The Mystery of Certain Lactobacillus acidophilus Strains in the Treatment of Gastrointestinal Symptoms of COVID-19: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Research
3. Results and Discussion
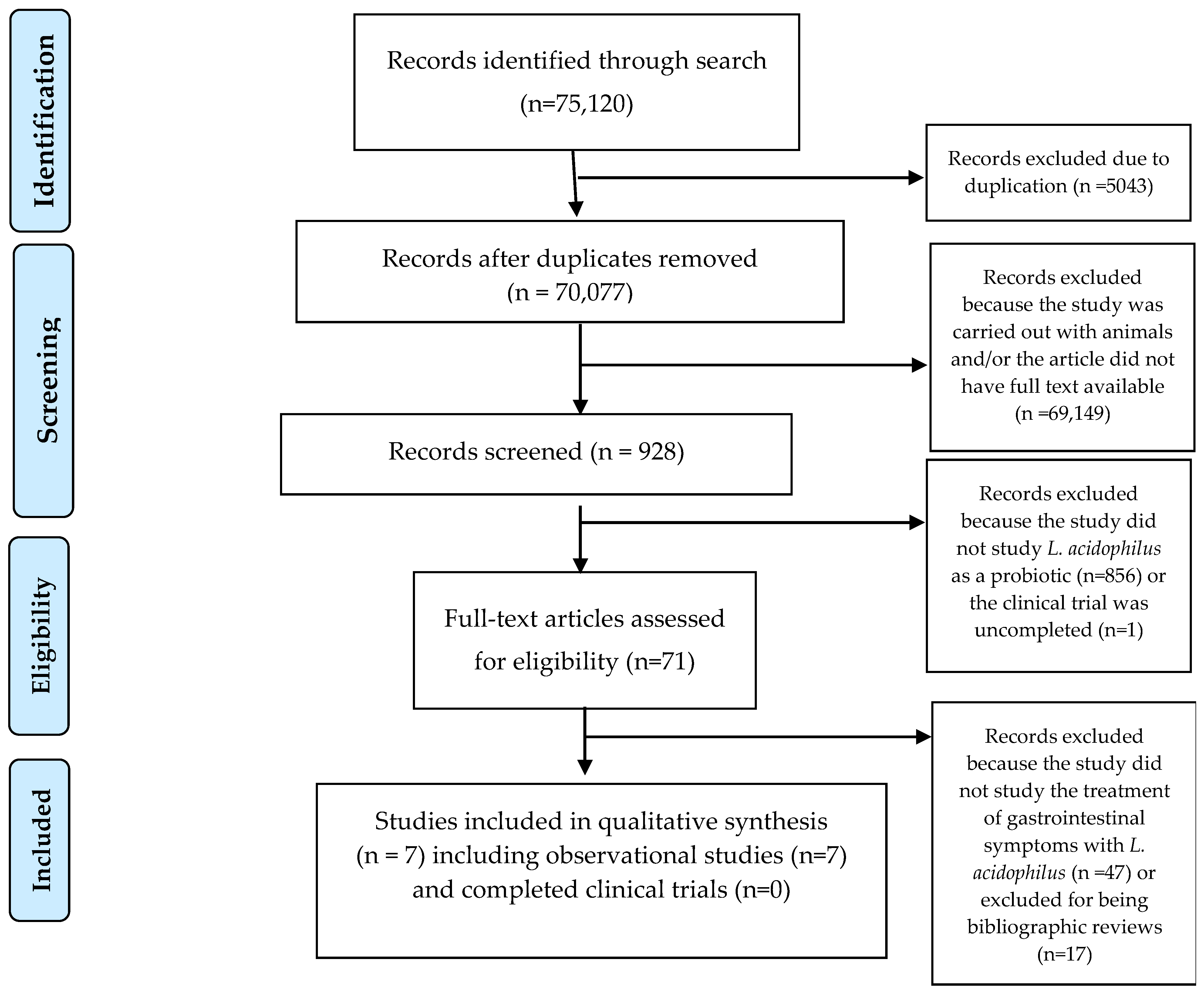
3.1. Selection of Results
3.2. Selected Studies
3.3. Mechanism of Action of L. acidophilus in Counteracting Gastrointestinal Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| COVID-19 | Coronavirus disease |
| CRP | C-reactive protein |
| GABA | Gamma-aminobutyric acid |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IgA | Immunoglobulin A |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| LAB | Lactic acid bacteria |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| NAC | N-acetyl-cysteine |
| PICO | Population, Intervention, Comparator, Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRN | Pro re nata |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SCFAs | Short-chain fatty acids |
| TNF-α | Necrosis factor-alpha |
| WHO | World Health Organization |
References
- Fernández-de-las-Peñas, C.; Raveendran, A.V.; Giordano, R.; Arendt-Nielsen, L. Long COVID or Post-COVID-19 Condition: Past, present and future research directions. Microorganisms 2023, 11, 2959. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, P.; Wilkins, M.W. Severe acute respiratory syndrome (SARS): A review. Clin. Med. 2004, 4, 152–160. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; Shehata, M.; El Shesheny, R.; Samy, A.M.; Kayali, G.; Ali, M.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): State of the science. Microorganisms 2020, 8, 991. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classification of 2019-nCoV and designation SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19–11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 12 March 2025).
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Boselli, P.M.; Soriano, J.M. COVID-19 in Italy: Is the mortality analysis a way to estimate how the epidemic lasts? Biology 2023, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. Epidemiology and pathogenesis of coronavirus disease (COVID-19). J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Slabakova, Y.; Gerasoudis, S.; Miteva, D.; Peshevska-Sekulovska, M.; Batselova, H.; Snegarova, V.; Vasilev, G.V.; Vasilev, G.H.; Sekulovski, M.; Lazova, S.; et al. SARS-CoV-2 Variant-Specific Gastrointestinal Symptoms of COVID-19: 2023 Update. Gastroenterol. Insights 2023, 14, 431–445. [Google Scholar] [CrossRef]
- Vernia, F.; Ashktorab, H.; Cesaro, N.; Monaco, S.; Faenza, S.; Sgamma, E.; Viscido, A.; Latella, G. COVID-19 and Gastrointestinal Tract: From Pathophysiology to Clinical Manifestations. Medicina 2023, 59, 1709. [Google Scholar] [CrossRef]
- Rodrigues, R.; Costa de Oliveira, S. The Impact of Angiotensin-Converting Enzyme 2 (ACE2) Expression Levels in Patients with Comorbidities on COVID-19 Severity: A Comprehensive Review. Microorganisms 2021, 9, 1692. [Google Scholar] [CrossRef]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Zhou, Y. Prevalence of comorbidities in Wuhan novel coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Du, B. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef] [PubMed]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Diet, Probiotics and Their Impact on the Gut Microbiota during the COVID-19 Pandemic. Nutrients 2021, 13, 3172. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Koh, G.C.H.; Car, J. COVID-19: A remote assessment in primary care. BMJ 2020, 368, m1182. [Google Scholar] [CrossRef]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Sheng, J.F. Clinical findings in patients infected with SARS-CoV-2 outside Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Qiu, Y. Managing Coronavirus Disease 2019 (COVID-19): The Experience of Zhejiang. J. Zhejiang Univ. (Med. Sci.) 2020, 49, 147–157. [Google Scholar]
- Wei, X.S.; Wang, X.; Niu, Y.R.; Ye, L.L.; Peng, W.B.; Wang, Z.H.; Wang, X.R. Clinical Features of SARS-CoV-2 Infected Pneumonia with Diarrhea. SSRN 2020. Available online: https://ssrn.com/abstract=3546120 (accessed on 12 March 2025).
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.C.; Shoham, S. Guidelines from the Infectious Diseases Society of America on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, 78, e83–e102. [Google Scholar] [CrossRef]
- Ji, L.-N.; Chao, S.; Wang, Y.-J.; Li, X.-J.; Mu, X.-D.; Lin, M.-G.; Jiang, R.-M. Clinical Features of Pediatric Patients with COVID-19: A Report of Two Family Cluster Cases. World J. Pediatr. 2020, 16, 267–270. [Google Scholar] [CrossRef]
- Jarocki, P.; Komoń-Janczara, E.; Glibowska, A.; Dworniczak, M.; Pytka, M.; Korzeniowska-Kowal, A.; Wzorek, A.; Kordowska-Wiater, M. Molecular Pathways for Specific Identification of the Lactobacillus Casei Group at the Species, Subspecies, and Strain Levels. Int. J. Mol. Sci. 2020, 21, 2694. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Barragán, P.J.; Serna, L. Review of Lactobacillus in the Food Industry and Their Culture Media. Rev. Colomb. Biotecnol. 2019, 21, 63–76. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and Intestinal Diseases: Mechanisms of Action and Clinical Applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Elements for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for the Reporting of Systematic Reviews and Meta-Analyses of Studies Evaluating Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, 65–94. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 March 2025).
- ClinicalTrials.gov. Monitoring the Efficacy of a Probiotic Dietary Supplement SmartProbio C in Patients with Severe COVID-19 Infection. Available online: https://clinicaltrials.gov/study/NCT05474144 (accessed on 16 March 2025).
- ClinicalTrials.gov. Synbiotic Therapy of Gastrointestinal Symptoms During COVID-19 Infection. Available online: https://clinicaltrials.gov/study/NCT04420676 (accessed on 16 March 2025).
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case Rep. 2020, 30, 101063. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, Y.; Qi, W. The small intestine, an underestimated site of SARS-CoV-2 infection: From Red Queen effect to probiotics. Preprints 2020. [Google Scholar] [CrossRef]
- Saviano, A.; Potenza, A.; Siciliano, V.; Petruzziello, C.; Tarli, C.; Migneco, A.; Nasella, F.; Franceschi, F.; Ojetti, V. COVID-19 Pneumonia and Gut Inflammation: The Role of a Mix of Three Probiotic Strains in Reducing Inflammatory Markers and Need for Oxygen Support. J. Clin. Med. 2022, 11, 3758. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.; Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in COVID-19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Haller, R.; Feldbacher, N.; Habisch, H.; Žukauskaitė, K.; Madl, T.; Stadlbauer, V. Probiotic Therapy of Gastrointestinal Symptoms During COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled, Remote Study. Nutrients 2024, 16, 3970. [Google Scholar] [CrossRef]
- Hassan, S.O.A.; Hassan, A.N.E.-D.; Mohamed, M.S.; Al Ashram, M.N.B.; Nesim, M.M.; Allam, M.F. The effects of probiotic Lactobacillus acidophilus and colchicine on the control of symptoms, duration, and disease progression of mild and moderate cases of COVID-19: Randomized controlled clinical trial. Microbes Infect. Dis. 2024, 5, 901–910. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. Antipathogenic activity of probiotics against Salmonella Typhimurium and Clostridium difficile in anaerobic batch culture systems: Is it due to synergies in probiotic mixtures or the specificity of single strains? Anaerobe 2013, 24, 60–65. [Google Scholar] [CrossRef]
- Medellin-Pena, M.J.; Griffiths, M.W. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157: H7 colonization. Appl. Environ. Microbiol. 2009, 75, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Guha, D.; Naik, A.K.; Banerjee, A.; Tambat, S.; Chawla, S.; Aich, P. Probiotics Lactobacillus acidophilus and Bacillus clausii modulate gut microbiota in Th1- and Th2-biased mice to ameliorate Salmonella typhimurium-induced diarrhea. Probiotics Antimicrob. Proteins 2019, 11, 887–904. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and Bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. Biomed. Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Karimi, R.; Hosseinzadeh, D. Probiotics and Gastro-Intestinal Disorders: Augmentation, Enhancement, and Strengthening of Epithelial Lining. In Probiotics; CRC Press: Boca Raton, FL, USA, 2024; pp. 188–215. [Google Scholar]
- di Vito, R.; Conte, C.; Traina, G. A multi-strain probiotic formulation improves intestinal barrier function by the modulation of tight and adherent junction proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef]
- Yadav, A.K.; Tyagi, A.; Kumar, A.; Panwar, S.; Grover, S.; Saklani, A.C.; Batish, V.K. Adhesion of lactobacilli and their anti-infectivity potential. Crit. Rev. Food Sci. Nutr. 2017, 57, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, G.; Zhang, J.; Pei, C.; Chen, Y.; Gong, J.; Liao, Q. Live Lactobacillus acidophilus alleviates ulcerative colitis via the SCFAs/mitophagy/NLRP3 inflammasome axis. Food Funct. 2022, 13, 2985–2997. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The functional roles of Lactobacillus acidophilus in different physiological and pathological processes. J. Microbiol. Biotechnol. 2022, 32, 1226. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Paturi, G.; Phillips, M.; Jones, M.; Kailasapathy, K. Immune enhancing effects of Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 in mice. Int. J. Food Microbiol. 2007, 115, 115–118. [Google Scholar] [CrossRef]
- Walana, W.; Ye, Y.; Li, M.; Wang, J.; Wang, B.; Cheng, J.W.; Li, F. IL-8 antagonist, CXCL8 (3-72) K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed. Pharmacother. 2018, 103, 253–261. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.; Choi, J.W.; Park, S.H.; Cho, M.L.; Kwok, S.K. Lactobacillus acidophilus supplementation exerts a synergistic effect on tacrolimus efficacy by modulating Th17/Treg balance in lupus-prone mice via the SIGNR3 pathway. Front. Immunol. 2021, 12, 696074. [Google Scholar] [CrossRef]
- Ayesiga, I.; Iqbal, S.; Kyejjusa, Y.; Okoboi, J.; Omara, T.; Adelina, T.; Kahwa, I. Key regulatory aspects of prebiotics, probiotics, and synbiotics in the management of metabolic disorders. In Synbiotics in Metabolic Disorders; CRC Press: Boca Raton, FL, USA, 2024; pp. 214–244. [Google Scholar]
- Zhao, W.; Liu, Y.; Kwok, L.Y.; Cai, T.; Zhang, W. The immune regulatory role of Lactobacillus acidophilus: An updated meta-analysis of randomized controlled trials. Food Biosci. 2020, 36, 100656. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Lang, U.E.; Brühl, A.B.; Schneider, E. Exploring the Therapeutic Potential of Gamma-Aminobutyric Acid in Stress and Depressive Disorders through the Gut–Brain Axis. Biomedicines 2023, 11, 3128. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can psychobiotics “mood” ify gut? An updated systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2020, 39, 1395–1410. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The effects of probiotics and prebiotics on mental disorders: A review on depression, anxiety, Alzheimer, and autism spectrum disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Sarid, L.; Zanditenas, E.; Ye, J.; Trebicz-Geffen, M.; Ankri, S. Insights into the mechanisms of Lactobacillus acidophilus activity against Entamoeba histolytica by using thiol redox proteomics. Antioxidants 2022, 11, 814. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Patel, M.; Adnan, M.; Jahan, S.; Saxena, J.; Alshahrani, M.M.; Abdelgadir, A.; Bardakci, F.; Sachidanandan, M.; Badraoui, R.; et al. Bacteriocin-Nanoconjugates (Bac10307-AgNPs) Biosynthesized from Lactobacillus acidophilus-Derived Bacteriocins Exhibit Enhanced and Promising Biological Activities. Pharmaceutics 2023, 15, 403. [Google Scholar] [CrossRef]
- Wei, Q.; Song, L.Y.; Rao, R.; Yang, H.W.; Wen, Y.P.; Lv, L.; Wang, L. The impact of combined therapy with Lactobacillus acidophilus and montmorillonite powder on the inflammatory response in pediatric rotavirus enteritis. Int. Arch. Allergy Immunol. 2024, 1–7. [Google Scholar] [CrossRef]
- Steyer, A.; Mičetić-Turk, D.; Fijan, S. The Efficacy of Probiotics as Antiviral Agents for the Treatment of Rotavirus Gastrointestinal Infections in Children: An Updated Overview of Literature. Microorganisms 2022, 10, 2392. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Abou Hachem, M.; Svensson, B. Mucin- and carbohydrate-stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. J. Proteomics 2017, 163, 102–110. [Google Scholar] [CrossRef]
- Mei, L.; Chen, Y.; Wang, J.; Lu, J.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus fermentum Stimulates Intestinal Secretion of Immunoglobulin A in an Individual-Specific Manner. Foods 2022, 11, 1229. [Google Scholar] [CrossRef]
- Săsăran, M.O.; Mărginean, C.O.; Adumitrăchioaiei, H.; Meliț, L.E. Pathogen-Specific Benefits of Probiotic and Synbiotic Use in Childhood Acute Gastroenteritis: An Updated Review of the Literature. Nutrients 2023, 15, 643. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Mishra, V.N.; Kumari, N.; Pathak, A.; Chaturvedi, R.K.; Gupta, A.K.; Chaurasia, R.N. Possible Role for Bacteriophages in the Treatment of SARS-CoV-2 Infection. Int. J. Microbiol. 2020, 2020, 8844963. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Reddy, L. Gastrointestinal Microbiota Dysbiosis Associated with SARS-CoV-2 Infection in Colorectal Cancer: The Implication of Probiotics. Gastroenterol. Insights 2022, 13, 35–39. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef]
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics Regulating Inflammation via NLRP3 Inflammasome Modulation: A Potential Therapeutic Approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef]
- Synodinou, K.D.; Nikolaki, M.D.; Triantafyllou, K.; Kasti, A.N. Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk. Microorganisms 2022, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
| Reference/ Article | 1 a | 2 a | 3 a | 4 a | 5 a | 6 a | 7 a | 8 a | 9 a | Quality Rating b |
|---|---|---|---|---|---|---|---|---|---|---|
| Ji et al. [23] | + | + | ? | + | + | + | ? | + | + | 7 |
| Horowitz et al. [34] | + | + | + | + | + | + | − | + | + | 8 |
| Feng et al. [35] | + | + | − | + | + | − | − | − | − | 4 |
| Saviano et al. [36] | + | + | + | + | + | + | + | + | + | 9 |
| Gutiérrez et al. [37] | + | + | + | + | + | + | + | + | + | 9 |
| Horvarth et al. [38] | + | + | + | + | + | + | + | + | + | 9 |
| Hassan et al. [39] | + | + | + | + | + | + | − | − | + | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertola, B.; Cotolí-Crespo, A.; San Onofre, N.; Soriano, J.M. The Mystery of Certain Lactobacillus acidophilus Strains in the Treatment of Gastrointestinal Symptoms of COVID-19: A Review. Microorganisms 2025, 13, 944. https://doi.org/10.3390/microorganisms13040944
Bertola B, Cotolí-Crespo A, San Onofre N, Soriano JM. The Mystery of Certain Lactobacillus acidophilus Strains in the Treatment of Gastrointestinal Symptoms of COVID-19: A Review. Microorganisms. 2025; 13(4):944. https://doi.org/10.3390/microorganisms13040944
Chicago/Turabian StyleBertola, Belén, Amparo Cotolí-Crespo, Nadia San Onofre, and Jose M. Soriano. 2025. "The Mystery of Certain Lactobacillus acidophilus Strains in the Treatment of Gastrointestinal Symptoms of COVID-19: A Review" Microorganisms 13, no. 4: 944. https://doi.org/10.3390/microorganisms13040944
APA StyleBertola, B., Cotolí-Crespo, A., San Onofre, N., & Soriano, J. M. (2025). The Mystery of Certain Lactobacillus acidophilus Strains in the Treatment of Gastrointestinal Symptoms of COVID-19: A Review. Microorganisms, 13(4), 944. https://doi.org/10.3390/microorganisms13040944







