Abstract
The skin cannot be considered as just a barrier that protects against physical, chemical, and biological damage; it is a complex and dynamic ecosystem that varies across lifespans. Interest in the relationship between physical activity and skin microbiota has grown significantly in recent years. The skin microbiota has a crucial role in skin functions and physiology, and an imbalance, known as dysbiosis, is correlated with several diseases, such as inflammatory bowel disease (IBD), infectious disease, obesity, allergic disorders, and type 1 diabetes mellitus. Among the causes of dysbiosis, the practice of physical exercise, especially in contact sports, including wrestling, artistic gymnastics, and boating, certainly represents a predisposing factor for infectious disease. This review aims to provide an overview of the skin microbiota and its regulation, focusing on interactions between physical exercise and skin microbiota, the antimicrobial peptides (AMPs) as regulators of skin microbiota, and the impact of probiotics supplementation on physical performance.
1. Introduction
The skin has long been considered a barrier for protective action against physical, chemical, and biological damage. The spread of the research in metagenomics has revealed that almost all human body niches host microbes, including the skin. Thus, being colonized by bacteria, fungi, and viruses, skin cannot be considered to be just a wall; it is a complex ecosystem whose composition depends on mutual adaptation with the host and can be influenced by environmental and nutritional stimuli [1]. As for other body sites, the skin microbiota is crucial to ensuring skin physiological functions because dysbiotic status has been described in several diseases. Characterizing the skin microbiome may be useful in these conditions and may clarify the complex interplay between the human host and its microbes (Figure 1).
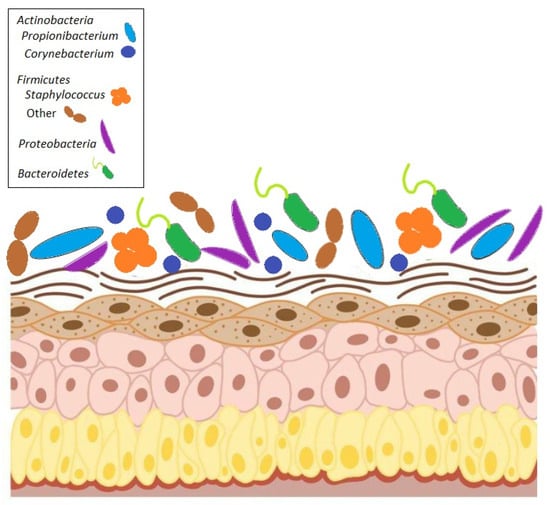
Figure 1.
Composition of human skin microbiome. The microbiome consists of four major phyla: Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria.
The main bacterial phyla that make up the skin microbiome fall into four different phyla: Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria. Distributions of bacteria change depending on the skin site’s physiology, with specific bacteria being associated with moist, dry, and sebaceous environmental conditions. In general, bacterial diversity seems to be lowest in sebaceous sites, suggesting that there is selection for specific subsets of organisms that can tolerate conditions in these areas. Propionibacterium spp. is the dominant organism in these and other sebaceous areas, which confirms that Propionibacterium spp. is a lipophilic resident of the pilosebaceous unit [2]. Staphylococcus and Corynebacterium spp. are the most abundant organisms colonizing moist areas, suggesting that these organisms prefer areas of high humidity [2,3]. Staphylococci occupy an aerobic niche on the skin and probably use the urea in sweat as a nitrogen source. Corynebacteria are highly fastidious and slow-growing organisms in culture, and, as such, their role as skin microorganisms has been underappreciated until recently. The most diverse skin sites are the dry areas, with mixed representations from the phyla Actinobacteria [2,3,4]. These sites include the forearm, buttock, and various parts of the hand.
However, it must be underlined that the skin microbiota is extremely variable and can be influenced by several factors, such as ethnicity, gender, delivery mode, age, occupation, diet, hygiene habits, skin product usage, medication usage, climate, pollution, geographical location, and UV exposure [5].
Despite these variables, across our lifespan, skin colonization is driven by nutrient availability and environmental conditions. Indeed, based on body features, the skin takes on four different environments, i.e., sebaceous, moist, dry, and foot, that drive specific microbial colonization in such different skin niches [6]. In particular, in the sebaceous sites (face, chest, and back), the production of lipid-rich sebum induces the colonization of lipophilic taxa, such as Cutibacterium and Malassezia; moist sites (groin, elbow crease, and axilla) are characterized by the presence of sweat glands that promote Staphylococcus spp. and Corynebacterium spp. colonization; and dry sites (abdomen, palms, and forearm) feature a great variability in taxa composition but a low microbial abundance [7] (Table 1).
The skin microbiome composition varies across different body sites due to differences in regional skin anatomy. Some skin regions, such as the groin, axillary vault, and web, are partially occluded. These regions are higher in temperature and humidity, encouraging the growth of microorganisms that thrive in moist conditions (for example, Gram-negative bacilli, Coryneforms, and Staphylococcus aureus). The density of sebaceous glands is another factor that influences the skin microbiota, depending on the region. Areas with a high density of sebaceous glands, such as the face, chest, and back, encourage the growth of lipophilic microorganisms (for example, Propionibacterium spp. and Malassezia spp.) [8]. Compared with other skin sites, arm and leg skin is relatively desiccated and experiences large fluctuations in surface temperature. These areas are found to harbor quantitatively fewer organisms than moist areas of the skin surface [9].

Table 1.
Microbial composition of each area of skin (data pooled from Grice et al. [10]).
Table 1.
Microbial composition of each area of skin (data pooled from Grice et al. [10]).
| Skin sites and Physiology | Alpha Diversity | Beta Diversity | Microbial Composition |
|---|---|---|---|
| Dry (hypothenar palm, volar forearm) | High | High interpersonal variation | Actinobacteria (Propionibacterium 13% and Corynebacterium 15%) Firmicutes, Proteobacteria (41%) and Bacteroidetes (14%) |
| Moist (Nare, antecubital fossa, inguinal crease, popliteal fossa) | Low | Low | Colonized predominantly by Firmicutes like Staphylococcus (21%), Corynebacterium spp. (28%), and Proteobacteria (26%) |
| Sebaceous (cheek, glabella, external auditory canal, occiput, back) | Lower | Lower | Colonized predominantly by Propionibacterium spp. (46%) and Staphylococcus (16%) |
While it is true that microbial colonization depends on the physiological properties of the skin in different body sites, it must be noted that the skin’s physiology varies over time according to individual maturation and development. This means that the skin microbiota must be considered as a dynamic community that varies across lifespans.
From a functional perspective, as mentioned above, the mutual relationship between the skin microbiota and the human host is required for human health [11].
Indeed, the skin microbiota has been adapted to metabolize several host molecules, including proteins, carbohydrates, and sebum lipids, and produces bioactive compounds, such as antimicrobial peptides, phenol-soluble modulins, and antibiotics [5]. These metabolic activities are required for skin microbiota functions: immunity and inflammation regulation, protection against pathogens colonization, immune system shaping and adaptation during early life, maintenance of skin integrity and functions, UV protection, wound healing, and odor production.
For instance, the skin microbiota can produce short-chain fatty acids (SCFAs) through the metabolic processes of commensal bacteria like C. acnes and S. epidermidis. These SCFAs influence the production of cytokines and T cell activation, regulate the immune system, and have anti-inflammatory properties that maintain the integrity of the skin barrier [5]. Additionally, the pH and hydration level of the skin are influenced by these acid metabolites in conjunction with other microbial metabolites, like lactic acid and other organic acids [12]. Skin pH is required for several important skin functions, including barrier permeability, desquamation, and antimicrobial activities; thus, a microbial alteration may affect them.
A given person’s skin contains microorganisms that most likely come from a variety of places, such as inanimate objects, people, pets, cosmetics, air, and water [13,14,15,16]. Our current understanding of the relative contributions from these potential sources is only beginning. Human–surface and human-human contacts have long been acknowledged in medical literature as potent vectors of microbial dispersal [17,18]. Shaking hands, as well as hand contact with other body parts and room surfaces, has been found in culture-based studies to be potent vectors of health service infections, including Klebsiella spp. and methicillin-resistant S. aureus (MRSA) [19,20]. Since human contact with surfaces—specifically, other people’s skin surfaces—has been demonstrated to transmit specific microbial taxa, it is conceivable that human-to-human interactions result in the sharing of skin microbial communities.
The health of athletes and the impact of professional factors on it is currently receiving a lot of attention worldwide. However, practically all athletes experience skin issues at some point in their athletic careers [21], particularly concerning infectious skin diseases [22]. Purulent inflammatory skin diseases are at the top of the general hierarchy of infectious pathology in athletes [23]. Athletes’ ability to perform at a high level can be negatively impacted by the development of pathological conditions that arise from cases of infectious skin diseases caused by microbial transmission during training or competition [24]. Fungal infections, such as ringworm; viral infections, such as herpes gladiatorum in wrestlers, which is caused by HSV-1; and bacterial infections, such as impetigo, caused by Staphylococci or Streptococci, including methicillin-resistant S. aureus (MRSA), are among the most common infections seen in athletes [25,26].
2. Interactions Between Physical Exercise and Skin Microbiota
Athletes involved in contact sports, such as judo, rugby, and wrestling, belong to the population most vulnerable to skin infections caused by bacteria, viruses, or fungi [27,28,29].
There are several mechanisms by which infectious agents may be spread among athletes. Direct contact transmission involves person-to-person contact in which infectious agents are physically transferred from one person to a susceptible host. Indirect contact transmission occurs when a susceptible host meets contaminated objects or fomites, such as equipment, towels, or clothing. A type of indirect contact is droplet transmission, which occurs when droplets containing infectious agents are generated through coughing, sneezing, or talking and are deposited on the host’s conjunctivae, nasal mucosa, or mouth after being propelled in the air a short distance [30].
In contact sports, athletes frequently suffer from skin diseases [27,31]. For instance, wrestlers are at higher risk of developing dermatophytosis (Tinea corporis, Tinea pedis), impetigo, and HSV infection [32,33]. Shifts in the skin microbiome can accompany this high susceptibility rate because these changes in the bacterial community may cause a predisposition to infection. Intense skin-to-skin contact creates perfect conditions for the transmission of infectious agents [34].
These infections, which occur because of the dysbiosis of the skin microbiota, can be transmitted from one athlete to another directly through contact or indirectly through contaminated objects, such as towels, mats, and equipment.
Sports equipment and the environment in which physical activity takes place, not just direct contact between athletes, can influence and alter the health of the skin microbiota. This can increase athletes’ susceptibility to skin infections [35].
The predominant genera identified on surfaces, such as benches, elliptical handles, floors, free weights, and mats, include Pseudomonas and Acinetobacter, followed by Staphylococcus, Corynebacterium, and Micrococcus [36]. Each surface within a facility where physical activity occurs harbors a distinct microbial community, and the surfaces that come in contact frequently with athletes’ skin exhibit more dynamic and diverse microbial communities characterized by a non-random distribution. Notably, the microbial diversity presents in gyms and on sports equipment is significantly shaped by the microbiomes of the athletes rather than being influenced by the geographical location of the facility [37]. For example, gym mats, which are frequently in contact with athletes’ hands and feet, are a source/reservoir of opportunistic pathogenic microorganisms, such as Staphylococcus, Corynebacterium, and Enhydrobacter [38].
Symptoms resulting from the infections commonly include lesions, blisters, or sores, leading to abstention from training and competition. For this reason, it is important to protect skin health and, consequently, the skin microbiota, as this can impact athletic recovery and performance. Additionally, it has been observed that maintaining healthy skin enhances athletes’ confidence, demonstrating a positive impact on physical and mental health [39].
Bacterial infections affect athletes through direct contact. However, some authors highlight the roles of mats and equipment in spreading infections [40]. The treatment of deep bacterial infections may be challenging due to much higher skin colonization rates with methicillin-resistant S. aureus among athletes [41]. Common viral infections that afflict athletes include those caused by the Herpes simplex virus (HSV), Human papillomavirus (HPV), and Molluscum contagiosum virus (MCV). HSV can occur in any location, especially in contact sports. HSV infection can be easily contracted, resulting in massive outbreaks during athletic training camps [42,43]. Herpetic keratitis (involvement of the cornea) can lead to scarring, and repeated lesions can lead to permanent corneal opacity, requiring a corneal transplant to maintain good vision. Retinal necrosis leading to blindness may also occur. Retinal necrosis due to HSV infection is the most common cause of blindness of contagious origin in the USA [44,45,46].
Athletes are at high risk of fungal infections caused by dermatophytes due to increased exposure to pathogens (e.g., swimmers through contact with water, wrestlers through using mats) and repeated exposure to mechanical factors (e.g., micro-injuries of the skin of runners’ feet). This infection has been widely reported in wrestlers and is often referred to as Tinea gladiatorum, Trichophytosis gladiatorum, and Tinea corporis gladiatorum [32,47,48,49]. T. capitis is less common in athletes. This condition mostly affects people who practice sports that entail close contact among the athletes and those using protective equipment, e.g., jockeys and hockey players [50,51,52]. Tinea versicolor, a condition caused by a fungus (Malassezia furfur), is another relatively frequent superficial skin infection [53]. Its occurrence is associated with individual predisposition, excessive sweating, humidity, and closefitting clothing.
Exposure to different sports environments revealed that skin microbiome composition was enriched with methicillin-susceptible S. aureus (MSSA) and contained methicillin-resistant S. aureus (MRSA) (football locker room and weight room). The fingertip location of S. aureus recovery from football players was significant when compared with both other locations in football players, fingertips in wrestlers, and the control group. All S. aureus isolates recovered from athletes in our study were resistant to clindamycin, which is often the drug of choice for soft tissue infections. Trimethoprim/sulfamethoxazole is another drug of choice for soft tissue infections, but half of the isolates we recovered were resistant to it. If the bacteria are resistant to the administered antibiotic, the result may be septicemia; therefore, the clinician should be careful to select an antibiotic to which the infection is susceptible [54,55,56,57].
Pathogenic bacteria are the most responsible for skin infections and soft tissue infections (SSTIs) in the athletic population and include S. aureus. S. aureus can cause several types of infections, especially in athletes involved in sports like judo and wrestling [58,59]. These bacterial infections, from superficial skin infections to more serious invasive infections, usually affect the nostrils, upper airways, digestive tract, skin, and genital mucosa. Recently, S. aureus infections have demonstrated resistance to methicillin or similar penicillin antibiotics, which make treatment and a cure difficult. These strains are referred to as MRSA. In addition to bacteria, viruses are also responsible for skin infections. Viral infections can occur in athletes, and in particular, verruca (warts), molluscum contagiosum, and herpes simplex are the most widespread. Swimmers could be particularly susceptible to the plantar verruca, and in this case, the only ways to prevent the spread of the wart are to treat it with liquid nitrogen and topical keratolytic/salicylic acid preparations and for the athletes to use sandals when using shared showers [60]. Molluscum contagiosum is characterized by discrete papules and can be found particularly in wrestlers. M. contagiosum is highly contagious through direct skin contact, so to avoid its spread among athletes, it must be treated promptly with destructive methods like liquid nitrogen [60]. HSV types I and 2 are common infectious agents in humans. The term Herpes gladiatorum (HG) became widely spread in 1989 when 60 wrestlers contracted the virus. It has been reported that 94% to 97% of HSV infections in wrestlers are caused by type 1 [61]. The timely identification and treatment of affected individuals and their exclusion from the activity to avoid contact with other wrestlers can avoid its spread.
Finally, fungal organisms can also significantly affect athletes. For example, tinea pedis can affect many athletes because its growth is favored by warm and humid environments. For this reason, runners, skaters, and long-distance walkers may be particularly at risk. Affected athletes should be treated with antifungal agents and wear shoes in shared spaces to reduce transmission [60].
Several studies have evaluated the correlation between physical exercise and the skin microbiome. A study conducted on 15 wrestlers, comparing the composition of the skin microbiome before and after training, demonstrated that the main cause of its alterations was not due to the sharing of training mats but to the transmission of microorganisms following physical contact between athletes [62]. A study by Meadow on flat-track roller derby players showed that their own skin microbial community composition characterized each team membership. This aspect could be because each team was from a different geographic area associated with a different climate, urban setting, outdoor microbiota, and also a very different environmental microbiota [31]. The most interesting aspect was that, following the competition, the players’ microbial communities became more like each other.
Physical exercise has been demonstrated to modulate immune function, thereby strengthening the body’s defenses against infections interacting with skin microbiota. By modulating the immune system and decreasing levels of pro-inflammatory cytokines, exercise has been observed to alleviate symptoms such as skin thickness and redness, which are common in inflammatory skin diseases.
While physical activity confers numerous health benefits, it also presents certain skin types, particularly in terms of increased susceptibility to infections [63].
The molecular mechanisms by which physical activity influences skin microbiota include improved blood flow to the skin, promotion of skin hydration, positive effects on the hormonal system, increased collagen production in the dermal layer, and positive influences on the mechanical properties of the skin [64]. Physical activity stimulates the release of interleukin-15, which prevents mitochondrial dysfunction and promotes mitochondrial biosynthesis. This, in turn, may correlate with better skin hydration in physically active individuals [65,66,67,68,69]. Proper skin hydration improves its natural barrier function, facilitating protection against internal and external irritants, thereby preventing the development of common skin conditions, such as atopic dermatitis, contact dermatitis, psoriasis, and acne [65,70,71].
The Centers for Disease Control and Prevention [72], the Infectious Diseases Society of America [73], and some athletic associations, such as the National Athletics Trainers Association [74] and National Collegiate Athletic Association [75], have all developed guidelines to avoid bacterial and fungal infections in locker rooms, private and school gyms, and fitness centers. The presence of open wounds, abrasions, or lacerations should be a reason for the athlete’s exclusion from common pools, and shared spaces should be sanitized after each use. Some studies also revealed that, in individuals with S. aureus SSTIs, the addition of chlorhexidine or immersion to normal routine hygiene measures in a bathtub containing a diluted solution of household bleach bubble bath can represent a valid tool for fighting microbial colonization and prevention. Adequate cleanliness and hygiene are a crucial point in this process; in fact, long pauses in competitive and training activities caused by infections can lead to a decline in athletic performance. For this, athletic trainers and sports doctors have an important role in educating athletes about preventing these types of infections [76].
3. Regulators of Skin Microbiota: Antimicrobial Peptides (AMPs)
The skin microbiome is an ecosystem consisting of multiple microbial species essential for the maintenance of skin physiology and immunity, with a crucial role in regulating its homeostasis. Dysbiosis of the microbiota can cause skin inflammation and the subsequent development of skin diseases. Therefore, there are specific mechanisms that control and shape the microbiota, enabling a proper balance of its composition [77]. An important regulatory role in the composition of the microbiome is played by host antimicrobial peptides (AMPs). Small peptides known as AMPs are widely expressed on the skin. They originate in the deeper layers of the epidermis and are then transported to the stratum corneum, where they function as multifunctional effector molecules that connect innate and adaptive immune responses, as well as serve as the body’s first line of defense against possible pathogens [78].
AMPs have a crucial role in regulating the skin microbiota. HBD-2 exhibits high activity against Gram-negative bacteria, such as P. aeruginosa and E. coli, as well as against the yeast C. albicans [79,80]. Another beta-defensin, hBD-3, has a very broad range of activity, and it acts in low doses and remains effective, even in high salt concentrations [81]. Also, the inducible AMP called CAMP (cathelicidin antimicrobial peptide) encodes for an 18 kDa precursor, which is proteolytically processed to a 37 amino acid-containing peptide, termed LL-37. LL-37 is the only human cathelicidin, and it is also expressed in the skin [82]. LL-37 shows broad antimicrobial activity against Gram-positive and Gram-negative bacteria and fungi, including yeasts [83,84], in contrast to hBD-2, hBD-3, and LL-37, which are only weakly expressed in healthy skin. Gläser et al. identified the S100 protein psoriasin (S100 A7) as an abundant AMP of healthy skin [85]. Psoriasin exhibits antimicrobial activity, especially against E. coli, and its reduced form has been reported to be active against various fungi [86]. Harder et al. isolated a new AMP from the stratum corneum of healthy skin with high antimicrobial activity against a wide range of microorganisms [87]. Because of its structural similarity, this AMP was assigned to the ribonuclease A superfamily and is referred to as ribonuclease 7 (RNase7). RNase 7 is a cationic, lysine-rich 14.5 kDa protein with a broad spectrum of antimicrobial activities and very potent ribonuclease activity. RNase 7 is abundant in human skin, and pro-inflammatory cytokines and bacteria can further induce its expression [87,88,89,90,91]. The inactivation of psoriasin on the skin surface enhances the growth of applied E. coli [85]. The antibody-based inactivation of RNase7 reveals its crucial role in controlling the cutaneous growths of S. aureus, Corynebacterium amycolatum, E. faecium, and P. aeruginosa. The antibody-based neutralization of hBD-3 reveals the important role of hBD-3 in restricting S. aureus growth [90,92,93,94].
Also, AMPs increase the production of chemokines and cytokines, draw immune cells to the infection site, alter the responses of Toll-like receptors, and bind and deactivate bacterial endotoxins, all of which promote wound healing and angiogenesis [95,96]. In addition to directly eliminating pathogens, AMPs regulate immune responses and interfere in cell differentiation, re-epithelialization, and their cooperative interactions with the skin microbiota.
The key regulatory mechanisms through which AMPs modulate skin immunity and prevent infections include: (1) protection from microbial infection (broad spectrum of antimicrobial activity against bacteria, yeast, fungi, protozoa, and viruses); (2) improvement of skin barrier homeostasis (regulation of the normal skin microflora composition); (3) modulation of inflammation responses (controlling the production of various cytokines/chemokines), and (4) promotion of wound healing. AMPs also initiate a potent host response to skin infection, resulting in cytokine/chemokine production, inflammation, and a cellular response [97,98]. After exposure to microbe-derived molecules, monocytes and lymphocytes stimulate the epidermal expression of hBDs [99]. The hBD-2, hBD-3, and hBD- 4, but not hBD-1, stimulate keratinocytes to produce proinflammatory cytokines and chemokines through the G protein-coupled receptor (GPCR) and phospholipase C (PLC) signaling pathways [100]. Furthermore, hBDs induce keratinocyte migration and proliferation, which involves EGFR, a signal transducer and activator of transcription (STAT)1 and STAT3 activation. Cathelicidin, LL-37, also synergizes with endogenous inflammatory mediators to enhance the induction of specific inflammatory effects through a complex mechanism involving multiple pathways, such as GPCR, EGFR, and TLR [101,102]. As a result, cathelicidin peptides increase cell migration and the secretion of cytokines (IL-6, IL-8, IL-10, IL-18, and IP-10) and chemokines (MCP-1, MIP3α, and RANTES) from activated cells. Also, dermcidin-1L (DCD-1L) stimulates keratinocytes to generate cytokines (TNF- α, IL-8, and IP-10) and chemokines (MIP3α) through both G protein and p38/MAPK pathways [103].
All of these factors are critical for preserving an ideal and functional skin barrier [104,105]. The antimicrobial capacity of AMPs, together with their epithelial expression, makes their roles as regulators and shapers of the microbiota likely, and the main classes are reported in Table 2. The most widely characterized families of AMPs in the skin are defensins and cathelicidins. Defensins are small cationic peptides; they have β-sheet structures with cysteine-rich residues that form characteristic disulfide bridges [106]. The main defensins are α- and β-defensins, according to the position of the disulfide bridges.

Table 2.
The main AMPs of the skin and their major targets.
Human α-defensins (HNPs) are mainly produced in neutrophils. In particular, there is a high expression of HNP1-3 and more moderate activity of HNP4. Among them, HNP2 has important bactericidal activity against S. aureus, compared to HNP1, 3, and 4 [107].
Human β-defensins (hBDs) are released by epithelial cells, such as keratinocytes, activated monocytes/macrophages, and dendritic cells [107]. Among β-defensins, hBD-2 has bacteriostatic activity against Gram-negative bacteria, such as P. aeruginosa and E. coli, as well as against the yeast C. albicans, while hBD-3 is very potent against C. albicans, E. coli, S. pyogenes, P. aeruginosa, and E. faecium. In turn, hBD-2 and hBD-3 are induced by proinflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor (TNF-α)/interferon-γ (IFN-γ), but also by microbial, injury, and UV-B stimuli [101]. Cathelicidins, also called LL-37, are small, cationic, amphipathic peptides consisting of 12 to 80 amino acids; LL-37 is the only human cathelicidin also expressed on the skin and shows broad antimicrobial activity against Gram-positive and Gram-negative bacteria [78]. The skin defense barrier also includes other less common antimicrobial peptides found in skin cells, such as psoriasin, RNase7, and dermcidin.
When applied at lower concentrations, psoriasin, found in the keratinocytes of psoriasis patients, is an extremely effective AMP against E. coli and is active against S. aureus at higher concentrations [108]. It is upregulated in psoriasis and chronic wounds, and pro-inflammatory cytokines can induce its expression in keratinocytes, and it is a potent modulator of neutrophil activation [109]. RNase7, produced in keratinocytes, is involved in protecting the skin from infection caused by S. aureus and has antimicrobial activities against E. coli, P. aeruginosa, E. faecium, and P. acnes [52]. Finally, dermcidin is secreted by sweat glands and has antimicrobial activities against S. aureus, E. coli, and C. albicans [109].
Despite such evidence, the impact of skin-derived AMPs on the microbiota is yet to be defined, but there is growing evidence that AMPs can modulate and balance the microbiota. This hypothesis is based on the direct antimicrobial activity of AMPs against microbiota members and microbiota-regulated AMP expressions in the skin. Members of the skin microbiota and their secreted products detected by host keratinocytes and immune cells induce the upregulation of genes involved in the immune system and inflammatory response, including several AMPs, indicating that the microbiota contributes to providing the skin with constant AMP-mediated antimicrobial action. One study showed that the stimulation of keratinocytes with S. epidermidis or its secreted factors induces the expression of many AMPs, such as ß-defensins hBD-2 and hBD-3 or RNase7. S. epidermidis stimulation of nasal keratinocytes also increases the expressions of hBD-3, RNase 7, and LL-37. Interestingly, the inductions of hBD-3 and RNase 7 by S. epidermidis highlight a possible mechanism of how skin commensals, such as S. epidermidis, amplify the innate immune response in the presence of infection [110,111,112,113,114]. The Gram-negative mucosal bacterium R. mucosa is a member of the healthy skin microbiota, and its absence in atopic dermatitis (AD), a skin infection caused by S. aureus, can trigger the disease. Skin treatment of AD patients treated with R. mucosa reduces disease severity and the S. aureus burden. Interestingly, R. mucosa induces the expressions of hBD-2 and LL-37 in keratinocytes, suggesting that AMP induction could be used to treat AD patients [115,116]. The above studies document that members of the skin microbiota also induce the expressions of various AMPs. This points to the presence of a feedback loop that regulates the precise AMPs–microbiota relationship and homeostasis: the microbiota induces AMP expression, which leads to the better control of AMP-mediated growth of microbiota members and results in a decrease in microbiota abundance, followed by reduced AMP induction [109]. The skin microbiota could represent an interesting opportunity to develop new therapies to improve skin health and treat skin infections. It has been observed that skin diseases are associated with a reduced diversity of the microbiota with specific microorganisms; using a cocktail of different strains as therapy would better suit the personal pathological situation of the recipients, offering therapeutic advantages [109].
The applied microbiota can be: (1) alive (probiotics): a probiotic is a living microorganism that, when added in sufficient amounts, exerts a beneficial effect on the host [117]. (2) Tyndallized or thermos-killed bacteria (postbiotics): bacterial cell structures, enzymes and excreted bacterial factors are added, but the bacteria do not replicate anymore. (3) Cell lysates or physically killed bacteria (postbiotics): the bacteria are destroyed, and the cell contents and cell walls are in the solution. The bacteria do not replicate anymore, but the enzymes can still be active. (4) Purified enzymes: single or groups of bacterial enzymes are purified and added. (5) Fermentation products or supernatants: the bacteria are not added, but the supernatants containing their antioxidants, amino acids, lipids, and/or vitamins are added. Methods 1–5 have multiple advantages over a skin microbiome transplant, with the main advantage being that the process is easily scalable and thus industrially applicable. For method 1 (application of live probiotics), highly concentrated bacteria can be applied; thus, a higher efficacy can be obtained, compared to a complete skin microbiome transplant. Pro- or postbiotics can be applied in a skin emollient, cream, or suitable medium for skin. There are also a series of drawbacks associated with the use of pro- and postbiotics. Bacteria are cultured in sugar-rich media; it can therefore be more difficult for the bacteria to adjust to a sebum-rich environment. Skin engraftment is not easy; the applied bacteria compete with the skin resident microbiome of the deeper skin layers. The application of high amounts of bacteria could lead to a skin immune reaction, with irritation and side effects as a result. A third method of changing the skin microbiome is through prebiotic stimulation. In this process, prebiotics are supplemented to the skin to stimulate the growth of specific health-promoting microbes. A prebiotic is an ingredient with a bioselective activity that exerts a beneficial effect on the host and attempts to improve the host’s health [118]. There are several advantages to this method. There is no need to work with living bacteria; thus, there is a reduced chance of a skin immune reaction. The method has an indirect mechanism of action. Prebiotics are typically well-defined compounds for which side effects are well-studied. The INCI name and safety sheets are normally available. There are also disadvantages. The indirect method has less direct results. Prebiotics could also stimulate non-targeted, low-abundance bacteria. The effect of prebiotics can be unpredictable, given the variability in the skin microbiome, physiology, and immune response in different individuals. All methods have their advantages and disadvantages. Scientific research is currently being conducted using several of these methods to treat common skin disorders.
Paetzold et al., through their studies, have shown the possibility of developing specific probiotic solutions based on the healthy skin microbiota that can convert a diseased microbiota status to a healthier one in the recipient [119]. In addition to the application of skin-derived commensals, different studies have shown how the use of probiotics containing non-skin-derived probiotics, such as lactic acid bacteria, improves skin diseases, such as AD or acne vulgaris [120,121].
4. Conclusions
This review highlights the intricate relationship between physical activity and skin microbiota, outlining the advantages and potential obstacles related to it. The skin hosts an intricate community of microorganisms (the skin microbiome), which is preeminent for maintaining skin health and protecting against pathogens. The skin microbiome’s composition is influenced by host physiology, lifestyle, and environmental factors, such as personal hygiene, physical activity, climate, and geography. Microbes can be moved to the skin through contact with various surfaces, other people, pets, cosmetics, or the environment. Physical activity is effective in maintaining skin mechanics, promoting immune defense, and fostering skin microbiota vitality. Athletes face unique challenges that can change the microbial composition of their skin. These challenges include exposure to various terrains, harsh weather conditions, crowded living spaces, limited access to sanitation, field exercises, and their typical work environments. External factors can disrupt the skin’s microbial balance, leading to skin health issues and increased vulnerability to infections. Athletes frequently encounter skin and soft tissue infections (SSTIs) due to proximity and frequent physical contact.
The impact of skin health not only affects individuals but also places a financial strain on the healthcare system. This highlights the importance of understanding and addressing the factors that influence the skin microbiome, which plays a very important role in preventing infections and maintaining overall health during physical activities. By studying the modulation of the skin microbiome, researchers can develop improved hygiene protocols and skincare strategies that enhance the health and well-being of athletes and others in demanding environments. Additionally, these insights can contribute to better infection control and hygiene practices beyond the realm of athletics.
Author Contributions
Conceptualization, O.S., S.L. and R.P.; data curation, O.S., C.M., M.C., A.G., S.L. and R.P.; writing—original draft preparation, O.S., C.M., M.C., L.G., V.D., B.L., S.L. and R.P.; writing—review and editing, O.S., C.M., M.C., A.G., A.V., L.I., I.V., C.S., L.G., I.L.M., R.D.L., G.F., P.R., V.D., B.L., S.L. and R.P.; visualization, O.S., C.M., M.C., A.G., A.V., L.I., I.V., C.S., P.R., I.L.M., R.D.L., G.F., V.D., B.L., S.L. and R.P.; supervision, O.S., S.L. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tseng, C.H.; Pei, Z.; Blaser, M.J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Q.; Zhong, Q.; Duan, C.; Krutmann, J.; Wang, J.; Xia, J. Skin Microbiome, Metabolome and Skin Phenome, from the Perspectives of Skin as an Ecosystem. Phenomics 2022, 2, 363–382. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef]
- Townsend, E.C.; Kalan, L.R. The dynamic balance of the skin microbiome across the lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Roth, R.R.; James, W.D. Microbial ecology of the skin. Annu. Rev. Microbiol. 1988, 42, 441–464. [Google Scholar] [CrossRef]
- Marples, M. A Seminal and Comprehensive Work of Classical Dermatological Microbiology. In The Ecology of the Human Skin; Charles C Thomas: Bannerstone House, Springfield, IL, USA, 1965. [Google Scholar]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Cui, L.; Jia, Y.; Cheng, Z.W.; Gao, Y.; Zhang, G.L.; Li, J.Y.; He, C.F. Advancements in the maintenance of skin barrier/skin lipid composition and the involvement of metabolic enzymes. J. Cosmet. Dermatol. 2016, 15, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Kembel, S.W.; Jones, E.; Kline, J.; Northcutt, D.; Stenson, J.; Womack, A.M.; Bohannan, B.J.; Brown, G.Z.; Green, J.L. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. Multidiscip. J. Microb. Ecol. 2012, 6, 1469–1479. [Google Scholar] [CrossRef]
- Pessoa-Silva, C.L.; Dharan, S.; Hugonnet, S.; Touveneau, S.; Posfay-Barbe, K.; Pfister, R.; Pittet, D. Dynamics of bacterial hand contamination during routine neonatal care. Infect. Control Hosp. Epidemiol. 2004, 25, 192–197. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Davis, M.F.; Iverson, S.A.; Baron, P.; Vasse, A.; Silbergeld, E.K.; Lautenbach, E.; Morris, D.O. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect. Dis. 2012, 12, 703–716. [Google Scholar] [CrossRef]
- Estes, K.R. Skin infections in high school wrestlers: A nurse practitioner’s guide to diagnosis, treatment, and return to participation. J. Am. Assoc. Nurse Pract. 2015, 27, 4–10. [Google Scholar] [CrossRef]
- Ilgen, D.E.; Metin, E. Characteristics of sports-related dermatoses for different types of sports: A cross-sectional study. J. Dermatol. 2005, 32, 620–625. [Google Scholar]
- Grosset-Janin, X.N.; Saraux, A. Sport and infectious risk: A systematic review of the literature over 20 years. Med. Et. Mal. Infect. 2012, 42, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Cain, G.; Naji, O.; Goff, J. Skin infections in athletes: Treating the patient, protecting the team. J. Fam. Pract. 2013, 62, 284–291. [Google Scholar] [PubMed]
- Ahmadinejad, Z.; Razaghi, A.; Noori, A.; Hashemi, S.; Asghari, R.; Ziaee, V. Prevalence of Fungal Skin Infections in Iranian Wrestlers. Asian J. Sports Med. 2013, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Wells, W.J.; Klein, R.; Sylvester, T.; Sunenshine, R. Outbreak of skin lesions among high school wrestlers-Arizona, 2014. Morb. Mortal. Wkly. Rep. 2015, 64, 559–560. [Google Scholar]
- Wilson, E.K.; deWeber, K.; Berry, J.W.; Wilckens, J.H. Cutaneous Infections in Wrestlers. Sports Health 2013, 5, 423–437. [Google Scholar] [CrossRef]
- Pecci, M.; Comeau, D.; Chawla, V. Skin conditions in the athlete. Am. J. Sports Med. 2009, 37, 406–418. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Laneri, S.; Franco, A.; De Biasi, M.G.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Gragnano, F.; Mazzaccara, C.; et al. Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis Among Athletes. Antibiotics 2020, 9, 332. [Google Scholar] [CrossRef]
- Adams, B.B. Which skin infections are transmitted between athletes? West. J. Med. 2001, 174, 352–353. [Google Scholar] [CrossRef][Green Version]
- Minooee, A.; Wang, J.; Gupta, G.K. Sports: The Infectious Hazards. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Meadow, J.F.; Bateman, A.C.; Herkert, K.M.; O’Connor, T.K.; Green, J.L. Significant changes in the skin microbiome mediated by the sport of roller derby. PeerJ 2013, 1, e53. [Google Scholar] [CrossRef]
- Adams, B.B. Tinea corporis gladiatorum. J. Am. Acad. Dermatol. 2002, 47, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Dienst, W.L., Jr.; Dightman, L.; Dworkin, M.S.; Thompson, R.K.; Howe, W.B. Pinning down skin infections: Diagnosis treatment and prevention in wrestlers. Physician Sports Med. 1997, 25, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, D.; Bagłaj-Oleszczuk, M.; Maj, J. Infectious diseases of the skin in contact sports. Adv. Clin. Exp. Med. 2020, 29, 1491–1495. [Google Scholar] [CrossRef]
- Turecek, S.; Brymer, E.; Rahimi-Golkhandan, S. The relationship between physical activity environment, mental wellbeing, flourishing and thriving: A mixed method study. Psychol. Sport Exerc. 2025, 76, 102769, ISSN: 1469-0292. [Google Scholar] [CrossRef]
- Mukherjee, N.; Dowd, S.E.; Wise, A.; Kedia, S.; Vohra, V.; Banerjee, P. Diversity of bacterial communities of fitness center surfaces in a U.S. metropolitan area. Int. J. Env. Res. Public. Health 2014, 11, 12544–12561. [Google Scholar] [CrossRef]
- Wood, M.; Gibbons, S.M.; Lax, S.; Eshoo-Anton, T.W.; Owens, S.M.; Kennedy, S.; Gilbert, J.A.; Hampton-Marcell, J.T. Athletic equipment microbiota are shaped by interactions with human skin. Microbiome 2015, 3, 25. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, C.; Liang, H.; Zhen, Y.; Zhou, R.; Han, Y.; Liang, Z.Q. A microbiome study reveals the potential relationship between the bacterial diversity of a gymnastics hall and human health. Sci. Rep. 2022, 12, 5663. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Short Communication: Could the Skin Microbiome Affect Sports Recovery and Performance? Preprints 2022, 2022100124. [Google Scholar]
- Young, L.M.; Motz, V.A.; Markey, E.R.; Young, S.C.; Beaschler, R.E. Recommendations for best disinfectant practices to reduce the spread of infection via wrestling mats. J. Athl. Train. 2017, 52, 82–88. [Google Scholar] [CrossRef]
- Champion, A.E.; Goodwin, T.A.; Brolinson, P.G.; Werre, S.R.; Prater, M.R.; Inzana, T.J. Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from healthy university student athletes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 33. [Google Scholar] [CrossRef]
- Belongia, E.A.; Goodman, J.L.; Holland, E.J.; Andres, C.W.; Homann, S.R.; Mahanti, R.L.; Mizener, M.W.; Erice, A.; Osterholm, M.T. An outbreak of herpes gladiatorum at a high-school wrestling camp. N. Eng. J. Med. 1991, 325, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.J.; Bergfeld, W.F. Skin diseases of football and wrestling participants. Cutis 1977, 20, 333–341. [Google Scholar] [PubMed]
- Usatine, R.P.; Tinitigan, R. Nongenital herpes simplex virus. Am. Fam. Physician 2010, 82, 1075–1082. [Google Scholar] [PubMed]
- Jefferis, J.; Perera, R.; Everitt, H.; Van Weert, H.; Rietveld, R.; Glasziou, P.; Rose, P. Acute infective conjunctivitis in primary care: Who needs antibiotics? An individual patient data meta-analysis. Br. J. Gen. Pract. 2011, 61, e542–e548. [Google Scholar] [CrossRef]
- Johnson, R. Herpes gladiatorum and other skin diseases. Clin. Sports Med. 2004, 23, 473–484. [Google Scholar] [CrossRef]
- Beller, M.; Gessner, B.D. An outbreak of tinea corporis gladiatorum on a high school wrestling team. J. Am. Acad. Dermatol. 1994, 31 Pt 1, 197–201. [Google Scholar] [CrossRef]
- Ilkit, M.; Ali Saracli, M.; Kurdak, H.; Turac-Bicer, A.; Yuksel, T.; Karakas, M.; Schuenemann, E.; Abdel-Rahman, S.M. Clonal outbreak of Trichophyton tonsurans tinea capitis gladiatorum among wrestlers in Adana, Turkey. Med. Mycol. 2009, 48, 480–485. [Google Scholar] [CrossRef]
- Kaushik, N.; Pujalte, G.G.; Reese, S.T. Superficial fungal infections. Prim. Care 2015, 42, 501–516. [Google Scholar] [CrossRef]
- John, A.M.; Schwartz, R.A.; Janniger, C.K. The kerion: An angry tinea capitis. Int. J. Dermatol. 2018, 57, 3–9. [Google Scholar] [CrossRef]
- Hay, R.J. Tinea capitis: Current status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef]
- Auchus, I.C.; Ward, K.M.; Brodell, R.T.; Brents, M.J.; Jackson, J.D. Tinea capitis in adults. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- Renati, S.; Cukras, A.; Bigby, M. Pityriasis versicolor. BMJ 2015, 350, h1394. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.V.; Treston, J.; O’Sullivan, A.L. Hand-to-hand: Preventing MRSA. Nurse Pract. 2006, 31, 16–23. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Mascola, L.; Bancroft, E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg. Infect. Dis. 2005, 11, 526–532. [Google Scholar] [CrossRef]
- Cohen, P.R. Cutaneous community-acquired methicillin-resistant Staphylococcus aureus infection in participants of athletic activities. South. Med. J. 2005, 98, 596–602. [Google Scholar] [CrossRef]
- Kazakova, S.V.; Hageman, J.C.; Matava, M.; Srinivasan, A.; Phelan, L.; Garfinkel, B.; Boo, T.; McAllister, S.; Anderson, J.; Jensen, B.; et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 2005, 352, 468–475. [Google Scholar] [CrossRef]
- Bergfeld, W.F.; Taylor, J.S. Trauma, sports, and the skin. Am. J. Ind. Med. 1985, 8, 403–413. [Google Scholar] [CrossRef]
- Sosin, D.M.; Gunn, R.A.; Ford, W.L.; Skaggs, J.W. An outbreak of furunculosis among high school athletes. Am. J. Sports Med. 1989, 17, 828–832. [Google Scholar] [CrossRef]
- Powell, F.C. Sports dermatology. J. Eur. Acad. Dermatol. Venereol. 1994, 3, 1–15. [Google Scholar] [CrossRef]
- Anderson, B.J. The epidemiology and clinical analysis of several outbreaks of herpes gladiatorum. Med. Sci. Sports Exerc. 2003, 35, 1809–1814. [Google Scholar] [CrossRef]
- Martykanova, D.S.; Davletova, N.C.; Zemlenuhin, I.A.; Volchkova, V.I.; Mugallimov, S.M.; Ahatov, A.M.; Laikov, A.V.; Markelova, M.I.; Boulygina, E.A.; Lopukhov, L.V.; et al. Skin Microbiota in Contact Sports Athletes and Selection of Antiseptics for Professional Hygiene. Biomed. Res. Int. 2019, 2019, 9843781. [Google Scholar] [CrossRef] [PubMed]
- Szatkowska, J.; Teofilak, M.; Śpiołek, O.; Siwiec, J.; Smyl, N.; Kędziora, F.; Wąsowicz, A.; Słowikowska, A.; Sztyler-Krąkowska, M.; Fabian, D. The Role of Physical Activity in Enhancing and Preserving Skin Health. J. Educ. Health Sport 2024, 76, 56455. [Google Scholar] [CrossRef]
- Nakagawa, N.; Shimizu, N.; Sugawara, T.; Sakai, S. The relationship between habitual physical activity and skin mechanical properties. Ski. Res. Technol. 2021, 27, 353–357. [Google Scholar] [CrossRef]
- Oizumi, R.; Sugimoto, Y.; Aibara, H. The Potential of Exercise on Lifestyle and Skin Function: Narrative Review. JMIR Dermatol. 2024, 7, e51962. [Google Scholar] [CrossRef]
- Ryosuke, O.; Yoshie, S.; Hiromi, A. The association between activity levels and skin moisturizing function in adults. Dermatol. Rep. 2021, 13, 8811. [Google Scholar]
- Lu, C.Y.; Lee, H.C.; Fahn, H.J.; Wei, Y.H. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat. Res. 1999, 423, 11–21. [Google Scholar] [CrossRef]
- Safdar, A.; Bourgeois, J.M.; Ogborn, D.I.; Little, J.P.; Hettinga, B.P.; Akhtar, M.; Thompson, J.E.; Melov, S.; Mocellin, N.J.; Kujoth, G.C.; et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. USA 2011, 108, 4135–4140. [Google Scholar] [CrossRef]
- Crane, J.D.; MacNeil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.E.; Raha, S.; Steinberg, G.R.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Lebwohl, M.; Herrmann, L.G. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis 2005, 76 (Suppl. 6), 7–12. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003, 52, 793–795. [Google Scholar]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.M.; Basler, R.S.; Foley, J.; Scarlata, C.; Vasily, D.B. National Athletic Trainers’ Association position statement: Skin diseases. J. Athl. Train. 2010, 45, 411–428. [Google Scholar] [CrossRef]
- National Collegiate Athletic Association. 2014–2015 NCAA Sports Medicine Handbook. 2014. Available online: http://www.ncaapublications.com/productdownloads/MD15.pdf (accessed on 6 April 2025).
- Mitchell, J.J.; Jackson, J.M.; Anwar, A.; Singleton, S.B. Bacterial Sport-Related Skin and Soft-Tissue Infections (SSTIs): An Ongoing Problem Among a Diverse Range of Athletes. JBJS Rev. 2017, 5, e4. [Google Scholar] [CrossRef]
- Zeeuwen, P.L.J.M.; Grice, E.A. Skin microbiome and antimicrobial peptides. Exp. Dermatol. 2021, 30, 1362–1365. [Google Scholar] [CrossRef]
- Clausen, M.L.; Agner, T. Antimicrobial Peptides, Infections and the Skin Barrier. Curr. Probl. Dermatol. 2016, 49, 38–46. [Google Scholar]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Schröder, J.M.; Harder, J. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Ganz, T. Cathelicidins: A family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 2002, 9, 18–22. [Google Scholar] [CrossRef]
- Frohm, M.; Agerberth, B.; Ahangari, G.; Ståhle-Bäckdahl, M.; Lidén, S.; Wigzell, H.; Gudmundsson, G.H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997, 272, 15258–15263. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar] [CrossRef]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005, 6, 57–64. [Google Scholar] [CrossRef]
- Simanski, M.; Rademacher, F.; Schröder, L.; Schumacher, H.M.; Gläser, R.; Harder, J. IL-17A and IFN-gamma synergistically induce RNase7 expression via STAT3 in primary keratinocytes. PLoS ONE 2013, 8, e59531. [Google Scholar] [CrossRef]
- Harder, J.; Schröder, J.M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002, 277, 46779–46784. [Google Scholar] [CrossRef]
- Rademacher, F.; Dreyer, S.; Kopfnagel, V.; Gläser, R.; Werfel, T.; Harder, J. The antimicrobial and immunomodulatory function of RNase 7in skin. Front. Immunol. 2019, 10, 2553. [Google Scholar] [CrossRef]
- Rademacher, F.; Simanski, M.; Schröder, L.; Mildner, M.; Harder, J. The role of RNase 7 in innate cutaneous defense against Pseudomonas aeruginosa. Exp. Dermatol. 2017, 26, 227–233. [Google Scholar] [CrossRef]
- Rademacher, F.; Simanski, M.; Harder, J. RNase 7 in Cutaneous Defense. Int. J. Mol. Sci. 2016, 17, 560. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; DiRosario, J.D.; McHugh, K.M.; McGillivary, G.; Justice, S.S.; Carpenter, A.R.; Baker, P.B.; Harder, J.; Hains, D.S. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011, 80, 174–180. [Google Scholar] [CrossRef]
- Walter, S.; Rademacher, F.; Kobinger, N.; Simanski, M.; Gläser, R.; Harder, J. RNase 7 participates in cutaneous innate control of Corynebacterium amycolatum. Sci. Rep. 2017, 7, 13862. [Google Scholar] [CrossRef]
- Simanski, M.; Dressel, S.; Gläser, R.; Harder, J. RNase 7 protects healthy skin from Staphylococcus aureus colonization. J. Invest. Dermatol. 2010, 130, 2836–2838. [Google Scholar] [CrossRef] [PubMed]
- Koten, B.; Simanski, M.; Gläser, R.; Podschun, R.; Schröder, J.M.; Harder, J. RNase 7 contributes to the cutaneous defense against Enterococcus faecium. PLoS ONE 2009, 4, e6424. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chertov, O.; Bykovskaia, S.N.; Chen, Q.; Buffo, M.J.; Shogan, J.; Anderson, M.; Schroder, J.M.; Wang, J.M.; Howard, O.M.; et al. Beta defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 286, 525–528. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Gallo, R.L.; Huttner, K.M. Antimicrobial peptides: An emerging concept in cutaneous biology. J. Invest. Dermatol. 1998, 111, 739–743. [Google Scholar] [CrossRef]
- Namjoshi, S.; Caccetta, R.; Benson, H.A. Skin peptides: Biological activity and therapeutic opportunities. J. Pharm. Sci. 2008, 97, 2524–2542. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Thapa, D.R.; Rosenthal, A.; Liu, L.; Roberts, A.A.; Ganz, T. Differential regulation of β-defensin expression in human skin by microbial stimuli. J. Immunol. 2005, 174, 4870–4879. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Annals Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef]
- Tokumaru, S.; Sayama, K.; Shirakata, Y.; Komatsuzawa, H.; Ouhara, K.; Hanakawa, Y.; Yahata, Y.; Dai, X.; Tohyama, M.; Nagai, H.; et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005, 175, 4662–4668. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Suzuki, A.; Ushio, H.; Nagaoka, I.; Ogawa, H.; Okumura, K. The human antimicrobial peptide dermcidin activates normal human keratinocytes. Br. J. Dermatol. 2009, 160, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The human beta-defensins (-1, -2, 3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Iwabuchi, K.; Matsuda, H.; Ogawa, H.; Nagaoka, I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 2002, 14, 421–426. [Google Scholar] [CrossRef]
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–382. [Google Scholar] [CrossRef]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef]
- Madsen, P.; Rasmussen, H.H.; Celis, J.E. Molecular cloning, occurrence, and expression of a novel partially secreted protein ‘psoriasin’ that is highly up-regulated in psoriatic skin. J. Invest. Dermatol. 1991, 97, 701–712. [Google Scholar] [CrossRef]
- Rademacher, F.; Glaser, R.; Harder, J. Antimicrobial peptides and proteins: Interaction with the skin microbiota. Exp. Dermatol. 2021, 30, 1496–1508. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Simanski, M.; Erkens, A.S.; Rademacher, F.; Harder, J. Staphylococcus epidermidis-induced interleukin-1 beta and human beta-defensin-2 expression in human keratinocytes is regulated by the host molecule A20 (TNFAIP3). Acta Derm. Venereol. 2019, 99, 181–187. [Google Scholar] [CrossRef]
- Park, K.; Ommori, R.; Imoto, K.; Asada, H. Epidermal growth factor receptor inhibitors selectively inhibit the expressions of human beta-defensins induced by Staphylococcus epidermidis. J. Dermatol. Sci. 2014, 75, 94–99. [Google Scholar] [CrossRef]
- Ommori, R.; Ouji, N.; Mizuno, F.; Kita, E.; Ikada, Y.; Asada, H. Selective induction of antimicrobial peptides from keratinocytes by staphylococcal bacteria. Microb. Pathog. 2013, 56, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Götz, F.; Peschel, A.; Schaller, M.; Schittek, B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Invest. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef]
- FAO; WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria: Report of a Joint FAO WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. In Proceedings of the Joint FAO/WHO Expert Consultation on Evaluation of Health, Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, American Córdoba Park Hotel, Córdoba, Argentina, 1–4 October 2001. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin microbiome modulation induced by probiotic solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef]
- Franca, K. Topical probiotics in dermatological therapy and skin care: A concise review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp. Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).