The Gut–Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review
Abstract
1. Introduction
2. Methods
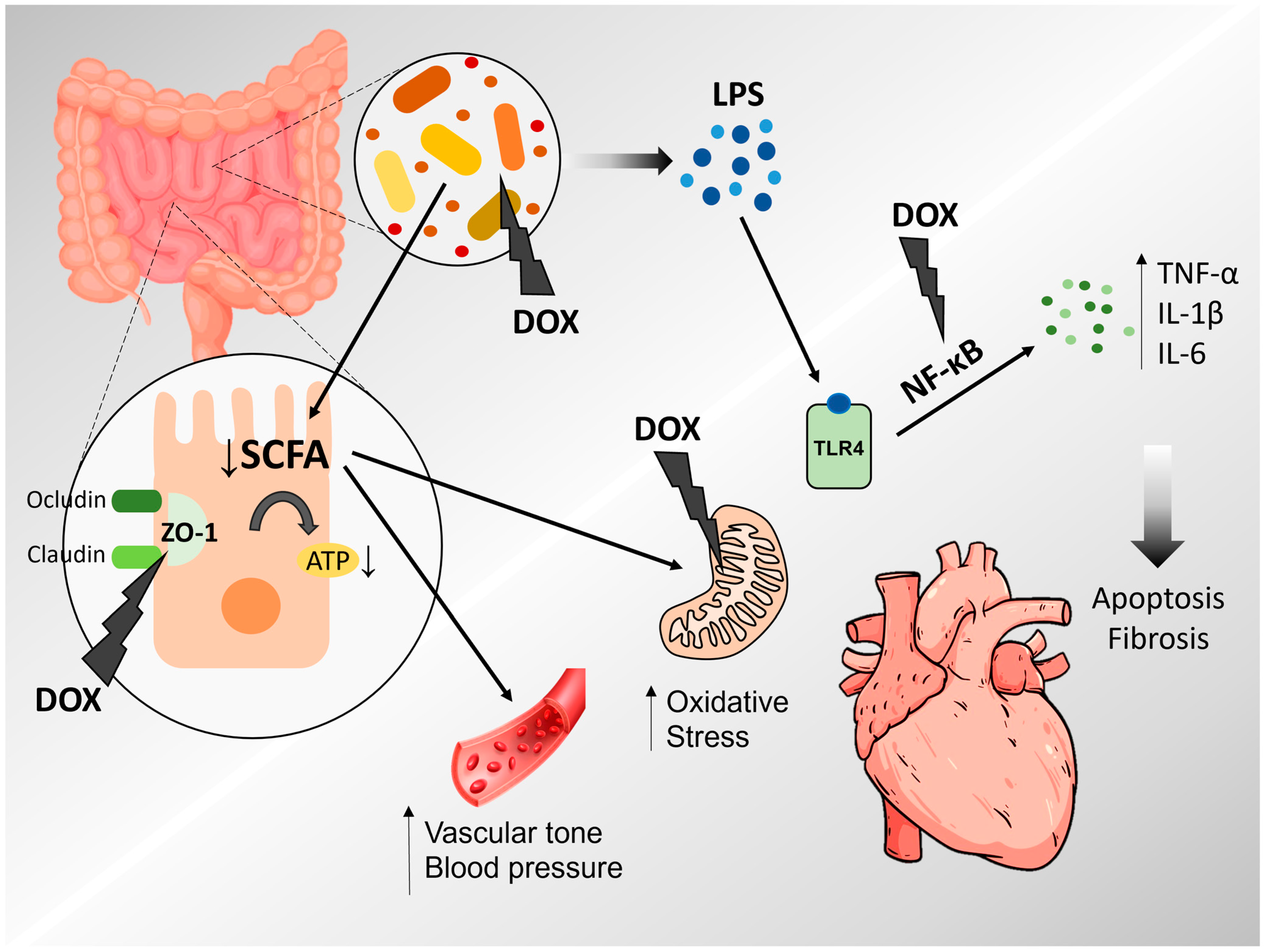
3. Doxorubicin-Induced Cardiotoxicity
4. Microbiota, Intestinal Permeability and Cardiovascular Diseases
5. Doxorubicin-Induced Cardiotoxicity and Inflammation
6. Interventions to Target Gut Microbiota in Cardiovascular Diseases
7. Interventions on DOX-Induced Cardiotoxicity by Targeting the Gut Microbiota and Intestinal Permeability
7.1. Polyphenols
7.2. Zinc (II)–Curcumin Complexes
7.3. Emodin
7.4. Fecal Microbiota Transplantation
7.5. Probiotics
7.6. Bacterial Membrane Protein Nanodrug
8. Conclusions, Final Considerations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.M.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Tonon, C.R.; Monte, M.G.; Balin, P.S.; Fujimori, A.S.S.; Ribeiro, A.P.D.; Ferreira, N.F.; Vieira, N.M.; Cabral, R.P.; Okoshi, M.P.; Okoshi, K.; et al. Liraglutide pretreatment does not improve acute doxorubicin-induced cardiotoxicity in rats. Int. J. Mol. Sci. 2024, 25, 5833. [Google Scholar] [CrossRef]
- Carrillo-Salinas, F.J.; Ngwenyama, N.; Anastasiou, M.; Kaur, K.; Alcaide, P. Heart inflammation. Am. J. Pathol. 2019, 189, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, B.M.; Zhang, C.; Khodade, V.S.; Keceli, G.; McGinity, C.; Paolocci, N.; Toscano, J.P. Hydropersulfides (RSSH) attenuate doxorubicin-induced cardiotoxicity while boosting its anticancer action. Redox Biol. 2023, 60, 102625. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, X.S.; Xian, C.J. Chemotherapy-induced intestinal microbiota dysbiosis impairs mucosal homeostasis by modulating Toll-like receptor signaling pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef]
- An, L.; Wuri, J.; Zheng, Z.; Li, W.; Yan, T. Microbiota modulate doxorubicin induced cardiotoxicity. Eur. J. Pharm. Sci. 2021, 166, 105977. [Google Scholar] [CrossRef]
- Rahman, M.d.M.; Islam, F.; Or-Rashid, M.d.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Nozaki, N.; Shishido, T.; Takeishi, Y.; Kubota, I. Modulation of doxorubicin-induced cardiac dysfunction in Toll-like receptor-2–knockout mice. Circulation 2004, 110, 2869–2874. [Google Scholar] [CrossRef]
- Riad, A.; Bien, S.; Gratz, M.; Escher, F.; Heimesaat, M.M.; Bereswill, S.; Krieg, T.; Felix, S.B.; Schultheiss, H.P.; Kroemer, H.K.; et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur. J. Heart Fail. 2008, 10, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Francisqueti-Ferron, F.V.; Nakandakare-Maia, E.T.; Siqueira, J.S.; Ferron, A.J.T.; Vieira, T.A.; Bazan, S.G.Z.; Correa, C.R. The role of gut dysbiosis-associated inflammation in heart failure. Rev. Assoc. Médica Bras. 2022, 68, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Kitai, T.; Tang, W.H.W. Gut microbiota in cardiovascular disease and heart failure. Clin. Sci. 2018, 132, 85–91. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Zhu, P.; Nasser, M.I.; Zhang, X.; Zhao, M. Implication of gut microbiota in cardiovascular diseases. Oxidative Med. Cell. Longev. 2020, 2020, 5394096. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Sandek, A.; Bjarnason, I.; Volk, H.D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012, 157, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bastos, P.; Picazo, Ó.; Fontes-Villalba, M.; Pareja-Galeano, H.; Lindeberg, S.; Martínez-Selles, M.; Lucia, A.; Emanuele, E. Serum zonulin and endotoxin levels in exceptional longevity versus precocious myocardial infarction. Aging Dis. 2018, 9, 317. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Kiechl, S.; Dunzendorfer, S.; Schratzberger, P.; Egger, G.; Oberhollenzer, F.; Willeit, J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease. J. Am. Coll. Cardiol. 1999, 34, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. On validly published names, correct names, and changes in the nomenclature of phyla and genera of prokaryotes: A guide for the perplexed. npj Biofilm. Microbiomes 2024, 10, 20. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W.; et al. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Feng, W.; Liu, Q.; Zhou, S.; Liu, Q.; Cai, L. The gut microbiota and its interactions with cardiovascular disease. Microb. Biotechnol. 2020, 13, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Wojtacki, J.; Lewicka-Nowak, E.; Leśniewski-Kmak, K. Anthracycline-induced cardiotoxicity: Clinical course, risk factors, pathogenesis, detection and prevention—Review of the literature. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2000, 6, 411–420. [Google Scholar]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive oxygen species, oxidative damage and cell death. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–55. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Kalivendi, S.V.; Kotamraju, S.; Zhao, H.; Joseph, J.; Kalyanaraman, B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. J. Biol. Chem. 2001, 276, 47266–47276. [Google Scholar] [CrossRef]
- Vásquez-Vivar, J.; Martasek, P.; Hogg, N.; Masters, B.S.S.; Pritchard, K.A.; Kalyanaraman, B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 1997, 36, 11293–11297. [Google Scholar] [CrossRef]
- Schirone, L.; D’Ambrosio, L.; Forte, M.; Genovese, R.; Schiavon, S.; Spinosa, G.; Iacovone, G.; Valenti, V.; Frati, G.; Sciarretta, S. Mitochondria and doxorubicin-induced cardiomyopathy: A complex interplay. Cells 2022, 11, 2000. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; de Boer, R.A. From inflammation to fibrosis—Molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Padrão, A.I.; Duarte, J.A.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. Role of inflammation and redox status on doxorubicin-induced cardiotoxicity in infant and adult CD-1 male mice. Biomolecules 2021, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-induced systemic inflammation is driven by upregulation of Toll-like receptor TLR4 and endotoxin leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef]
- Shaker, R.A.; Abboud, S.H.; Assad, H.C.; Hadi, N. Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. BMC Pharmacol. Toxicol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Li-Qiu, M.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Zhang, S.; You, Z.Q.; Yang, L.; Li, L.; Wu, Y.P.; Gu, L.Q.; Xin, Y.F. Protective effect of Shenmai injection on doxorubicin-induced cardiotoxicity via regulation of inflammatory mediators. BMC Complement. Altern. Med. 2019, 19, 317. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The dynamic interplay between cardiac inflammation and fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Cawthray, J.F.; Jamieson, G.; Fox, J.A.; Orvig, C. Iron(III)-binding of the anticancer agents doxorubicin and vosaroxin. Dalton Trans. 2015, 44, 2348–2358. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. The effects of doxorubicin on cardiac calcium homeostasis and contractile function. J. Cardiol. 2022, 80, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Garcia, L.R.; Queiroz, D.A.R.; Lazzarin, T.; Tonon, C.R.; Balin, P.S.; Polegato, B.F.; Paiva, S.A.R.; Azevedo, P.S.; Minicucci, M.F. Oxidative stress as a therapeutic target of cardiac remodeling. Antioxidants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G.; Jiang, W.; Lamont, S.; et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Roediger, W.E.W. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 316, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: Prevention and therapeutic implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Willemsen, L.E.M. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions1. J. Anim. Sci. 2012, 90 (Suppl. S4), 266–268. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Tack, J.; Farre, R. The role of intestinal permeability in gastrointestinal disorders and current methods of evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Arango-González, A.; Lara-Guzmán, O.J.; Rivera, D.A.; Álvarez, R.; Salazar-Serrano, D.; Muñoz-Durango, K.; Escobar, J.S.; Sierra, J.A. Putative intestinal permeability markers do not correlate with cardiometabolic health and gut microbiota in humans, except for peptides recognized by a widely used zonulin ELISA kit. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 112–123. [Google Scholar] [CrossRef]
- Abreu, M.T.; Fukata, M.; Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 2005, 174, 4453–4460. [Google Scholar] [CrossRef]
- Hug, H.; Mohajeri, M.; La Fata, G. Toll-like receptors: Regulators of the immune response in the human gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Chagnon, F.; Metz, C.N.; Bucala, R.; Lesur, O. Endotoxin-induced myocardial dysfunction: Effects of macrophage migration inhibitory factor neutralization. Circ. Res. 2005, 96, 1095–1102. [Google Scholar] [CrossRef]
- Cheng, C.K.; Huang, Y. The gut-cardiovascular connection: New era for cardiovascular therapy. Med. Rev. 2021, 1, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of Tumor Necrosis Factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Bouras, G.; Giannopoulos, G.; Hatzis, G.; Alexopoulos, D.; Leventopoulos, G.; Deftereos, S. Inflammation and chronic heart failure: From biomarkers to novel anti-inflammatory therapeutic strategies. Med. Chem. 2014, 10, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in heart failure. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kribbs, S.B.; Clubb, F.J.; Michael, L.H.; Didenko, V.V.; Hornsby, P.J.; Seta, Y.; Oral, H.; Spinale, F.G.; Mann, D.L. Pathophysiologically relevant concentrations of Tumor Necrosis Factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998, 97, 1382–1391. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nakano, M.; Bednarczyk, J.L.; McIntyre, B.W.; Entman, M.; Mann, D.L. Tumor Necrosis Factor-α provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997, 95, 1247–1252. [Google Scholar] [CrossRef]
- Li, Y. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc. Res. 1999, 42, 162–172. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Dutka, M.; Bobiński, R.; Ulman-Włodarz, I.; Hajduga, M.; Bujok, J.; Pająk, C.; Ćwiertnia, M. Various aspects of inflammation in heart failure. Heart Fail. Rev. 2020, 25, 537–548. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to Tumor Necrosis Factor-α, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) Trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Duan, J.; Hu, J.; Qi, Y.; Kang, L.; Wang, K.; Chen, J.; Wu, X.; Xu, B.; Gu, R. Colchicine alleviates inflammation and improves diastolic dysfunction in heart failure rats with preserved ejection fraction. Eur. J. Pharmacol. 2022, 929, 175126. [Google Scholar] [CrossRef]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-chain fatty acids in gut–heart axis: Their role in the pathology of heart failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-chain fatty acid receptors and blood pressure regulation: Council on hypertension mid-career award for research excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Zhang, N.; Wei, W.Y.; Li, L.L.; Ma, Z.G.; Tang, Q.Z. Osteocrin attenuates inflammation, oxidative stress, apoptosis, and cardiac dysfunction in doxorubicin-induced cardiotoxicity. Clin. Transl. Med. 2020, 10, e124. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Li, J.; Li, E. Dexmedetomidine reduces the inflammation and apoptosis of doxorubicin-induced myocardial cells. Exp. Mol. Pathol. 2020, 113, 104371. [Google Scholar] [CrossRef]
- Peng, W.; Rao, D.; Zhang, M.; Shi, Y.; Wu, J.; Nie, G.; Xia, Q. Teneligliptin prevents doxorubicin-induced inflammation and apoptosis in H9c2 cells. Arch. Biochem. Biophys. 2020, 683, 108238. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q. Catalpol ameliorates doxorubicin-induced inflammation and oxidative stress in H9C2 cells through PPAR-γ activation. Exp. Ther. Med. 2020, 20, 1003–1011. [Google Scholar] [CrossRef]
- Wan, Y.; He, B.; Zhu, D.; Wang, L.; Huang, R.; Zhu, J.; Wang, C.; Gao, F. Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Arch. Biochem. Biophys. 2021, 712, 109050. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Li, X.; Yang, F.; Huang, T.; Qiu, C.; Peng, K.; Wang, Z.; Yang, Y.; Lan, C. Amentoflavone mitigates doxorubicin-induced cardiotoxicity by suppressing cardiomyocyte pyroptosis and inflammation through inhibition of the STING/NLRP3 signalling pathway. Phytomedicine 2023, 117, 154922. [Google Scholar] [CrossRef] [PubMed]
- Akolkar, G.; Da Silva Dias, D.; Ayyappan, P.; Bagchi, A.K.; Jassal, D.S.; Salemi, V.M.C.; Irigoyen, M.C.; Angelis, K.; Singal, P.K. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H795–H809. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wei, S.; Jiang, C.; Xiao, Z.; Liu, J.; Peng, W.; Zhang, B.; Li, W. Involvement of Abnormal Gut Microbiota Composition and Function in Doxorubicin-Induced Cardiotoxicity. Front. Cell Infect. Microbiol. 2022, 12, 808837. [Google Scholar] [CrossRef]
- Lin, H.; Meng, L.; Sun, Z.; Sun, S.; Huang, X.; Lin, N.; Zhang, J.; Lu, W.; Yang, Q.; Chi, J.; et al. Yellow Wine Polyphenolic Compound Protects Against Doxorubicin-Induced Cardiotoxicity by Modulating the Composition and Metabolic Function of the Gut Microbiota. Circ. Heart Fail. 2021, 14, e008220. [Google Scholar] [CrossRef]
- Wu, R.; Mei, X.; Wang, J.; Sun, W.; Xue, T.; Lin, C.; Xu, D. Zn(ii)-Curcumin supplementation alleviates gut dysbiosis and zinc dyshomeostasis during doxorubicin-induced cardiotoxicity in rats. Food Funct. 2019, 10, 5587–5604. [Google Scholar] [CrossRef]
- Cray, P.; Sheahan, B.J.; Cortes, J.E.; Dekaney, C.M. Doxorubicin increases permeability of murine small intestinal epithelium and cultured T84 monolayers. Sci. Rep. 2020, 10, 21486. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef]
- Taladrid, D.; de Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension- and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Robles-Vera, I.; de la Visitación, N.; Toral, M.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Vargas, F.; Duarte, J.; Jiménez, R. Changes in Gut Microbiota Induced by Doxycycline Influence in Vascular Function and Development of Hypertension in DOCA-Salt Rats. Nutrients 2021, 13, 2971. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Du, M.M.; Yu, X.J.; Bai, J.; Xia, W.J.; Dai, Z.M.; Li, C.X.; Li, Y.; Su, Q.; et al. Curcumin ameliorates hypertension via gut-brain communication in spontaneously hypertensive rat. Toxicol. Appl. Pharmacol. 2021, 429, 115701. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tang, X.; Li, F.; Zhu, J.; Wu, M.; Wei, X.; Wang, Y. Green and Oolong Tea Extracts with Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022, 9, 892801. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Nelson, J.W.; Eskew, J.R.; Ganesan, A.; Ajami, N.J.; Petrosino, J.F.; Bryan, R.M.; Durgan, D.J. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension 2018, 72, 1141–1150. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J.; et al. Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Mol. Nutr. Food Res. 2020, 64, 1900616. [Google Scholar] [CrossRef]
- Deng, J.; Luo, K.; Xia, C.; Zhu, Y.; Xiang, Z.; Zhu, B.; Tang, X.; Zhang, T.; Shi, L.; Lyu, X.; et al. Phytochemical composition of Tibetan tea fermented by Eurotium cristatum and its effects on type 1 diabetes mice and gut microbiota. Heliyon 2024, 10, e27145. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, C.; Wan, L.; Peng, L.; Wen, S.; Fang, W.; Chen, H.; Wang, K.; Yang, X.; Huang, J.; et al. (−)-Epicatechin ameliorates type 2 diabetes mellitus by reshaping the gut microbiota and Gut–Liver axis in GK rats. Food Chem. 2024, 447, 138916. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, D.; Zhang, H.; Guo, H.; Liao, Y.; Lian, C.; Yao, Y.; Gao, H.; Huang, Y. Theabrownin as a Potential Prebiotic Compound Regulates Lipid Metabolism via the Gut Microbiota, Microbiota-Derived Metabolites, and Hepatic FoxO/PPAR Signaling Pathways. J. Agric. Food Chem. 2024, 72, 8506–8520. [Google Scholar] [CrossRef]
- Salamat, S.; Jahan-Mihan, A.; Tabandeh, M.R.; Mansoori, A. Randomized clinical trial evaluating the efficacy of synbiotic supplementation on serum endotoxin and trimethylamine N-oxide levels in patients with dyslipidaemia. Arch. Med. Sci. Atheroscler. Dis. 2024, 9, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiang, M.; Xin, L.; Zhang, Y.; Wang, Y.; Shen, Z.; Li, L.; Cui, X. Qiliqiangxin Modulates the Gut Microbiota and NLRP3 Inflammasome to Protect Against Ventricular Remodeling in Heart Failure. Front. Pharmacol. 2022, 13, 905424. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Kobayashi, M.; Ito, M.; Matsui, H.; Ohashi, K.; Murohara, T.; Takeda, J.I.; Ueyama, J.; Hirayama, M.; Ohno, K. Soy protein β-conglycinin ameliorates pressure overload-induced heart failure by increasing short-chain fatty acid (SCFA)-producing gut microbiota and intestinal SCFAs. Clin. Nutr. 2024, 43, 124–137. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.; Pereira, A.G.; Todo, M.C.; Fujimori, A.S.S.; Santos, P.P.; Dantas, D.; Fernandes, A.A.; Zanati, S.G.; Hassimotto, N.M.A.; Zornoff, L.A.M.; et al. Pera orange (Citrus sinensis) and Moro orange (Citrus sinensis (L.) Osbeck) juices attenuate left ventricular dysfunction and oxidative stress and improve myocardial energy metabolism in acute doxorubicin-induced cardiotoxicity in rats. Nutrition 2021, 91–92, 111350. [Google Scholar] [CrossRef]
- Dantas, D.; Pereira, A.G.; Fujimori, A.S.S.; Ribeiro, A.P.D.; Silva, C.C.V.A.; Monte, M.G.; Corrêa, C.R.; Fernandes, A.A.; Bazan, S.G.Z.; Azevedo, P.S.; et al. Doxycycline Attenuates Doxorubicin-Induced Cardiotoxicity by Improving Myocardial Energy Metabolism in Rats. J. Cardiovasc. Dev. Dis. 2022, 9, 254. [Google Scholar] [CrossRef]
- Modesto, P.N.; Polegato, B.F.; Santos, P.P.; Grassi, L.D.V.; Molina, L.C.C.; Bazan, S.G.Z.; Pereira, A.J.; Fernandes, A.A.H.; Fabro, A.T.; Androcioli, V.N.; et al. Green Tea (Camellia sinensis) Extract Increased Topoisomerase II β, Improved Antioxidant Defense, and Attenuated Cardiac Remodeling in an Acute Doxorubicin Toxicity Model. Oxidative Med. Cell. Longev. 2021, 2021, 8898919. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Chen, X.; Wang, C.; Chen, X.; Liu, W.; Huang, K.; Chen, H.; Yang, J. Multi-walled carbon nanotubes exacerbate doxorubicin-induced cardiotoxicity by altering gut microbiota and pulmonary and colonic macrophage phenotype in mice. Toxicology 2020, 435, 152410. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, N.; Kan, J.; Tang, S.; Sun, R.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Polyphenols from Arctium lappa L. ameliorate doxorubicin-induced heart failure and improve gut microbiota composition in mice. J. Food Biochem. 2022, 46, e13731. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Tang, H.; Qiu, M.; Li, C.; Duan, C.; Wang, C.; Yang, J.; Zhou, X. Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice. Front. Pharmacol. 2019, 10, 107. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, J.; Zhong, Z.; Yang, J.; Lv, T.; Zhao, B.; Lin, H.; Chi, J.; Guo, H. Fecal Microbiota Transplantation in Mice Exerts a Protective Effect Against Doxorubicin-Induced Cardiac Toxicity by Regulating Nrf2-Mediated Cardiac Mitochondrial Fission and Fusion. Antioxid. Redox Signal. 2024, 41, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, J.; Hao, J.; Zhong, Z.; Wu, H.; Zhang, P.; Yang, J.; Guo, H.; Chi, J. Emodin ameliorates doxorubicin-induced cardiotoxicity by inhibiting ferroptosis through the remodeling of gut microbiota composition. Am. J. Physiol.-Cell Physiol. 2024, 326, C161–C176. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, S.; Fan, Q.; Xu, K.; Xu, Y.; Wu, F.; Zhang, X.; Wang, T.; Xia, Z. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity by regulating organic acid metabolism in gut microbiota. Front. Pharmacol. 2023, 14, 1286210. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xing, J.; Ma, X.; Zhang, W.; Wang, C.; Wang, Y.; Qi, X.; Liu, Y.; Jian, D.; Cheng, X.; et al. An orally administered bacterial membrane protein nanodrug ameliorates doxorubicin cardiotoxicity through alleviating impaired intestinal barrier. Bioact. Mater. 2024, 37, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Saper, R.B.; Rash, R. Zinc: An essential micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and cardiovascular disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Chen, Y.; Fan, X.; Han, J.; Zhong, L.; Zhang, Y.; Liu, Q.; Lin, J.; Huang, W.; Su, L.; et al. Emodin attenuates cardiomyocyte pyroptosis in doxorubicin-induced cardiotoxicity by directly binding to GSDMD. Phytomedicine 2023, 121, 155105. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xi, J.; Fang, J.; Zhang, B.; Cai, W. Aloe-emodin alleviates doxorubicin-induced cardiotoxicity via inhibition of ferroptosis. Free Radic Biol. Med. 2023, 206, 13–21. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Doublier, S.; Cirrincione, S.; Scardaci, R.; Botta, C.; Lamberti, C.; Giuseppe, F.D.; Angelucci, S.; Rantsiou, K.; Cocolin, L.; Pessione, E. Putative probiotics decrease cell viability and enhance chemotherapy effectiveness in human cancer cells: Role of butyrate and secreted proteins. Microbiol. Res. 2022, 260, 127012. [Google Scholar] [CrossRef]
- Khaleghi, M.; Khorrami, S.; Jafari-Nasab, T. Pediococcus acidilactici isolated from traditional cheese as a potential probiotic with cytotoxic activity against doxorubicin-resistant MCF-7 cells. 3 Biotech 2023, 13, 170. [Google Scholar] [CrossRef]
- Akbaba, M.; Gökmen, G.G.; Kışla, D.; Nalbantsoy, A. In vivo investigation of supportive immunotherapeutic combination of Bifidobacterium infantis 35624 and doxorubicin in murine breast cancer. Probiotics Antimicrob. Proteins 2023, 15, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Smoak, P.; Hydock, D.S.; Hayward, R.; O’Brien, K.; Lisano, J.K.; Boeneke, C.; Christensen, M.; Mathias, A. Milk and kefir maintain aspects of health during doxorubicin treatment in rats. J. Dairy Sci. 2019, 102, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaad, N.M.; Abd Elhameed, A.G.; El-Karef, A.; Ibrahim, T.M. Yogurt Containing the Probacteria Lactobacillus acidophilus combined with natural antioxidants mitigates doxorubicin-induced cardiomyopathy in rats. J. Med. Food 2015, 18, 950–959. [Google Scholar] [CrossRef] [PubMed]
| Study | Investigated Compound | Model | General Effects | Cardiac Effects | Microbiota and Intestinal Effects | Ref. |
|---|---|---|---|---|---|---|
| Wu et al., 2020 | Arctium lappa L. | Mice | ↓ OS, ↓ NO, ↓ TNF-α, ↓ casein kinase, ↓ LDH | Amelioration of morphological damage | ↓ Species richness and diversity of microbial community ↑ Lactobacillaceae, ↑ Ruminococcaceae, ↑ Roseburia ↓ Proteobacteria, ↓ Enterococcus, ↓ Erysipelatoclostridium ↓ Escherichia-Shigella | [131] |
| Lin et al., 2021 | Yellow wine | Rats | ↓ LDH, ↓ CK-MB, ↓ mitochondrial damage, ↓ TNF- α, IL-1β, IL-6, IL-8 | Improvement of LVEF, LVSF, LVPW ↓ collagen deposition ↓ cardiomyocite size, ↓ myocardial apoptosis | ↓ Bacterial species richness, ↓ Escherichia–Shigella, ↓ Dubosiella, ↓ Allobaculum ↑ Muribaculaceae, ↑ Ralstonia, ↑ Rikenellaceae | [107] |
| Huang et al., 2019 | Glabridin | Rats | ↓ IL-1β, ↓TNF-α, ↑ TGF-β, ↑ IL-10 | ↓ Bax, ↓ cleaved-caspase 9, ↓ cleaved-caspase 3 ↑ HAX-1, ↑ Bcl-2 | ↓ Desulfovibrio genus, ↑ Lactobacillus genus | [132] |
| Wu et al., 2019 | Zinc (II)-curcumin | Rats | ↓ CK, ↓ CK-MB, ↓ LDH, ↓ INF-γ, ↓ IL-6, ↓ TNF-α, ↓ IL-1β, ↓ MCP-1 | ↓ Cardiac apoptosis, ↑ LVEDP, ↓ fibrosis | ↑ Firmicutes, ↓ Bacteroidetes, ↓ LPS, ↑ ZO-1, ↑ occludins, ↓ inflammatory cells, ↑ crypt depth, ↑ TJ, ↑ goblet cells | [108] |
| An et al., 2020 | Fecal transplantation | Mice | ↓ NOX-2, ↓ TLR-2, ↓ IL-1β | ↑ LVFE, ↑ FS, ↓ perivascular and intersticial fibrosis | ↑ Goblet cells, ↓ intestinal ulcers, ↓ lymphocyte infiltration, ↑ TJ, ↑ ZO-1, ↓ LPS, ↑ microbiota richness | [13] |
| Zhou et al., 2024 | Fecal transplantation | Mice | ↓ MDA, ↑ SOD, ↓ Drp, ↑ MFN-2, improved mitochondrial complexes I and III, ↑ Nrf2 | ↑ LVFE, ↑ FS, ↓ collagen deposition, ↓ vacuolization, ↓ apoptosis | Alteration of p_Proteobacteria, c_Gammapro-teobacteria, o_Bacteroidales, and p_Bacteroidota | [133] |
| Hu et al., 2023 | Emodin | Mice | ↑ Nrf2, ↑ HO-1, ↑ NQO1 | ↓ Myocardial fibrosis, ↓ hypertrophy, and ↓ disorganization | ↑ Bacteroidota, ↓ Verrucomicrobiota | [134] |
| Zhao et al., 2023 | Apocynum venetum leaf extract | Mice | ↓ cardiac apoptosis, ↓ BNP | ↑ Ejection fraction, ↑ fractional shortening, ↓ myocardial fibrosis | ↑ Escherichia−Shigella, ↑ Akkermansia, ↑ Bacteroides, ↑ Clostridium, ↑ Ruminococcus, ↑ Enterobacter, ↑ Anaerotruncus, ↑ Enterorhabdus, ↑ Faecalibaculum, ↑ Romboutsia, and ↑ Halomonas | [135] |
| Li et al., 2024 | Bacterial membrane protein nanodrug | Mice | ↓ IL-1β, IFN-γ, TNF-α, NT-proBNP, T troponin, CKMB | ↑ Ejection fraction, ↑ fractional shortening, ↓ myocardial fibrosis, ↓ myocardial apoptosis | ↑ crypt depth, ↓ inflammatory cell infiltration, ↓ reduced LPS, ↓ zonulin ↑ short-chain fatty acid-producing bacteria, ↑ butyrate and pentanoic acids, changes in α and β-diversity | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonon, C.R.; Pereira, A.G.; Ferreira, N.F.; Monte, M.G.; Vieira, N.M.; Fujimori, A.S.S.; Ballin, P.d.S.; Paiva, S.A.R.d.; Zornoff, L.A.M.; Minicucci, M.F.; et al. The Gut–Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review. Microorganisms 2025, 13, 855. https://doi.org/10.3390/microorganisms13040855
Tonon CR, Pereira AG, Ferreira NF, Monte MG, Vieira NM, Fujimori ASS, Ballin PdS, Paiva SARd, Zornoff LAM, Minicucci MF, et al. The Gut–Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review. Microorganisms. 2025; 13(4):855. https://doi.org/10.3390/microorganisms13040855
Chicago/Turabian StyleTonon, Carolina Rodrigues, Amanda Gomes Pereira, Natália Fernanda Ferreira, Marina Gaiato Monte, Nayane Maria Vieira, Anderson Seiji Soares Fujimori, Paola da Silva Ballin, Sergio Alberto Rupp de Paiva, Leonardo Antonio Mamede Zornoff, Marcos Ferreira Minicucci, and et al. 2025. "The Gut–Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review" Microorganisms 13, no. 4: 855. https://doi.org/10.3390/microorganisms13040855
APA StyleTonon, C. R., Pereira, A. G., Ferreira, N. F., Monte, M. G., Vieira, N. M., Fujimori, A. S. S., Ballin, P. d. S., Paiva, S. A. R. d., Zornoff, L. A. M., Minicucci, M. F., & Polegato, B. F. (2025). The Gut–Heart Axis and Its Role in Doxorubicin-Induced Cardiotoxicity: A Narrative Review. Microorganisms, 13(4), 855. https://doi.org/10.3390/microorganisms13040855







