Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review
Abstract
1. Introduction
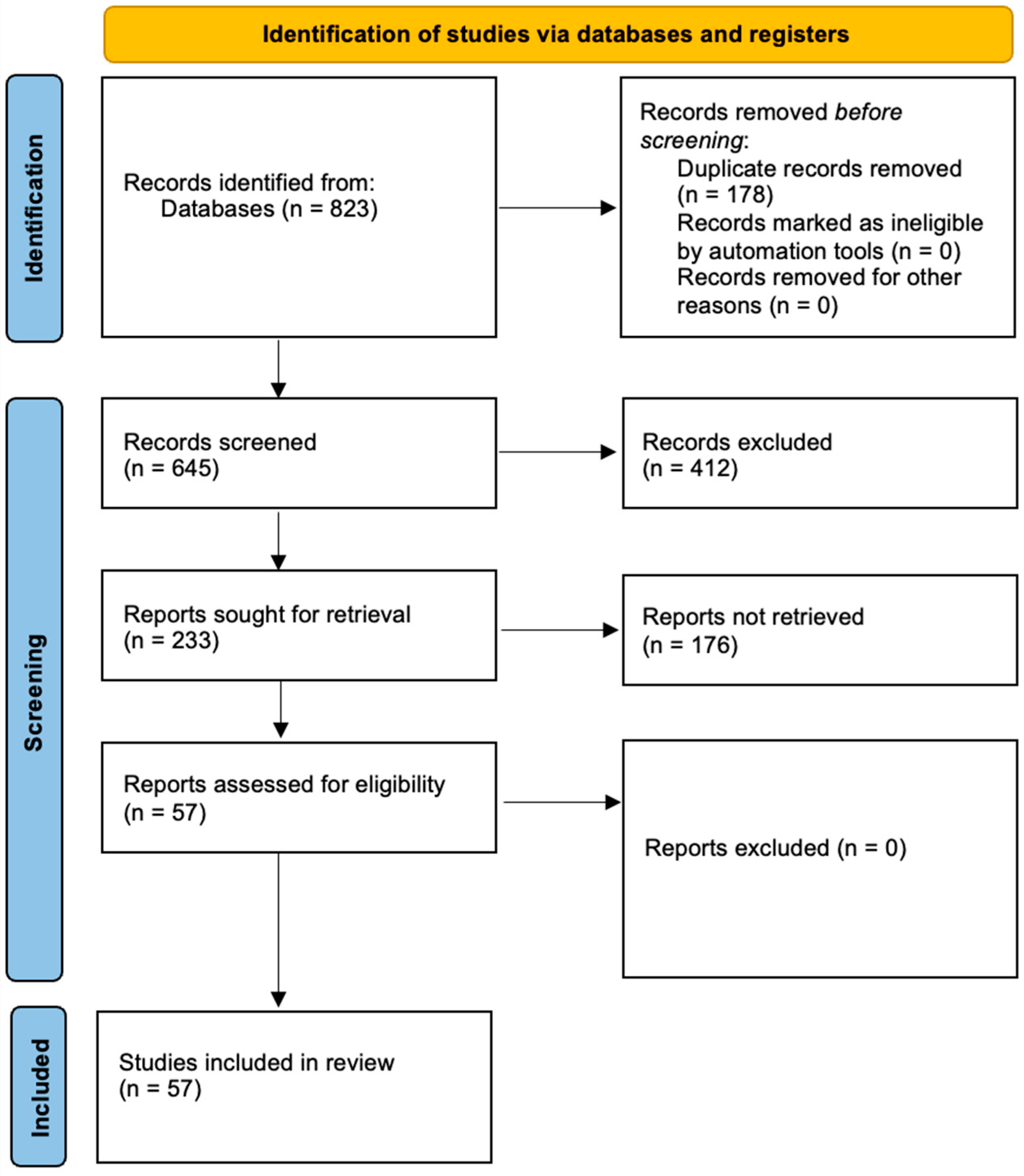
2. Methods
3. Results and Discussion
3.1. Comparative Analysis of the Effectiveness of Er:YAG Laser Against Single-Species Candida Biofilms
3.2. Comparison of Er:YAG Laser and Er,Cr:YSGG Laser, Its Antifungal Capabilities in Dentistry
3.3. The Effect of Laser on Candida albicans and Non-abicans Forms
3.4. Clinical Prospects for the Use of Er:YAG and Er,Cr:YSGG in Candidiasis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Deeb, J.; Smith, J.; Belvin, B.; Lewis, J.; Grzech-Leśniak, K.; Grzech-Leśniak, K. Er:YAG Laser Irradiation Reduces Microbial Viability When Used in Combination with Irrigation with Sodium Hypochlorite, Chlorhexidine, and Hydrogen Peroxide. Microorganisms 2019, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Deeb, J.; Kitten, T.; Carrico, C.; Grzech-Leśniak, K. The In Vitro Effect of Laser Irradiation (Er:YAG and CO2) and Chemical Reagents (Hydrogen Peroxide, Sodium Hypochlorite, Chlorhexidine, or Sodium Fluoride) Alone or in Combination on Reducing Root Caries Bacteria. Int. J. Mol. Sci. 2022, 23, 15732. [Google Scholar] [CrossRef]
- Datla, V.; Uppalapati, L.; Pilli, H.; Mandava, J.; Kantheti, S.; Komireddi, S.; Chandolu, V. Effect of ultrasonic and Er,Cr:YSGG laser-activated irrigation protocol on dual-species root canal biofilm removal: An in vitro study. J. Conserv. Dent. Endod. 2024, 27, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Golge, M.; Aydın, U.; Manay, A. Antibacterial effects of ER: YAG-PIPS, ER, CR: YSGG laser and conventional irrigation on enterococcus faecalis and Candida albicans. Ann. Med. Res. 2019, 26, 2748–2753. [Google Scholar] [CrossRef]
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of sensitivity of selected Candida strains on antimicrobial photodynamic therapy using diode laser 635 nm and toluidine blue—In vitro research. Photodiagn. Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef]
- Tyczkowska-Sieroń, E.; Kałużewski, T.; Grabiec, M.; Kałużewski, B.; Tyczkowski, J. Genotypic and phenotypic changes in Candida albicans as a result of cold plasma treatment. Int. J. Mol. Sci. 2020, 21, 8100. [Google Scholar] [CrossRef]
- Staniszewska, M. Virulence factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Bartusik-Aebisher, D.; Latos, W.; Cieślar, G.; Sieroń, K.; Kwiatek, S.; Oleś, P.; Kwiatek, B.; Aebisher, D.; Krupka, M.; et al. Clinical Trials and Basic Research in Photodynamic Diagnostics and Therapies from the Center for Laser Diagnostics and Therapy in Poland. Photochem. Photobiol. 2020, 96, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2024, 17, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilowski, Ł.; Wiench, R.; Polakiewicz-Gilowska, A.; Dwornicka, K. Necrotizing sialometaplasia of the palatal mucosa in patient with history of anorexia: Review and case report. Am. J. Otolaryngol. 2014, 35, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Zięba, N.; Turski, R.; Misiołek, M.; Wiench, R. Hypericin-Mediated Photodynamic Therapy for Head and Neck Cancers: A Systematic Review. Biomedicines 2025, 13, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sebbane, N.; Steinberg, D.; Keinan, D.; Sionov, R.; Farber, A.; Sahar-Helft, S. Antibacterial Effect of Er:YAG Laser Irradiation Applied by a New Side-Firing Spiral Tip on Enterococcus faecalis Biofilm in the Tooth Root Canal—An Ex Vivo Study. Appl. Sci. 2022, 12, 12656. [Google Scholar] [CrossRef]
- Assery, N.; Alomeir, N.; Zeng, Y.; Xiao, J.; Tsigarida, A. The Effect of Er:YAG Laser Treatment on Biofilm Formation on Titanium and Zirconia Disc Surfaces. J. Periodontol. 2022, 94, 344–353. [Google Scholar] [CrossRef]
- Kung, J.; Wang, W.; Chiang, Y.; Yang-Wang, Y.; Wang, Y.; Chen, W.; Shih, C. The Antibacterial and Remineralization Effect of Silver-Containing Mesoporous Bioactive Glass Sealing and Er-YAG Laser on Dentinal Tubules Treated in a Streptococcus mutans Cultivated Environment. Pharmaceuticals 2021, 14, 1124. [Google Scholar] [CrossRef]
- Lotfali, E.; Ghasemi, R.; Fattahi, A.; Keymaram, M.; Shafiei, M.; Norouzi, M.; Ayatollahi, A. Activities of Nanoparticles Against Fluconazole-Resistant Candida parapsilosis in Clinical Isolates. Assay Drug Dev. Technol. 2021, 19, 501–507. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida auris: A comparison between planktonic and biofilm susceptibility to antifungal drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Delavy, M.; Cerutti, L.; Croxatto, A.; Prod’Hom, G.; Sanglard, D.; Greub, G.; Coste, A. Machine learning approach for Candida albicans fluconazole resistance detection using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Front. Microbiol. 2020, 10, 3000. [Google Scholar] [CrossRef]
- Cleare, L.; Li, K.; Abuzeid, W.; Nacharaju, P.; Friedman, J.; Nosanchuk, J. NO Candida auris: Nitric oxide in nanotherapeutics to combat emerging fungal pathogen Candida auris. J. Fungi 2020, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Momeni, E.; Didehdar, M.; Sarlak, E.; Safari, M. In vitro effect of a high-intensity laser on Candida albicans colony count. J. Lasers Med. Sci. 2022, 13, e59. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Hirota, T.; Ohsugi, Y.; Shimohira, T.; Toyoshima, K.; Katagiri, S.; Iwata, T.; Aoki, A. Effects of low-level Er:YAG laser irradiation on proliferation and gene expression in primary gingival fibroblasts isolated from mouse maxilla. J. Biophotonics 2023, 17, e202300166. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, J.; Ribeiro, Í.; De Oliveira Santos, I.; Soares, R.; Valentim-Silva, J.; Maggi, L.; De Albuquerque Pereira, W.; Rodriguez, A. In vitro Effect of the Association of Therapeutic Ultrasound to Fluconazole on the Growth Inhibition of Candida albicans. Arch. Curr. Res. Int. 2024, 24. [Google Scholar] [CrossRef]
- Osamah, R.; Jawad, H. In vitro investigation of the antifungal and 940 nm diode laser effects on inhibition of Candida albicans isolated from the oral cavity. Iraqi J. Laser 2023, 22, 44–51. [Google Scholar] [CrossRef]
- Wang, C.; Lee, B.; Jhang, Y.; Huang, C.; Fu, K.; Lai, C.; Kuo, M.; Chen, Y. Effect of Er:YAG laser on surface properties, bacterial and lipopolysaccharide clearance, and human gingival fibroblast adhesion: An in-vitro peri-implantitis model. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Ambati, S.; Pham, T.; Lewis, Z.; Lin, X.; Meagher, R. DectiSomes: Glycan targeting of liposomal drugs improves the treatment of disseminated candidiasis. Antimicrob. Agents Chemother. 2021, 66, e01467-21. [Google Scholar] [CrossRef]
- Hetta, H.F.; Melhem, T.; Aljohani, H.M.; Salama, A.; Ahmed, R.; Elfadil, H.; Alanazi, F.E.; Ramadan, Y.N.; Battah, B.; Rottura, M.; et al. Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways. Pharmaceuticals 2025, 18, 364. [Google Scholar] [CrossRef]
- Pinna, A.; Donadu, M.G.; Dore, S.; Serra, R.; Sacchi, M.; Boscia, G.; Bozó, A.; Kovács, R. In Vitro Activity of a New Ophthalmic Spray Containing Biosecur® Citrus Extract (Oftasecur®) Against Candida auris and Candida albicans and Preformed Biofilm on Contact Lenses. Vision 2025, 9, 12. [Google Scholar] [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian Essential Oil of Ruta graveolens against Nosocomial Antifungal Resistant Candida Strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef]
- Matulić, N.; Bago, I.; Sušić, M.; Gjorgievska, E.; Kotarac Knežević, A.; Gabrić, D. Comparison of Er:YAG and Er,Cr:YSGG laser in the treatment of oral leukoplakia lesions refractory to the local retinoid therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.; Taher, H.; Alalawi, A. Histological evaluation of the surgical margins of oral soft tissue incisions using a dual-wavelength diode laser and an Er, Cr:YSGG laser; an ex vivo study. J. Appl. Oral Sci. 2024, 32, e20230419. [Google Scholar] [CrossRef]
- Fathi-Hafshejani, P.; Tinker, H.; Freel, K.; Mahjouri-Samani, M.; Hasim, S. Effects of TiS2 on Inhibiting Candida albicans Biofilm Formation and Its Compatibility with Human Gingival Fibroblasts in Titanium Implants. ACS Appl. Bio Mater. 2023, 6, 436–444. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Ding, H.; Tsoi, J. Antifungal Ability of Novel Silane on Titanium Implant Surface. Coatings 2024, 14, 1277. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Kang, P.; Sanz-Miralles, E.; Li, J.; Linden, E.; Momen-Heravi, F. Efficacy of Er,Cr:YSGG Laser Application in Nonsurgical Treatment of Peri-implantitis: A Human Randomized Controlled Trial. Int. J. Periodontics Restor. Dent. 2023, 43, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Merigo, E.; Bufflier, P.; Rocca, J.; Chevalier, M.; Medioni, E.; Meng, Z.; Fornaini, C. Bactericidal effect of Er,Cr:YSGG laser irradiation on endodontic biofilm: An ex vivo study. J. Photochem. Photobiol. B 2021, 218, 112185. [Google Scholar] [CrossRef]
- Li, M.; Jia, J.; Wu, M.; Zhao, C.; Jia, L.; Shi, H.; Zhang, X. Clinical effectiveness of Er,Cr:YSGG lasers in non-surgical treatment of chronic periodontitis: A meta-analysis of randomized controlled trials. Lasers Med. Sci. 2020, 36, 889–901. [Google Scholar] [CrossRef]
- Talebi-Ardakani, M.R.; Torshabi, M.; Karami, E.; Arbabi, E.; Rezaei Esfahrood, Z. In Vitro Study of Er:YAG and Er, Cr:YSGG Laser Irradiation on Human Gingival Fibroblast Cell Line. Acta Med. Iran. 2016, 54, 251–255. [Google Scholar] [PubMed]
- Aiuto, R.; Dioguardi, M.; Angiero, F.; Peñarrocha-Diago, M.; Crippa, R. A clinical protocol for immediate dental implant placement in post-extraction-infected sites decontaminated with Er,Cr:YSGG laser. Minerva Dent. Oral Sci. 2023, 73, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Benfenati, S.; Galletti, P.; Zuchi, A.; Sava, C.; Trifan, M.; Piattelli, A.; Iezzi, G.; Chen, C.; Kim, D.; et al. Human Histologic Evaluations of the Use of Er,Cr:YSGG Laser to Decontaminate an Infected Dental Implant Surface in Preparation for Implant Reosseointegration. Int. J. Periodontics Restor. Dent. 2020, 40, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Klokkevold, P.; Damian, A.; Pham, C.; Mallya, S.; Lux, R. Clinical evaluation of Er,Cr:YSGG laser therapy used as an adjunct to non-surgical treatment of periodontitis: Twelve-month results from a pilot study. J. Periodontol. 2022, 93, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Turan, D.; Aksaray, S. One-Year Candida Data of the Central Mycology Laboratory: Which Sample, Which Species, How Resistant? Mikrobiyol. Bul. 2022, 56, 493–505. [Google Scholar] [CrossRef]
- Rudresh, S.; Nikhi, V.; Shakuntala, P.; Hansraj, C.; Karthik, B.; Tejaswini, N.; Shivaprakash, M. Antifungal susceptibility profile of Candida species isolated from women with vulvovaginal candidiasis. J. Lab. Physicians 2024, 16, 272–276. [Google Scholar] [CrossRef]
- Bayraktar, E.; Erel, C.; Akturk, H.; Erkan, I.; Hamid, R.; Alper, E.; Adaletli, I.; Urfalioglu, M. A novel objective evaluation method, shear wave elastography, in the treatment of atrophic vaginitis by nonablative intravaginal Er:YAG laser, a randomized-sham controlled pilot study. Menopause 2024, 31, 716–723. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Qin, J.; Lu, S.; Cai, W.; Li, J.; Huang, H.; Yang, S.; Xi, L. Comparison of a fractional 2940-nm Er:YAG laser and 5% amorolfine lacquer combination therapy versus a 5% amorolfine lacquer monotherapy for the treatment of onychomycosis: A randomized controlled trial. Lasers Med. Sci. 2020, 36, 147–152. [Google Scholar] [CrossRef]
- Mohammadi, S.; Shoorgashti, R.; Lotfali, E.; Lesan, S.; Ebrahimi, H. The Evaluation of 660, 810, and 940nm Laser Wavelengths on Nystatin-Resistant Candida albicans: An In-Vitro Study. Jundishapur J. Microbiol. 2024, 17, e144680. [Google Scholar] [CrossRef]
- Campos, W.G.; Esteves, C.V.; Gallo, C.B.; Domaneschi, C.; Aranha, A.C.C.; Lemos, C.A. Treatment of oral leukoplakia with CO2 laser (10,600 nm): Analysis of 37 cases. Braz Oral Res. 2022, 36, e014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pereira de Sá, N.; Zarnowski, R.; Huang, M.Y.; Fernandes, C.M.; Lanni, F.; Andes, D.R.; Del Poeta, M.; Mitchell, A.P. Biofilm-associated metabolism via ERG251 in Candida albicans. PLoS Pathog. 2024, 20, e1012225. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luan, X.; Xie, F.; Chang, W.; Lou, H. Erg6 Acts as a Downstream Effector of the Transcription Factor Flo8 To Regulate Biofilm Formation in Candida albicans. Microbiol. Spectr. 2023, 11, e00393-23. [Google Scholar] [CrossRef]
- Kramara, J.; Kim, M.-J.; Ollinger, T.L.; Ristow, L.C.; Wakade, R.S.; Zarnowski, R.; Wellington, M.; Andes, D.R.; Mitchell, A.G.; Krysan, D.J. Systematic analysis of the Candida albicans kinome reveals environmentally contingent protein kinase-mediated regulation of filamentation and biofilm formation in vitro and in vivo. mBio. 2024, 15, e0124924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Büyüktuna, S.A.; Hasbek, M.; Elaldı, N.; Gözel, M.G.; Çelik, C.; Engin, A.; Bakıcı, M.Z.; Bakır, M. Epidemiological analysis of nosocomial Candida infections: Experience of a university hospital. Cumhur. Med. J. 2019, 41, 318–327. [Google Scholar] [CrossRef][Green Version]
- Kermani, F.; Sadeghian, M.; Shokohi, T.; Hashemi, S.; Moslemi, D.; Davodian, S.; Abastabar, M.; Bandalizadeh, Z.; Faeli, L.; Seifi, Z.; et al. Molecular identification and antifungal susceptibility testing of Candida species isolated from oral lesions in patients with head and neck cancer undergoing radiotherapy. Curr. Med. Mycol. 2021, 7, 44–50. [Google Scholar] [CrossRef]
- Phommachan, K.; Keo-Oudone, C.; Nurcholis, M.; Vongvilaisak, N.; Chanhming, M.; Savanhnaly, V.; Bounphanmy, S.; Matsutani, M.; Kosaka, T.; Limtong, S.; et al. Adaptive Laboratory Evolution for Multistress Tolerance, including Fermentability at High Glucose Concentrations in Thermotolerant Candida tropicalis. Energies 2022, 15, 561. [Google Scholar] [CrossRef]
- Adnan, R.; Jawad, H. Antimicrobial photodynamic therapy using a low-power 650 nm laser to inhibit oral Candida albicans activity: An in vitro study. J. Med. Life 2024, 17, 28–34. [Google Scholar] [CrossRef]
- Hasanah, N.; Sufiawati, I.; Kusumadjati, A.; Sunardi, M. In Vitro Effect of Low-level Laser Therapy on Candida albicans Colonies Isolated from Patients Undergoing Radiotherapy for Head and Neck Cancer. J. Lasers Med. Sci. 2024, 15, e11. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Tempesta, A.; Limongelli, L.; Maiorano, E.; Benedicenti, S.; Favia, G. Nonsurgical Periodontal Treatment by Erbium:YAG Laser Promotes Regression of Gingival Overgrowth in Patient Taking Cyclosporine A: A Case Report. Photobiomodulation Photomed. Laser Surg. 2019, 37, 53–56. [Google Scholar] [CrossRef]
- Najafi, S.; Sheykhbahaei, N.; Khayamzadeh, M.; Gholizadeh, N. The effect of low level laser on number of Candida albicans colonies in-vitro: A new finding. BMC Oral Health 2019, 19, 104. [Google Scholar] [CrossRef]
- Moreschi, L.; Fuhr, M.; Saraçol, D. Photodynamic activity of porfyrinic derivatives front Candida albicans. Amaz. Sci. Health 2024, 12. [Google Scholar] [CrossRef]
- Cavallo, L.; Menotti, F.; Roana, J.; Costa, C.; Longo, F.; Pagano, C.; Curtoni, A.; Bondi, A.; Banche, G.; Allizond, V.; et al. Synergistic Effect of Essential Oils and Antifungal Agents in Fighting Resistant Clinical Isolates of Candida auris. Pharmaceutics 2024, 16, 957. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Luong, M. Update on therapeutic approaches for invasive fungal infections in adults. Ther. Adv. Infect. Dis. 2024, 11, 20499361231224980. [Google Scholar] [CrossRef]
- Alhamdan, M.; Basunbul, G. Oral Health-Related Quality of Life among Complete Denture Stomatitis Patients Treated with Methylene-Blue-Mediated Photodynamic Therapy. Appl. Sci. 2024, 14, 926. [Google Scholar] [CrossRef]
- Izoton, C.; Chagas, W.; Israel, M. Photodynamic therapy on the treatment of oral candidiasis in people living with HIV/AIDS: A promising approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e240. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, G.; Cardoso, A.; Ferreira, I.; Brazão-Silva, M.; Guimarães, D. Evaluation of Quality of Life and Oral Changes of Patients in Head and Neck Radiotherapy: Observational Study. J. Cancer Ther. 2021, 12, 641–653. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Taniguchi, Y.; Lin, T.; Ohsugi, Y.; Mikami, R.; Katagiri, S.; Meinzer, W.; Iwata, T. Current status of Er:YAG laser in periodontal surgery. Jpn. Dent. Sci. Rev. 2024, 60, 1–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, S.H.; Kim, O.J.; Chung, H.J.; Kim, O.S. Effect of a Er, Cr:YSGG laser and a Er:YAG laser treatment on oral biofilm-contaminated titanium. J. Appl. Oral Sci. 2020, 28, e20200528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashim, A.; Kheir El Din, N.H.; El-Khazragy, N.; Almalahy, H.G. Comparison of the efficacy of Er,Cr:YSGG laser on oral biofilm removal from implant surfaces with various application times for the treatment of peri-implantitis defects: Ex vivo study. BMC Oral Health 2024, 24, 980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AlMoharib, H.S.; Steffensen, B.; Zoukhri, D.; Finkelman, M.; Gyurko, R. Efficacy of an Er:YAG laser in the decontamination of dental implant surfaces: An in vitro study. J. Periodontol. 2021, 92, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Ho, K.; Ma, X.; Cho, Y.W.; Chen, C.Y.; Kim, D.M. Effect of Er,Cr:YSGG Laser Irradiation on the Surface Modification and Cell Adhesion on Titanium Discs: An In Vitro Study. Materials 2024, 17, 4899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, A.; Ghaffar, H.; Taib, H.; Hassan, A. A Review of Bacterial Colonization on Dental Implants With Various Hygiene Instruments. Cureus 2023, 15, e47483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, C.; Khan, A.; Meredith, N.; Walsh, L.J. Influence of eight debridement techniques on three different titanium surfaces: A laboratory study. Int. J. Dent. Hyg. 2023, 21, 238–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, S.H.; Kim, K.; Cho, S.; Chung, D.H.; Ahn, S.J. Variation in adhesion of Streptococcus mutans and Porphyromonas gingivalis in saliva-derived biofilms on raw materials of orthodontic brackets. Korean J. Orthod. 2022, 52, 278–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfergany, A.M.; Nasher, R.; Gutknecht, N. Calculus Removal and Root Surface Roughness When Using the Er:YAG or Er,Cr:YSGG Laser Compared with Conventional Instrumentation Method: A Literature Review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 197–226. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Er:YAG | Er,Cr:YSGG | Main Results |
|---|---|---|---|
| Wavelength (nm) | About 2940 | About 2780 | Both lasers operate in the mid-infrared range, which facilitates the ablation of fluid and biofilm |
| Type of study (in vitro/in vivo) | Used in biofilm research and clinical applications | Used mainly in experiments on dental and prosthetic surfaces | Both types of lasers are capable of reducing the number of viable Candida cells under the right parameters |
| Optimum power (W) | 1.5–5.0 (depending on the pulse mode) | 1.0–4.0 (adjustable for different modes) | Efficiency increases with increasing power, but at the same time, the risk of thermal damage to tissue increases |
| Exposure time (s) | 10–30 | 5–20 | Longer exposures usually provide better disinfection, but require control over the preservation of surrounding tissue |
| Penetration depth (mm) | Several millimeters, mostly surface action | Similar or slightly smaller | Limited depth of penetration is relevant for surface biofilms, which allows targeted destruction of the fungal layer without significant damage to the underlying layers |
| Candida form | Typical Lesions | The Main Factors of Virulence | Probable Sensitivity to Laser |
|---|---|---|---|
| C. albicans | Denture stomatitis, chronic atrophic candidiasis | High enzymatic activity, adhesion to mucous membranes | Considered to be fairly high with correct Er:YAG and Er,Cr:YSGG parameters |
| C. glabrata | Recurrent gum disease, possible drug-resistant forms | Weaker hyphal formation, but higher tolerance to some antimycotics | May require higher laser parameters for effective suppression |
| C. tropicalis | Ulcerative lesions of the tongue, erythematous lesions | Expressed ability to form biofilms | Potentially high sensitivity; in thick biofilms, careful longer exposures are required |
| C. parapsilosis | Frequent infections in immunocompromised patients | Active formation of biofilms on dentures | Needs to be investigated for optimal parameters; preliminary data indicate good potential |
| C. krusei | Rare but resistant gingival infections | Low adhesion, but high tolerance to some drugs | Limited information; requires tailored approaches and additional research |
| Study | Laser Type | Chemical Adjuncts | Microorganisms Targeted | Key Findings |
|---|---|---|---|---|
| Deeb et al. [1] | Er:YAG | NaOCl, CHX, H2O2 | Bacteria | Significant microbial reduction with laser + antiseptics |
| Reddy et al. [2] | Er:YAG, CO2 | NaOCl, CHX, H2O2, NaF | Bacteria | Combined treatments more effective than individual |
| Datla et al. [3] | Er,Cr:YSGG | C. albicans, S. aureus | Laser outperformed ultrasonic and syringe rinsing | |
| Golge et al. [4] | Er:YAG-PIPS, Er,Cr:YSGG | NaOCl | C. albicans | Er:YAG-PIPS + NaOCl most effective; Er,Cr:YSGG less effective |
| Valenti et al. [5] | Er:YAG | None | C. albicans, Streptococcus spp., Lactobacillus spp. | Laser less invasive, effective against Candida |
| Laser Type | Wavelength (nm) | Typical Power Range (W) | Typical Exposure Time (s) | Target Applications | Advantages |
|---|---|---|---|---|---|
| Er:YAG | 2940 | 1.5–5.0 | 10–30 | Deep fungal disinfection, biofilm matrix breakdown | High antifungal effect, effective in dense biofilms |
| Er,Cr:YSGG | 2780 | 1.0–4.0 | 5–20 | Tissue-sensitive applications, prosthetic surfaces | Lower thermal impact, better postoperative comfort |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembicka-Mączka, D.; Gryka-Deszczyńska, M.; Sitkiewicz, J.; Makara, A.; Fiegler-Rudol, J.; Wiench, R. Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review. Microorganisms 2025, 13, 942. https://doi.org/10.3390/microorganisms13040942
Dembicka-Mączka D, Gryka-Deszczyńska M, Sitkiewicz J, Makara A, Fiegler-Rudol J, Wiench R. Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review. Microorganisms. 2025; 13(4):942. https://doi.org/10.3390/microorganisms13040942
Chicago/Turabian StyleDembicka-Mączka, Diana, Magdalena Gryka-Deszczyńska, Jacek Sitkiewicz, Aleksander Makara, Jakub Fiegler-Rudol, and Rafał Wiench. 2025. "Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review" Microorganisms 13, no. 4: 942. https://doi.org/10.3390/microorganisms13040942
APA StyleDembicka-Mączka, D., Gryka-Deszczyńska, M., Sitkiewicz, J., Makara, A., Fiegler-Rudol, J., & Wiench, R. (2025). Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review. Microorganisms, 13(4), 942. https://doi.org/10.3390/microorganisms13040942






