Production and Characterization of Poly-γ-Glutamic Acid by Bacillus velezensis SDU
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the Bacteria
2.2. Optimization of γ-PGA Production
Selection of Factors Required for γ-PGA Production
2.3. Scale-Up Fermentation in Bioreactor
2.4. Purification of γ-PGA from Bacillus velezensis SDU
2.5. Analytical Methods for Biomass and Glycerol Quantification
2.6. Qualitative and Quantitative Analysis of γ-PGA
2.7. Determination of Amino Acid Configuration by Marfey’s Method
2.8. GPC-MALLS for Molecular Weight Determination of γ-PGA
2.9. Mark–Houwink Equation for Molecular Weight Determination of γ-PGA
2.10. Preparation of γ-PGA with Different Molecular Weights
2.11. The Scavenging Effect of the γ-PGA to the ·O2− Free Radicals
2.12. γ-PGA Tyrosinase Inhibition Rate Measurement
3. Results
3.1. Isolation and Characterization of Bacillus velezensis SDU
3.2. Results of the Optimization of γ-PGA Production
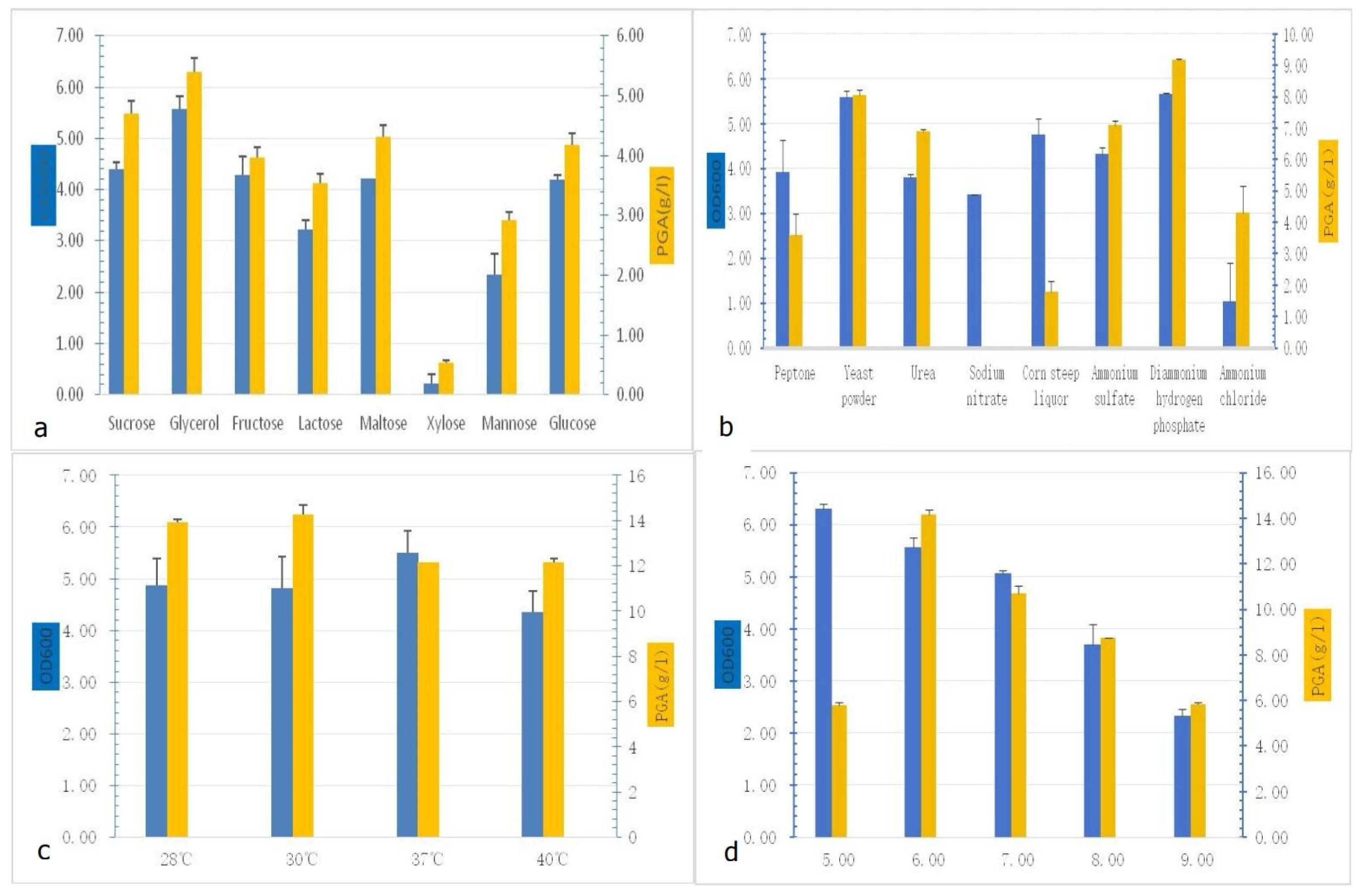
3.2.1. Results of the Selection of Factors Required for γ-PGA Production
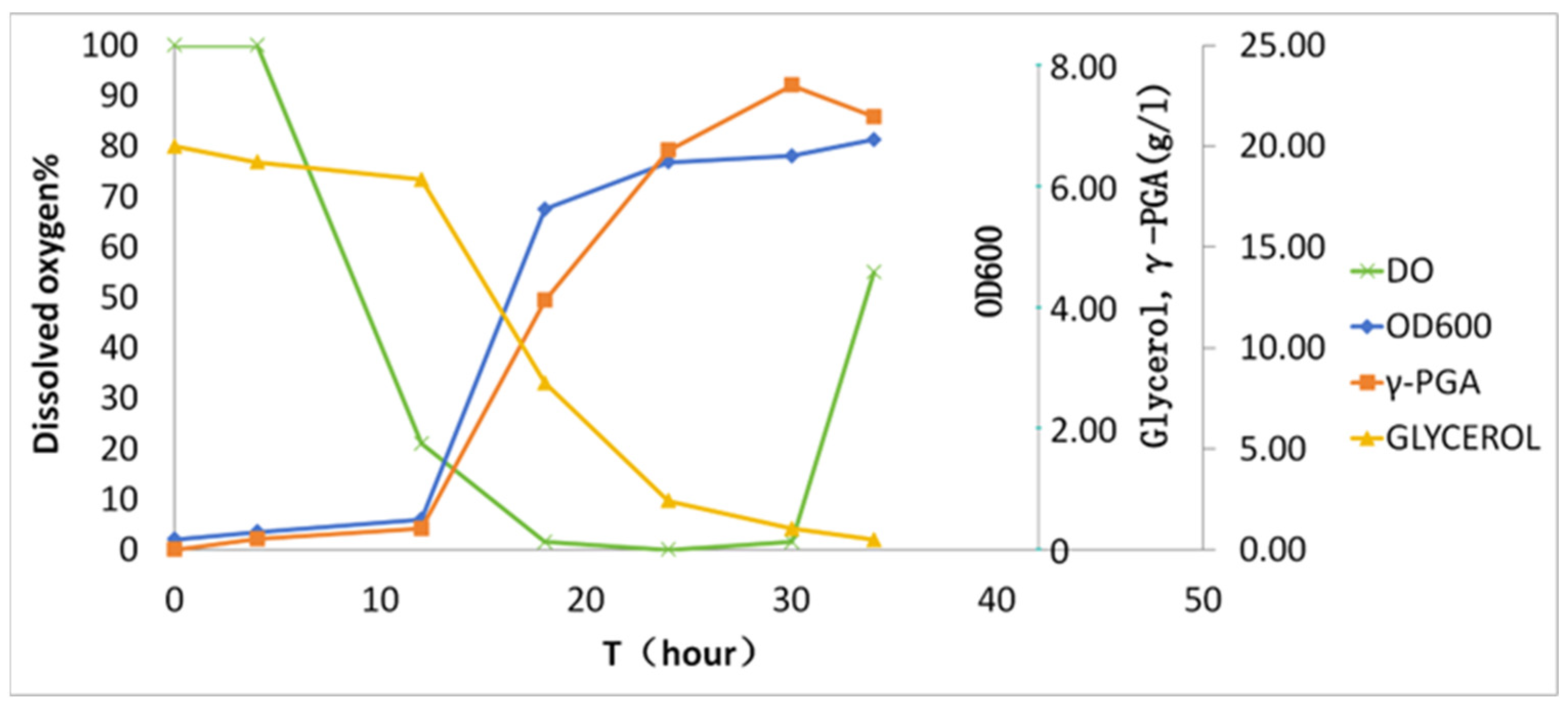
3.2.2. Scale-Up Fermentation in 50 L Bioreactor
3.2.3. Characterization of the γ-PGA in the Strain SDU
3.2.4. Preparation of Poly-γ-Glutamic Acid with Different Molecular Weights
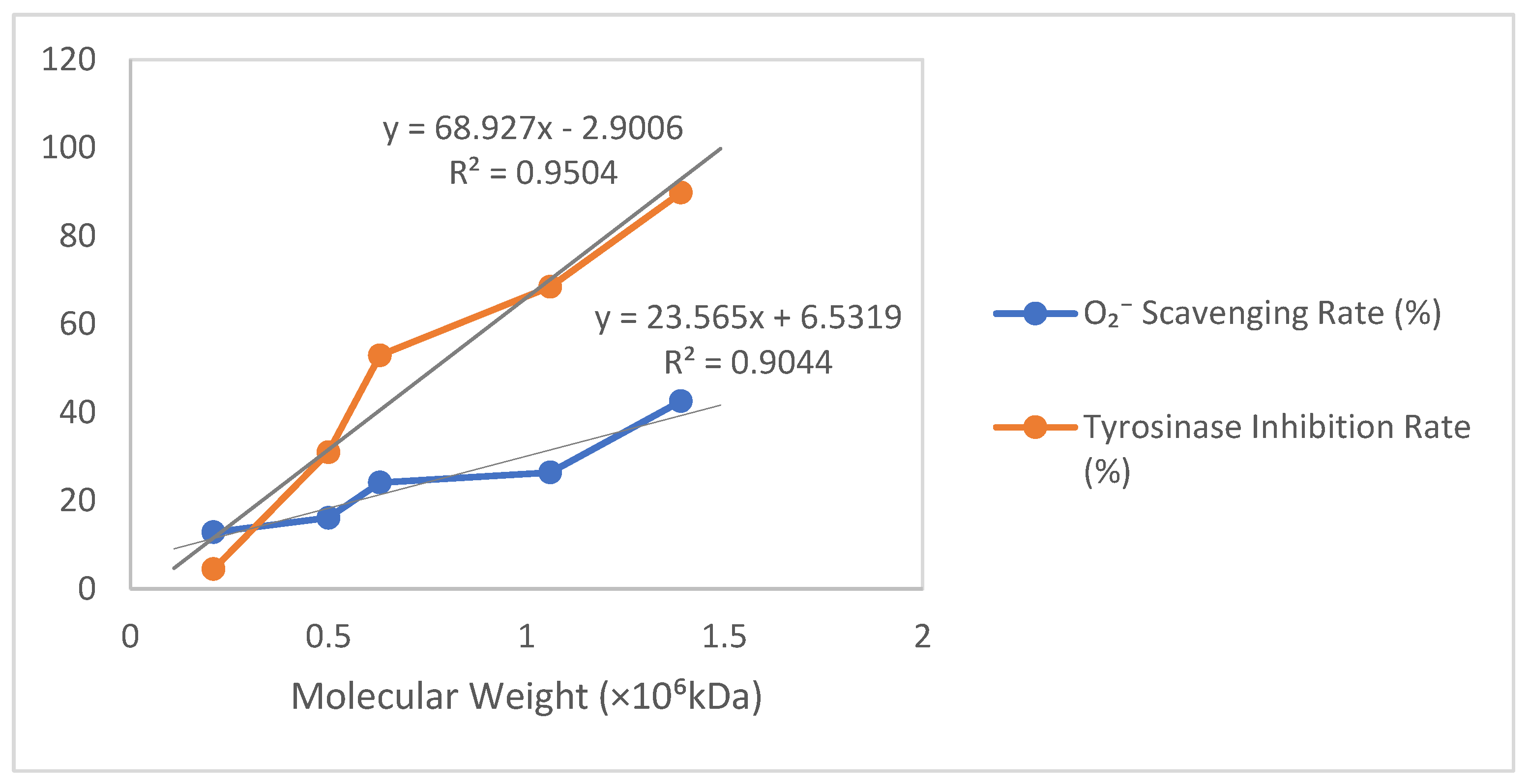
3.2.5. Results of ·O2− Clearance and Tyrosinase Inhibition by Different Molecular Weights of γ-PGA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebrahimzadeh Kouchesfahani, M.; Bahrami, A.; Babaeipour, V. Poly-γ-glutamic acid overproduction of Bacillus licheniformis ATCC 9945a by developing a novel optimum culture medium and glutamate pulse feeding using different experimental design approaches. Biotechnol. Appl. Biochem. 2024, 71, 565–583. [Google Scholar] [CrossRef]
- Tanimoto, H. Food Applications of Poly-Gamma-Glutamic Acid. In Amino-Acid Homopolymers Occurring in Nature; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Abdelnaby, T.; Li, Z.; Cao, W.; Xue, C. The effect of gamma-poly glutamic acid as a cryoprotectant on crayfish physicochemical and texture properties during frozen storage. LWT 2023, 184, 114905. [Google Scholar] [CrossRef]
- Johnson, L.C.; Akinmola, A.T.; Scholz, C. Poly(glutamic acid): From natto to drug delivery systems. Biocatal. Agric. Biotechnol. 2022, 40, 102292. [Google Scholar] [CrossRef]
- Sekine, T.; Nakamura, T.; Shimizu, Y.; Ueda, H.; Matsumoto, K.; Takimoto, Y.; Kiyotani, T. A new type of surgical adhesive made from porcine collagen and polyglutamic acid. J. Biomed. Mater. Res. 2001, 54, 305–310. [Google Scholar] [CrossRef]
- Wang, L.; Hu, D.; Kong, X.; Liu, J.; Li, X.; Zhou, K.; Zhao, H.; Zhou, C. Anionic polypeptide poly(γ-glutamic acid)-functionalized magnetic Fe3O4-GO-(o-MWCNTs) hybrid nanocomposite for high-efficiency removal of Cd(II), Cu(II) and Ni(II) heavy metal ions. Chem. Eng. J. 2018, 346, 38–49. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, J.T.; Wang, L.F.; Wu, S.; Zhang, G.Z.; Yu, H.-Q.; Ye, X.-D.; Shi, Q.-S. Conformations and molecular interactions of poly-γ-glutamic acid as a soluble microbial product in aqueous solutions. Sci. Rep. 2017, 7, 12787. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, Y.; Fu, C.; Sablani, S.S.; Huang, Z.; Han, C.; Li, D.; Sun, Z.; Qin, H. Antimicrobial activity of gamma-poly (glutamic acid), a preservative coating for cherries. Colloids Surf. B Biointerfaces 2023, 225, 113272. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Wei, X.; Hu, Z.; Zhu, F.; Xu, L.; Luo, M.; Liu, H. Production of ultra-high molecular weight poly-γ-glutamic acid with Bacillus licheniformis P-104 and characterization of its flocculation properties. Appl. Biochem. Biotechnol. 2013, 170, 562–572. [Google Scholar] [CrossRef]
- Park, C.; Choi, Y.-H.; Shin, H.-J.; Poo, H.; Song, J.J.; Kim, C.-J.; Sung, M.-H. Effect of high-molecular-weight poly-γ-glutamic acid from Bacillus subtilis (chungkookjang) on Ca solubility and intestinal absorption. J. Microbiol. Biotechnol. 2005, 15, 855–858. [Google Scholar]
- Lee, J.M.; Jang, W.J.; Park, S.H.; Kong, I.S. Antioxidant and gastrointestinal cytoprotective effect of edible polypeptide poly-γ-glutamic acid. Int. J. Biol. Macromol. 2020, 153, 616–624. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Singhal, R.S. Enhanced production of poly(gamma-glutamic acid) from Bacillus licheniformis NCIM 2324 by using metabolic precursors. Appl. Biochem. Biotechnol. 2009, 159, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Cryoprotection of probiotic bacteria with poly-γ-glutamic acid produced by Bacillus subtilis and Bacillus licheniformis. J. Genet. Eng. Biotechnol. 2016, 14, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Yamaguchi, F.; Yuasa, K.; Tahara, Y. Efficient Production of γ-Polyglutamic Acid by Bacillus subtilis (natto) in Jar Fermenters. Biosci. Biotechnol. Biochem. 1997, 61, 1684–1687. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Thapa, P.; Thapa, A.; Khadka, S.; Sapkota, S.; Panta, O.P.; Sharma, S.; Karki, T.B.; Poudel, P. Screening and characterization of potent poly glutamic acid producing Bacillus sp. isolated from Kinema, water and soil samples. Heliyon 2021, 7, e07715. [Google Scholar] [CrossRef]
- Moraes, L.P.; Alegre, R.M.; Brito, P.N. Optimisation of Poly(γ-Glutamic Acid) Production by Bacillus velezensis NRRL B—23189 in Liquid Fermentation with Molasses as the Carbon Source without Addition of Glutamic Acid. Int. Rev. Chem. Eng. 2014, 5, 130–135. [Google Scholar]
- Zhu, R.; Ma, X.; Liu, J. Optimization of γ-polyglutamic acid synthesis using response surface methodology of a newly isolated glutamate dependent Bacillus velezensis Z3. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2018, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, N.; Ke, L.; Zhao, X.; Qi, G. Microbial synthesis of poly-γ-glutamic acid (γ-PGA) with fulvic acid powder, the waste from yeast molasses fermentation. Biotechnol. Biofuels 2020, 13, 180. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Q.; Wang, Y.; Li, Y.; Jiang, Z. Efficient production of poly-γ-glutamic acid by Bacillus velezensis via solid-state fermentation and its application. Food Biosci. 2022, 46, 101575. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) glutamic acid: A unique microbial biopolymer with diverse commercial applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Soma, Y.; Tominaga, S.; Tokito, K.; Imado, Y.; Naka, K.; Hanai, T.; Takahashi, M.; Izumi, Y.; Bamba, T. Trace impurities in sodium phosphate influences the physiological activity of Escherichia coli in M9 minimal medium. Sci. Rep. 2023, 13, 17396. [Google Scholar] [CrossRef]
- Wang, D.; Hwang, J.-S.; Kim, D.-H.; Lee, S.; Kim, D.-H.; Joe, M.-H. A newly isolated Bacillus siamensis SB1001 for mass production of poly-γ-glutamic acid. Process Biochem. 2020, 92, 164–173. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Q.; Zhou, G.; Wang, L.; Chen, A.; Xie, X.; Huang, X.; Hu, W. Improved production of poly-γ-glutamic acid with low molecular weight under high ferric ion concentration stress in Bacillus licheniformis ATCC 9945a. Process Biochem. 2017, 56, 30–36. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Liu, S.; Li, H.; Ling, P.; Zhu, X. Poly-γ-glutamate from Bacillus subtilis inhibits tyrosinase activity and melanogenesis. Appl. Microbiol. Biotechnol. 2013, 97, 9801–9809. [Google Scholar] [CrossRef]
- Wang, L.M.; Chen, S.B.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Völker, F.; Hoffmann, K.; Halmschlag, B.; Maaß, S.; Büchs, J.; Blank, L.M. Citrate Supplementation Modulates Medium Viscosity and Poly-γ-Glutamic Acid Synthesis by Engineered B. subtilis 168. Eng. Life Sci. 2025, 25, e70009. [Google Scholar] [CrossRef]
- Shih, I.-L.; Wu, P.-J.; Shieh, C.-J. Microbial production of a poly(γ-glutamic acid) derivative by Bacillus subtilis. Process Biochem. 2005, 40, 2827–2832. [Google Scholar] [CrossRef]
- Ashiuchi, M.; Shimanouchi, K.; Nakamura, H.; Kamei, T.; Soda, K.; Park, C.; Sung, M.-H.; Misono, H. Enzymatic synthesis of high-molecular-mass poly-gamma-glutamate and regulation of its stereochemistry. Appl. Environ. Microbiol. 2004, 70, 4249–4255. [Google Scholar] [CrossRef]
- Quach, N.T.; Vu, T.H.N.; Nguyen, T.T.A.; Ha, H.; Ho, P.H.; Chu-Ky, S.; Nguyen, L.-H.; Van Nguyen, H.; Thanh, T.T.T.; Nguyen, N.A.; et al. Structural and genetic insights into a poly-γ-glutamic acid with in vitro antioxidant activity of Bacillus velezensis VCN56. World J. Microbiol. Biotechnol. 2022, 38, 173. [Google Scholar] [CrossRef]
- Sirisansaneeyakul, S.; Cao, M.; Kongklom, N.; Chuensangjun, C.; Shi, Z.; Chisti, Y. Microbial production of poly-γ-glutamic acid. World J. Microbiol. Biotechnol. 2017, 33, 173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Asada, Y.; Aida, T. Production of γ-Polyglutamic Acid by Bacillus licheniformis A35 under Denitrifying Conditions. Agric. Biol. Chem. 1989, 9, 53. [Google Scholar] [CrossRef]
- Ito, Y.; Tanaka, T.; Ohmachi, T.; Asada, Y. Glutamic Acid Independent Production of Poly(γ-glutamic acid) by Bacillus subtilis TAM-4, Bioscience. Biotechnol. Biochem. 1996, 8, 60. [Google Scholar] [CrossRef]
- Gao, W.; He, Y.; Zhang, F.; Zhao, F.; Huang, C.; Zhang, Y.; Zhao, Q.; Wang, S.; Yang, C. Metabolic engineering of Bacillus amyloliquefaciens LL3 for enhanced poly-γ-glutamic acid synthesis. Microb. Biotechnol. 2019, 5, 19. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, G.; Guo, Y.; Zhang, B.; Dong, M.; Wu, Y.; Wang, J.; Che, Z.; Liang, Z. Production of poly-γ-glutamic acid by a thermotolerant glutamate-independent strain and comparative analysis of the glutamate dependent difference. AMB Expr. 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Zhu, X.; Cai, J.; Zhang, A.; Hong, Y.; Huang, J.; Huang, L.; Xu, Z. High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresour. Technol. 2012, 116, 241–246. [Google Scholar] [CrossRef]
- Kongklom, N.; Luo, H.; Shi, Z.; Pechyen, C.; Chisti, Y.; Sirisansaneeyakul, S. Production of poly-γ-glutamic acid by glutamic acid-independent Bacillus licheniformis TISTR 1010 using different feeding strategies. Biochem. Eng. J. 2015, 100, 67–75. [Google Scholar] [CrossRef]


| Handling Time | 0 min | 10 min | 20 min | 30 min | 40 min |
|---|---|---|---|---|---|
| [η] | 24.57 | 17.95 | 9.82 | 7.51 | 2.75 |
| MW (kDa) | 1390 | 1060 | 630 | 500 | 210 |
| Molecular Weight (×106 kDa) | 1.39 | 1.06 | 0.63 | 0.50 | 0.21 |
|---|---|---|---|---|---|
| O2− Scavenging Rate (%) | 42.57 | 26.38 | 24.08 | 16.08 | 12.86 |
| Tyrosinase Inhibition Rate (%) | 89.86 | 68.46 | 52.94 | 30.97 | 4.50 |
| Isolate | Carbon Source (g·L−1) | Methods and Key Conditions | γ-PGA Production (g·L−1) | Conversion Efficiency (%) a | Productivity (g·L−1 h−1) | References |
|---|---|---|---|---|---|---|
| B. licheniformis A35 | Glucose (75) | 30 °C, 100 h | 8.50 | 16.00 | 11.33 | [33] |
| Bacillus subtilis TAM-4 | Glucose (75) | 30 °C, 96 h | 22.10 | 29.47 | 0.23 | [34] |
| B. amyloliquefaciens LL3 | Sucrose (50) | 37 °C, 44 h | 4.40 | 8.80 | 0.10 | [35] |
| B. subtilis GXG-5 | Glucose (25) | 50 °C, 34 h | 19.50 | 78.00 | 0.57 | [36] |
| Bacillus subtilis C10 | Glucose (80) and citric acid (20) | 32 °C, 32 h | 27.70 | 27.70 | 0.79 | [37] |
| B. licheniformis TISTR 1010 | Glucose (20) and citric acid (30) | 37 °C, 95 h | 27.50 | 55.00 | 0.29 | [38] |
| B. subtilis C1 | Glycerol (170) and citric acid (22) | 37 °C, 6 days | 21.40 | 0.11 | 0.15 | [29] |
| Bacillus velezensis SDU | Glycerol (25) and sodium citrate (3.4) | 30 °C, 30 h | 23.01 | 81.00 | 0.77 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Wang, H.; Jia, H.; Ni, H.; Xu, S.; Zhang, C.; Zhang, Y.; Wu, Y.; Tu, Q. Production and Characterization of Poly-γ-Glutamic Acid by Bacillus velezensis SDU. Microorganisms 2025, 13, 917. https://doi.org/10.3390/microorganisms13040917
Guo G, Wang H, Jia H, Ni H, Xu S, Zhang C, Zhang Y, Wu Y, Tu Q. Production and Characterization of Poly-γ-Glutamic Acid by Bacillus velezensis SDU. Microorganisms. 2025; 13(4):917. https://doi.org/10.3390/microorganisms13040917
Chicago/Turabian StyleGuo, Guangyao, Han Wang, Huiyuan Jia, Haiping Ni, Shouying Xu, Cuiying Zhang, Youming Zhang, Yuxia Wu, and Qiang Tu. 2025. "Production and Characterization of Poly-γ-Glutamic Acid by Bacillus velezensis SDU" Microorganisms 13, no. 4: 917. https://doi.org/10.3390/microorganisms13040917
APA StyleGuo, G., Wang, H., Jia, H., Ni, H., Xu, S., Zhang, C., Zhang, Y., Wu, Y., & Tu, Q. (2025). Production and Characterization of Poly-γ-Glutamic Acid by Bacillus velezensis SDU. Microorganisms, 13(4), 917. https://doi.org/10.3390/microorganisms13040917








