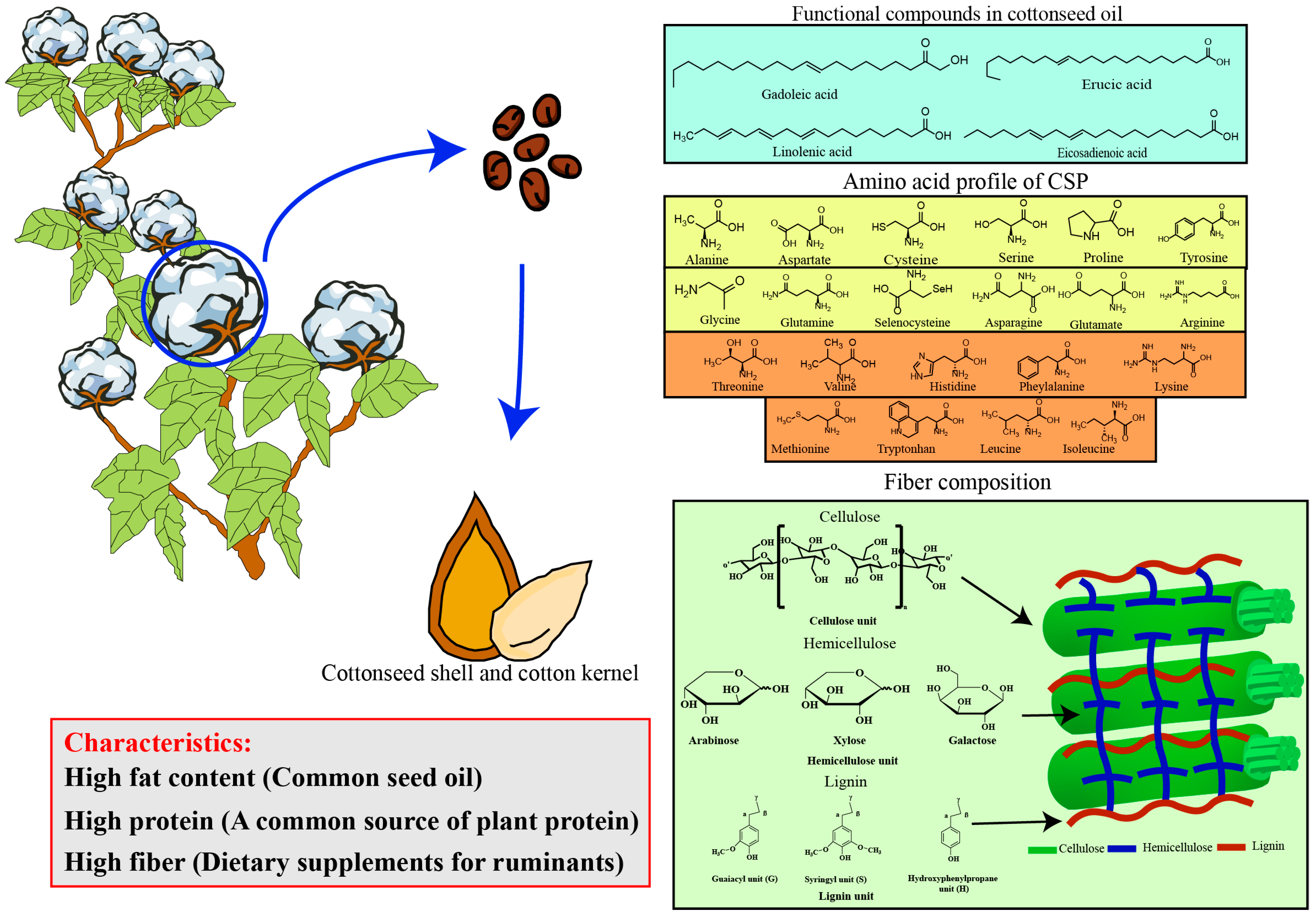
2. Chemical Composition, Structure, and Characteristics of Cottonseed
2.1. DCSM Obtained After Cottonseed Oil Extraction
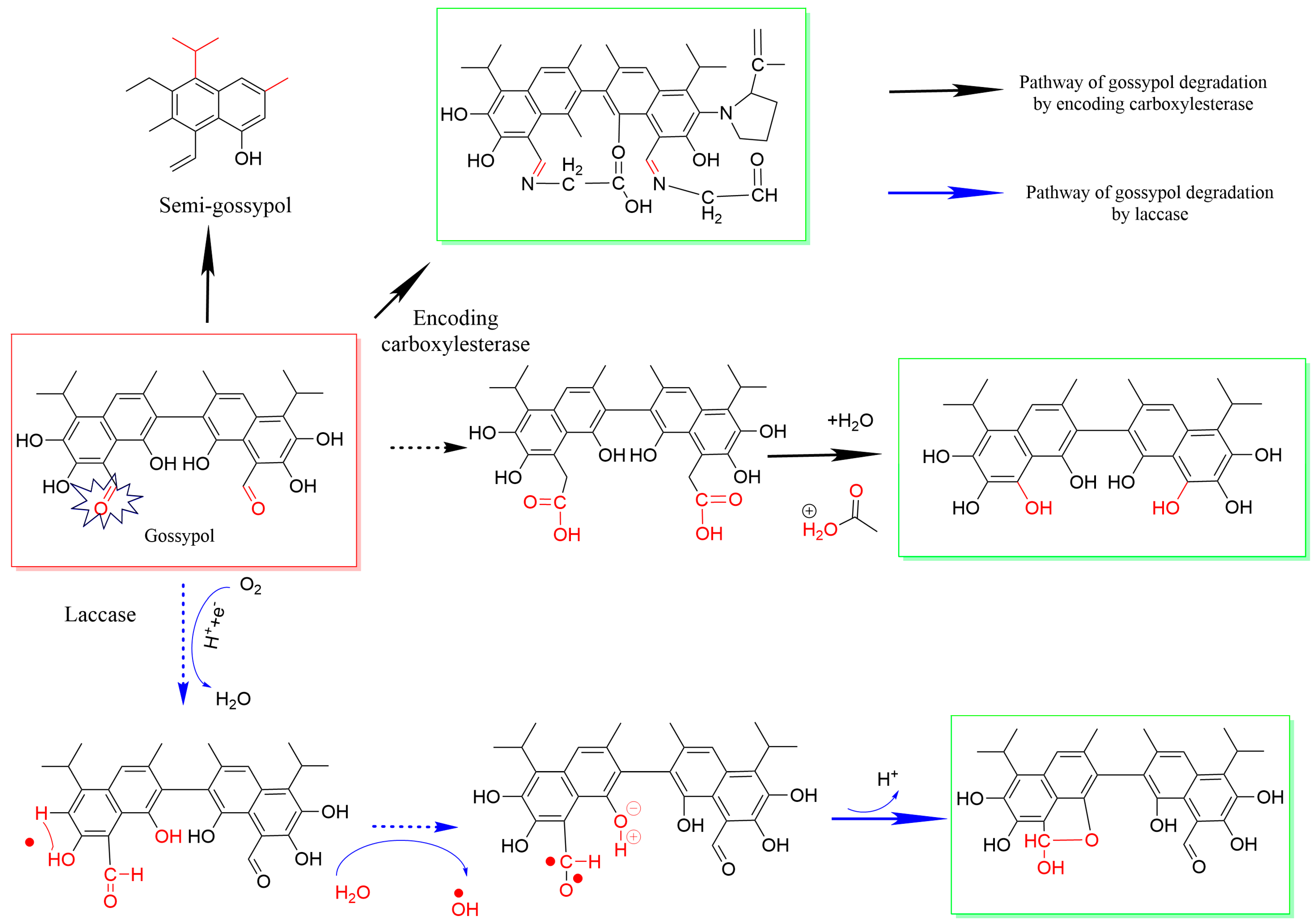
2.2. Antinutritional Factors—Gossypol
2.3. Green and Sustainable Plant Protein–Cottonseed Protein
3. Approaches for Efficient Utilization of DCSM
3.1. Physical Methods for the Degossypolization of DCSM
3.2. Chemical Methods for the Degossypolization of DCSM
3.3. Biological Methods for the Degossypolization of DCSM
3.4. Factors Affecting the Degradation of Gossypol by Solid-State Fermentation
3.5. Impact of Different Start Cultures on the Nutrient Content of DCSM
3.6. Application of Microbiome Engineering in Gossypol Degradation
4. Foods and Non-Food Applications of Cottonseed Protein
4.1. Cottonseed Protein as Food Source for Human Nutrition
4.2. DCSM as Animal Protein Source
4.3. Non-Food Application of DCSM and Cottonseed Protein
4.3.1. Cottonseed Protein Used as an Adhesive
4.3.2. Cottonseed Protein as Packaging Material
4.3.3. Cottonseed Protein as a Substrate for Industrial Production of Enzymes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Qanmber, G.; Wang, Z.; Yang, Z.; Li, F. Gossypium genomics: Trends, scope, and utilization for cotton improvement. Trends Plant Sci. 2020, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Data of Food and Agriculture Organization of United Nations. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 11 July 2024).
- Huang, G.; Huang, J.Q.; Chen, X.Y.; Zhu, Y.X. Recent advances and future perspectives in cotton research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A review of cottonseed protein chemistry and non-food applications. Sustain. Chem. 2020, 1, 256–274. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S. Cottonseed: A Sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, J.; Zou, G.; Zhang, X.; Shang, H.; Ji, B.; Bai, Y.; Qu, L.; Wei, Y. Enhancing the nutritional quality of defatted cottonseed meal by solid-state fermentation with probiotic microbes. Fermentation 2024, 10, 429. [Google Scholar] [CrossRef]
- Dolatkhah, B.; Ghorbani, G.R.; Alikhani, M.; Hashemzadeh, F.; Mahdavi, A.H.; Sadeghi-Sefidmazgi, A.; Erfani, H.; Rezamand, P. Effects of hydrolyzed cottonseed protein supplementation on performance, blood metabolites, gastrointestinal development, and intestinal microbial colonization in neonatal calves. J. Dairy Sci. 2020, 103, 5102–5117. [Google Scholar] [CrossRef]
- Bu, X.; Chen, A.; Lian, X.; Chen, F.; Zhang, Y.; Muhammad, I.; Ge, X.; Yang, Y. An evaluation of replacing fish meal with cottonseed meal in the diet of juvenile Ussuri catfish Pseudobagrus ussuriensis: Growth, antioxidant capacity, nonspecific immunity and resistance to Aeromonas hydrophila. Aquaculture 2017, 479, 829–837. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Sultan, Z.; Singh, R.; Dar, A.H. Classification, benefits, and applications of various anti-nutritional factors present in edible crops. J. Agric. Food Res. 2023, 14, 100902. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xu, Z.R.; Zhao, S.-H.; Jiang, J.F.; Wang, Y.; Yan, X.H. Optimization of process parameters for reduction of gossypol levels in cottonseed Meal by Candida tropicalis ZD-3 during solid substrate fermentation. Toxicon 2006, 48, 221–226. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. The use of cottonseed meal as a protein source for poultry: An updated review. World’s Poult. Sci. J. 2016, 72, 473–484. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Liu, Y.; Zheng, S.; Xu, S.; Xie, S.; Hu, Y. Gossypol is the main limiting factor in the application of cottonseed meal in grass carp feed production: Involvement of growth, intestinal physical and immune barrier, and intestinal microbiota. Water Biol. Secur. 2024, 3, 100287. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xu, Z.R.; Pan, X.L.; Yan, X.H.; Wang, Y. Advances in gossypol toxicity and processing effects of whole cottonseed in dairy cows feeding. Livest. Sci. 2007, 111, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Zhang, C.; Bian, Y.; Yao, W.; Xu, Z.; Wang, Y.; Li, X.; Leng, X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2022, 548, 737551. [Google Scholar] [CrossRef]
- Li, J.; Pradyawong, S.; He, Z.; Sun, X.S.; Wang, D.; Cheng, H.N.; Zhong, J. Assessment and application of phosphorus/calcium-cottonseed protein adhesive for plywood production. J. Clean. Prod. 2019, 229, 454–462. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.D.; Rodrigues, J.M.; Valadares, A.C.F.; Almeida, A.B.D.; Lima, T.M.D.; Takeuchi, K.P.; Alves, C.C.F.; Sousa, H.A.D.F.; Silva, E.R.D.; Dyszy, F.H. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Yue, H.; Zheng, Y.; Zheng, P.; Guo, J.; Fernández-Blázquez, J.P.; Clark, J.H.; Cui, Y. On the improvement of properties of bioplastic composites derived from wasted cottonseed protein by rational cross-linking and natural fiber reinforcement. Green Chem. 2020, 22, 8642–8655. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Z.; Ma, L.; Xu, Z.; Yu, J.; Li, F. Cotton stalk valorization towards bio-based materials, chemicals, and biofuels: A review. Renew. Sustain. Energy Rev. 2024, 202, 114651. [Google Scholar] [CrossRef]
- Tian, G.; Li, L.; Li, Y.; Wang, Q. Water-soluble poly(vinyl alcohol)/biomass waste composites: A new route toward ecofriendly materials. ACS Omega 2022, 7, 42515–42523. [Google Scholar] [CrossRef]
- Kaur, T.; Sharma, P.K.; Brar, A.S.; Choudhary, A.K.; Sharma, S.; Brar, H.S. Fiber quality, oil seed composition and fatty acid profiling of cotton (Gossypium hirsutum L.) seed as influenced by sub-surface drip-irrigation and foliar-fertilization strategy in semi-arid agro-ecology of south-Asia. J. Agric. Food Res. 2025, 19, 101604. [Google Scholar] [CrossRef]
- Zia, M.A.; Shah, S.H.; Shoukat, S.; Hussain, Z.; Khan, S.U.; Shafqat, N. Physicochemical features, functional characteristics, and health benefits of cottonseed oil: A review. Braz. J. Biol. 2022, 82, e243511. [Google Scholar] [CrossRef] [PubMed]
- Hinze, L.L.; Horn, P.J.; Kothari, N.; Dever, J.K.; Frelichowski, J.; Chapman, K.D.; Percy, R.G. Nondestructive measurements of cottonseed nutritional trait diversity in the U.S. national cotton germplasm collection. Crop Sci. 2015, 55, 770–782. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Sudduth, T.Q.; Condon, A.; Jenkins, T.C.; Calhoun, M.C. Nutrient content of whole cottonseed. J. Dairy Sci. 2005, 88, 1470–1477. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, A.L.; Owens, F.N. The effects of commercial processing method of cottonseed meal on site and extent of digestion in cattle. J. Anim. Sci. 1985, 60, 803–813. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kerkman, T.M.; Sullivan, H.M.; Dowd, M.K.; Funk, P.A. Effect of replacing soybean meal protein with protein from upland cottonseed, pima cottonseed, or extruded pima cottonseed on production of lactating dairy cows. J. Dairy Sci. 2013, 96, 2374–2386. [Google Scholar] [CrossRef]
- Pelitire, S.M.; Dowd, M.K.; Cheng, H.N. Acidic solvent extraction of gossypol from cottonseed meal. Anim. Feed Sci. Technol. 2014, 195, 120–128. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, X.; Wang, J.; Wang, H.; Qi, G.; Zhang, H.; Wu, S. Comparison of amino acid digestibility of soybean meal, cottonseed meal, and low-gossypol cottonseed meal between broilers and laying hens. Anim. Biosci. 2023, 36, 619–628. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Z.; Yang, H.; Wang, Z. Effects of cottonseed meal on growth performance, liver redox status, and serum biochemical parameters in goslings at 1 to 28 days of age. BMC Vet. Res. 2022, 18, 347. [Google Scholar] [CrossRef]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Liu, Z.; Guo, C.; Zhou, Y.; Sun, X. An overview of cotton gland development and its transcriptional regulation. Int. J. Mol. Sci. 2022, 23, 4892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shu, G.; Zhou, K.; You, Y.; Liu, R.; Chen, J.; Jiang, Q.; Yin, Y.; Chen, L. Long-term bound gossypol administration damages the liver and reproductive functions of male mice. Ann. Agric. Sci. 2024, 69, 100377. [Google Scholar] [CrossRef]
- Tao, A.; Wang, J.; Luo, B.; Liu, B.; Wang, Z.; Chen, X.; Zou, T.; Chen, J.; You, J. Research progress on cottonseed meal as a protein source in pig nutrition: An updated review. Anim. Nutr. 2024, 18, 220–233. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, A. Comparative studies on seed protein characteristics in eight lines of two Gossypium species. J. Cotton Res. 2019, 2, 6. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Cao, H. Protein profiling of water and alkali soluble cottonseed protein isolates. Sci. Rep. 2018, 8, 9306. [Google Scholar] [CrossRef]
- Delgado, E.; Valverde-Quiroz, L.; Lopez, D.; Cooke, P.; Valles-Rosales, D.; Flores, N. Characterization of soluble glandless cottonseed meal proteins based on electrophoresis, functional properties, and microscopic structure. J. Food Sci. 2019, 84, 2820–2830. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Sun, X.; Qin, A.; Wang, X.; Ge, X.; Liu, Z.; Guo, C.; Yu, X.; Zhang, X.; Lu, Y.; Yang, J. Spatiotemporal transcriptome and metabolome landscapes of cotton fiber during initiation and early development. Nat. Commun. 2025, 16, 858. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.J.; Ganjyal, G.M. Utilization of food processing by-products in extrusion processing: A review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Lin, S.M.; Zhou, X.M.; Zhou, Y.L.; Kuang, W.M.; Chen, Y.J.; Luo, L.; Dai, F.Y. Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish Shellfish Immunol. 2020, 103, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhao, C.; Zhu, J.; Hu, J.; Dong, X.; Sun, L. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 2018, 8, 113. [Google Scholar] [CrossRef]
- Soares Neto, C.B.; Conceição, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. A comparison of physical, chemical, biological and combined treatments for detoxification of free gossypol in crushed whole cottonseed. Waste Biomass Valorization 2021, 12, 3965–3975. [Google Scholar] [CrossRef]
- Gad, T.E.; El-Zalaki, E.M. Effect of various pressure cooking conditions on gossypol content of cottonseed meal and oil. Fette Seifen Anstrichm. 1980, 82, 450–453. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, Y.; Li, Y.; Zheng, J.; Zhang, J.; Kumar, M.; Li, F.; Ren, M. Multiple strategies to detoxify cottonseed as human food source. Front. Plant Sci. 2022, 13, 1080407. [Google Scholar] [CrossRef]
- Kadam, D.M.; Kumar, M.; Kasara, A. Application of high energy electromagnetic radiations in elimination of anti-nutritional factors from oilseeds. LWT 2021, 151, 112085. [Google Scholar] [CrossRef]
- Fatehi, P.; Alamouti, A.A.; Behgar, M.; Norouzian, M.A. Effect of electron irradiation on some physical, chemical and digestion properties of pistachio by-products. Radiat. Phys. Chem. 2020, 174, 108921. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Wholesomeness and safety aspects of irradiated foods. Food Chem. 2019, 285, 363–368. [Google Scholar] [CrossRef]
- Barraza, M.L.; Coppock, C.E.; Brooks, K.N.; Wilks, D.L.; Saunders, R.G.; Latimer, G.W. Iron sulfate and feed pelleting to detoxify free gossypol in cottonseed diets for dairy cattle. J. Dairy Sci. 1991, 74, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, D.; Sastry, V.R.B.; Agrawal, D.K. Detoxification of undecorticated cottonseed meal by various physical and chemical methods. Anim. Nutr. Feed Technol. 2002, 2, 117–126. [Google Scholar]
- Zhang, W.-J.; Xu, Z.R.; Zhao, S.-H.; Sun, J.-Y.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Technol. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Weng, X.Y.; Sun, J.Y. Biodegradation of free gossypol by a new strain of Candida tropicalis under solid state fermentation: Effects of fermentation parameters. Process Biochem. 2006, 41, 1663–1668. [Google Scholar] [CrossRef]
- Weng, X.Y.; Sun, J.Y. Kinetics of biodegradation of free gossypol by Candida tropicalis in solid-state fermentation. Biochem. Eng. J. 2006, 32, 226–232. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Huang, R.; Nie, C.; Niu, J.; Chen, C.; Zhang, W. Biodegradation of free gossypol by Helicoverpa armigera carboxylesterase expressed in Pichia pastoris. Toxins 2022, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, M.; Luo, X.; Fan, Y.; Zheng, Z.; He, Z.; Yin, R.; Meng, T.; Xu, S.; Pan, Y. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein. Front. Chem. 2020, 8, 583176. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.B.S.; Gomes, T.G.; Filho, E.X.F.; Fontes, W.; Ricart, C.A.O.; De Almeida, J.R.M.; De Siqueira, F.G.; Miller, R.N.G. An enzymatic and proteomic analysis of Panus lecomtei during biodegradation of gossypol in cottonseed. J. Fungi 2024, 10, 321. [Google Scholar] [CrossRef]
- Li, Q.; Chai, C.; Zhao, L. Biodegradation of endocrine disrupting chemicals with laccase isozymes from recombinant Pichia pastori. Catal. Lett. 2022, 152, 2625–2636. [Google Scholar] [CrossRef]
- Lin, J.; Li, T.; Yin, Y.; Wei, Y. Selected fungal fermentation of Astragali radix (Huangqi) alters its bioactive compound profile. Food Biosci. 2025, 63, 105781. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Ramos, C.L.; De Almeida, E.G.; Freire, A.L.; Freitas Schwan, R. Diversity of bacteria and yeast in the naturally fermented cotton seed and rice beverage produced by Brazilian Amerindians. Food Microbiol. 2011, 28, 1380–1386. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Liu, L.; Chang, Z.; Peng, N. Effective gossypol removal from cottonseed meal through optimized solid-state fermentation by Bacillus coagulans. Microb. Cell Fact. 2022, 21, 252. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Hao, Z.; Guo, X.; Zhu, B. Anaerobic solid-state fermentation with Bacillus subtilis for digesting free gossypol and improving nutritional quality in cottonseed meal. Front. Nutr. 2022, 9, 1017637. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Effect of lactic acid bacteria and yeast supplementation on anti-nutritional factors and chemical composition of fermented total mixed ration containing cottonseed meal or rapeseed meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joußen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, C.; Yang, S.; Ruan, Y.; Lyu, L.; Guo, X.; Wu, X.; Chen, Y. Exploring the impact of initial moisture content on microbial community and flavor generation in Xiaoqu baijiu fermentation. Food Chem. X 2023, 20, 100981. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Q.; Zhan, Y.; Mukhtar, H.; Fu, X.; Zhang, F.; Wang, Y.; Mou, H. A novel gossypol-degradation approach by Meyerozyma guilliermondii WST-M1 and its application in the development of cottonseed meal as feed resource. Ind. Crops Prod. 2024, 220, 119299. [Google Scholar] [CrossRef]
- Mougi, A. pH Adaptation stabilizes bacterial communities. npj Biodivers. 2024, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, M.; Elain, A.; Lendormi, T.; Le Fellic, M.; Le Treut, Y.; Sire, O. Keeping under control a liquid feed fermentation process for pigs: A reality scale pilot based study. Anim. Feed Sci. Technol. 2014, 194, 81–88. [Google Scholar] [CrossRef]
- Kabir, M.F.; Ju, L.-K. On optimization of enzymatic processes: Temperature effects on activity and long-term deactivation kinetics. Process Biochem. 2023, 130, 734–746. [Google Scholar] [CrossRef]
- Khalaf, M.; Meleigy, S. Reduction of free gossypol levels in cottonseed meal by microbial treatment. Int. J. Agric. Biol. 2008, 10, 1560–8530. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Dai, L.; Liu, Y.; Cheng, M.; Chen, L. Isolation and characterization of a novel gossypol-degrading bacteria Bacillus subtilis strain Rumen Bacillus Subtilis. Asian-Australas. J. Anim. Sci. 2018, 31, 63–70. [Google Scholar] [CrossRef]
- Sun, H.; Tang, J.; Yao, X.; Wu, Y.; Wang, X.; Liu, Y. Effects of replacement of fish meal with fermented cottonseed meal on growth performance, body composition and haemolymph indexes of pacific white shrimp, Litopenaeus vannamei Boone, 1931. Aquac. Res. 2016, 47, 2623–2632. [Google Scholar] [CrossRef]
- Wang, W.K.; Li, W.J.; Wu, Q.C.; Wang, Y.L.; Li, S.L.; Yang, H.J. Isolation and identification of a rumen Lactobacillus bacteria and its degradation potential of gossypol in cottonseed meal during solid-state fermentation. Microorganisms 2021, 9, 2200. [Google Scholar] [CrossRef]
- Tang, J.W.; Sun, H.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Australas. J. Anim. Sci. 2012, 25, 393–400. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Miao, L.; Meng, F.; Wu, L. Growth performance and toxic response of broilers fed diets containing fermented or unfermented cottonseed meal. J. Anim. Feed Sci. 2016, 25, 348–353. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, H.Y.; Zhuge, B.; Zhang, M.; Zhu, G.J. Isolation, identification and mutation breeding of high gossypol detoxification strain. Microbiol. China 2011, 38, 1166–1171. [Google Scholar]
- Dharmakar, P.; Aanand, S.; Kumar, J.; Ande, M.; Padmavathy, P.; Pereira, J.; Balakrishna, C. Solid-state fermentation of cottonseed meal with Saccharomyces cerevisiae for gossypol reduction and nutrient enrichment. Indian J. Anim. Res. 2023, 57, 868–874. [Google Scholar] [CrossRef]
- Sun, Z.T.; Liu, C.; Du, J.H. Optimisation of fermentation medium for the detoxification of free gossypol in cottonseed powder by Geotrichum candidum G07 in solid-state fermentation with response surface methodology. Ann. Microbiol. 2008, 58, 683–690. [Google Scholar] [CrossRef]
- Tang, X.; Xiang, R.; Chen, S.; Yang, S.; Hu, L.; Fang, R.; Li, A. Effects of fermented cottonseed meal and enzymatic hydrolyzed cottonseed meal on amino acid digestibility and metabolic energy in white leghorn rooster. Pak. J. Zool. 2018, 50, 957–962. [Google Scholar] [CrossRef]
- Vellaichamy, M. Optimization of solid state fermentation process for gossypol detoxification in heat sterilized cotton seed cake by mixed fungal cultures. Int. J. Food. Ferment. Technol. 2016, 6, 97. [Google Scholar] [CrossRef]
- Atia, A.; Abdel-Rahim, G. Detoxification treatments of free gossypol in cottonseed meal by microbial treatment of mixed cultures and biochemical evaluation on rabbits. J. Radiat. Res. Appl. Sci. 2009, 2, 397–415. [Google Scholar]
- Wang, Y.; Deng, Q.; Song, D.; Wang, W.; Zhou, H.; Wang, L.; Li, A. Effects of fermented cottonseed meal on growth performance, serum biochemical parameters, immune functions, antioxidative abilities, and cecal microflora in broilers. Food Agric. Immunol. 2017, 28, 725–738. [Google Scholar] [CrossRef]
- Mageshwaran, V.; Satankar, V.; Paul, S. Solid-state fermentation for gossypol detoxification and nutritive enrichment of cottonseed cake: A scale-up of batch fermentation process. BioResources 2024, 19, 1107–1118. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Enhancing the quality of total mixed ration containing cottonseed or rapeseed meal by optimization of fermentation conditions. Fermentation 2021, 7, 234. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Jazi, V.; Rezvani, M.R.; Mohebodini, H.; Soumeh, E.A.; Abdollahi, M.R. An investigation into the influence of fermented cottonseed meal on the productive performance, egg quality, and gut health in laying hens. Poult. Sci. 2024, 103, 103574. [Google Scholar] [CrossRef]
- Vellaichamy, M.; Shaikh, A.; Kathe, A. Optimization of process parameters for gossypol detoxification in chemical disinfected cottonseed cake by mixed fungal culture during solid state fermentation. J. Sci. Ind. Res. 2017, 76, 714–719. [Google Scholar]
- Li, Y.; Guo, B.; Li, C.; Wang, W.; Wu, Z.; Liu, G.; Cai, H. Isolation of a highly efficient antigenic-protein-degrading Bacillus amyloliquefaciens and assessment of its safety. Animals 2020, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules 2013, 3, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Duodu, C.P.; Adjei-Boateng, D.; Edziyie, R.E.; Agbo, N.W.; Owusu-Boateng, G.; Larsen, B.K.; Skov, P.V. Processing techniques of selected oilseed by-products of potential use in animal feed: Effects on proximate nutrient composition, amino acid profile and antinutrients. Anim. Nutr. 2018, 4, 442–451. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast proteins: The novel and sustainable alternative protein in food applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Lima, P.C.; Karimian, P.; Johnston, E.; Hartley, C.J. The use of Trichoderma spp. for the bioconversion of agro-industrial waste biomass via fermentation: A review. Fermentation 2024, 10, 442. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Zou, G.; Wei, Y.; Qu, L. Advancements and future directions in yellow rice wine production research. Fermentation 2024, 10, 40. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. CRISPR-based gene editing technology and its application in microbial engineering. Eng. Microbiol. 2023, 3, 100101. [Google Scholar] [CrossRef]
- Zou, G.; Nielsen, J.B.; Wei, Y. Harnessing synthetic biology for mushroom farming. Trends Biotechnol. 2023, 41, 480–483. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Nie, C.; Chen, C.; Zhang, W. Combined transcriptomics and cellular analyses reveal the molecular mechanism by which Candida tropicalis ZD-3 adapts to and degrades gossypol. Int. J. Biol. Macromol. 2024, 279, 135294. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Wen, S.; Wang, Y.; Pan, C.; Qu, L.; Yin, Y.; Wei, Y. Advancements in the biotransformation and biosynthesis of the primary active flavonoids derived from Epimedium. Molecules 2023, 28, 7173. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Tang, B.; Mijakovic, I.; Ji, X.J.; Qu, L.; Wei, Y. Discovery of novel alkaline-tolerant xylanases from fecal microbiota of dairy cows. Biotechnol. Biofuels Bioprod. 2023, 16, 182. [Google Scholar] [CrossRef]
- Fu, B.; Cheng, C.; Fan, J.; Yuan, J. Designing and Engineering Synthetic Microbiota to Utilize Plant Lignin-Based Biomass for the Synthesis of Bioactive Compounds. In Scale-Up and Chemical Process for Microbial Production of Plant-Derived Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2024; pp. 89–124. ISBN 978-0-443-15584-0. [Google Scholar]
- Wei, Y.; Niu, W.; Zhang, X.; Li, T.; Wang, L.; Xu, X.; Qu, L. The Synthetic Probiotic Microbiota and Their Potential Applications in the Production of Plant-Derived Products. In Scale-Up and Chemical Process for Microbial Production of Plant-Derived Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2024; pp. 137–152. ISBN 978-0-443-15584-0. [Google Scholar]
- Rathore, K.S.; Pandeya, D.; Campbell, L.M.; Wedegaertner, T.C.; Puckhaber, L.; Stipanovic, R.D.; Thenell, J.S.; Hague, S.; Hake, K. Ultra-low gossypol cottonseed: Selective gene silencing opens up a vast resource of plant-based protein to improve human nutrition. Crit. Rev. Plant Sci. 2020, 39, 1–29. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Y.; Xie, W.; Zhou, D.; Tang, S.; Kuang, M.; Wang, Y.; Du, S. Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Andrade-González, I.; Solís-Soto, A.; Medrano-Roldán, H.; Carrete, F.; Delgado, E. The effect of glandless cottonseed meal content and process parameters on the functional properties of snacks during extrusion cooking. Food Nutr. Sci. 2012, 3, 1716–1725. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Patil, S.; Saxena, S.; Patil, P.G.; Mageshwaran, V.; Punia, S.; Varghese, E.; Mahapatra, A.; Ashtaputre, N.; et al. Extraction of ultra-low gossypol protein from cottonseed: Characterization based on antioxidant activity, structural morphology and functional group analysis. LWT 2021, 140, 110692. [Google Scholar] [CrossRef]
- Jazi, V.; Boldaji, F.; Dastar, B.; Hashemi, S.R.; Ashayerizadeh, A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br. Poult. Sci. 2017, 58, 402–408. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Effects of phosphorus-containing additives on soy and cottonseed protein as wood adhesives. Int. J. Adhes. Adhes. 2017, 77, 51–57. [Google Scholar] [CrossRef]
- Verbeek, C.J.; Berg, L.E. Recent developments in thermo-mechanical processing of proteinous bioplastics. Recent Pat. Mater. Sci. 2009, 2, 171–189. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Chen, W.; Ding, J.; Yan, X.; Yan, W.; He, M.; Yin, G. Plasticization of cottonseed protein/polyvinyl alcohol blend films. Polymers 2019, 11, 2096. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Maurice, N. Role of Solid-State Fermentation to Enhance Cellulase Production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Srivastava, N., Srivastava, M., Mishra, P.K., Ramteke, P.W., Singh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–153. ISBN 978-0-444-64223-3. [Google Scholar]
- Zhang, H.; Sang, Q. Statistical optimization of cellulases production by Penicillium chrysogenum QML-2 under solid-state fermentation and primary application to chitosan hydrolysis. World J. Microbiol. Biotechnol. 2012, 28, 1163–1174. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Sharma, N.; Singh, S. Enhanced production of fibrinolytic protease from Bacillus cereus NS-2 using cotton seed cake as nitrogen source. Biocatal. Agric. Biotechnol. 2013, 2, 204–209. [Google Scholar] [CrossRef]


| Microorganisms | Optimum Conditions | Free Gossypol Removed (%) | Improvement in Protein Content (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Initial Moisture Content (%) | pH | Temperature (°C) | Duration of Fermentation (h) | Initial Inoculum Level (cells/g) | ||||
| Bacillus subtilis GH38 | 50 | 6.5 | 39 | 72 | 107 | 78.86 | 4.98 | [76] |
| Bacillus subtilis BJ–1 | 50 | – | 30 | 48 | 1% (v/w) | 59.47 | 7.63 | [77] |
| Bacillus coagulans S17 | 50 | – | 40 | 52 | 2.8 × 109 | 81.83 | 10.09 | [66] |
| Lactobacillus agilis WWK129 | 50 | – | 39 | 120 | 5% (v/w) | 80.0 | 7.12 | [78] |
| Bacillus subtilis BJ–1 | 50 | – | 30 | 48 | 1.4 × 108 | 74.4 | 8.58 | [79] |
| Bacillus subtilis M–15 | 50 | – | 25 | 336 | 109 | 96.5 | – | [67] |
| Candida tropicalis | 55 | 5.2 | 30 | 48 | 107 | 88.6 | 15.24 | [75] |
| Candida tropicalis ZD–3 | 50 | – | 30 | 48 | 10 g mycelia/Kg | 94.6 | 10.76 | [54] |
| Candida tropicalis ZAU–1 | 55 | 6.0 | 30 | 72 | 107 | 92.29 | – | [55] |
| Saccharomyces cerevisiae ZD–5 | 50 | – | 30 | 48 | 5 mL yeast | 88.51 | 11.09 | [54] |
| Candida utilis | 50 | – | 30 | 24 | 5.0 × 105 | 67.1 | 2.3 | [80] |
| Meyerozyma guilliermondii WST–M1 | 45 | – | 30 | 72 | 3.0 × 108 | 74.70 | 6.10 | [71] |
| Pichia pastoris Y–2 | 50 | – | 30 | 48 | 20 g mycelia/Kg | 58 | – | [81] |
| Saccharomyces cerevisiae | 50 | – | 28 | 48 | 60 mg yeast | 25 | – | [82] |
| Aspergillus niger ZD–8 | 50 | – | 30 | 48 | 10 g mycelia/Kg | 85.15 | 22.23 | [54] |
| Pycnoporus sanguineus CC400 | 60 | – | 28 | 360 | – | 98.95 | – | [46] |
| Geotrichum candidum G07 | 62.19 | – | 30 | 48 | 107 | 78.9 | – | [83] |
| Aspergillus niger | 50 | – | 28 | 48 | 10% (v/w) | – | 8.42 | [84] |
| Pleurotus sajor-caju and Saccharomyces cerevisiae | 70 | – | 30 | 48 | 106, 15% (v/w) | 83.6 | – | [85] |
| Saccharomyces cerevisae and Aspergillus niger | 55 | – | 30 | 48 | 106, 5% (v/w) | 90.2 | – | [86] |
| Bacillus subtilis ST-141 and Saccharomycetes N5 | 33.3 | – | 30 | 48 | 109, 0.5% (v/w) | 57.8 | 2.41 | [87] |
| Candida tropicalis and Saccharomycetes cerevisiae | 70 | – | 28 | 48 | 7.5% (v/w) | 83.6 | 67.5 | [88] |
| Bacillus clausii and Saccharomyces cariocanus | 50 | – | 32 | 60 | 109 and 5.0 × 109 | 36.50 | 17.45 | [89] |
| Saccharomyces cerevisiae, Bacillus subtilis and Lactiplantibacillus plantarum | 54.5 | 5.72 | 30 | 168 | 105, 1:1:1 | 89.14 | 8.02 | [90] |
| Candida tropicalis and Saccharomyces cerevisiae | 70 | – | 30 | 48 | 15% (v/w) | 79.50 | 13.40 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Li, T.; Zou, G.; Zhang, X.; Qu, L.; Wei, Y. Probiotic Fermentation of Defatted Cottonseed Meal for Sustainable Foods and Non-Food Applications. Microorganisms 2025, 13, 1020. https://doi.org/10.3390/microorganisms13051020
Yan Z, Li T, Zou G, Zhang X, Qu L, Wei Y. Probiotic Fermentation of Defatted Cottonseed Meal for Sustainable Foods and Non-Food Applications. Microorganisms. 2025; 13(5):1020. https://doi.org/10.3390/microorganisms13051020
Chicago/Turabian StyleYan, Zhanqiang, Tian Li, Gen Zou, Xiaoling Zhang, Lingbo Qu, and Yongjun Wei. 2025. "Probiotic Fermentation of Defatted Cottonseed Meal for Sustainable Foods and Non-Food Applications" Microorganisms 13, no. 5: 1020. https://doi.org/10.3390/microorganisms13051020
APA StyleYan, Z., Li, T., Zou, G., Zhang, X., Qu, L., & Wei, Y. (2025). Probiotic Fermentation of Defatted Cottonseed Meal for Sustainable Foods and Non-Food Applications. Microorganisms, 13(5), 1020. https://doi.org/10.3390/microorganisms13051020








