The Impact of Wheat Growth Stages on Soil Microbial Communities in a Rain-Fed Agroecosystem
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Abiotic Soil Analysis
2.4. Soil Biotic Parameters
2.5. Statistical Analysis
3. Results
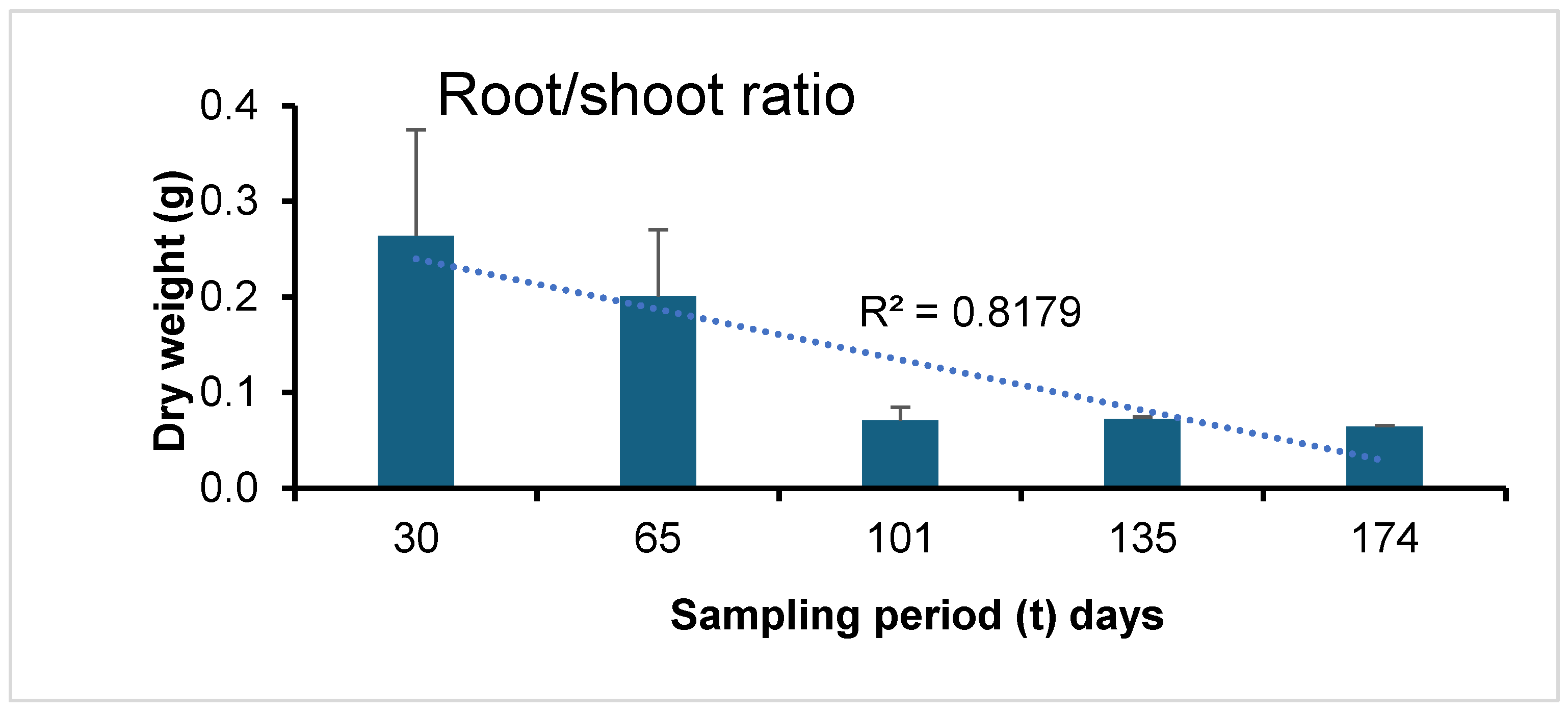
3.1. Plant Biomass
3.2. Microbial Community
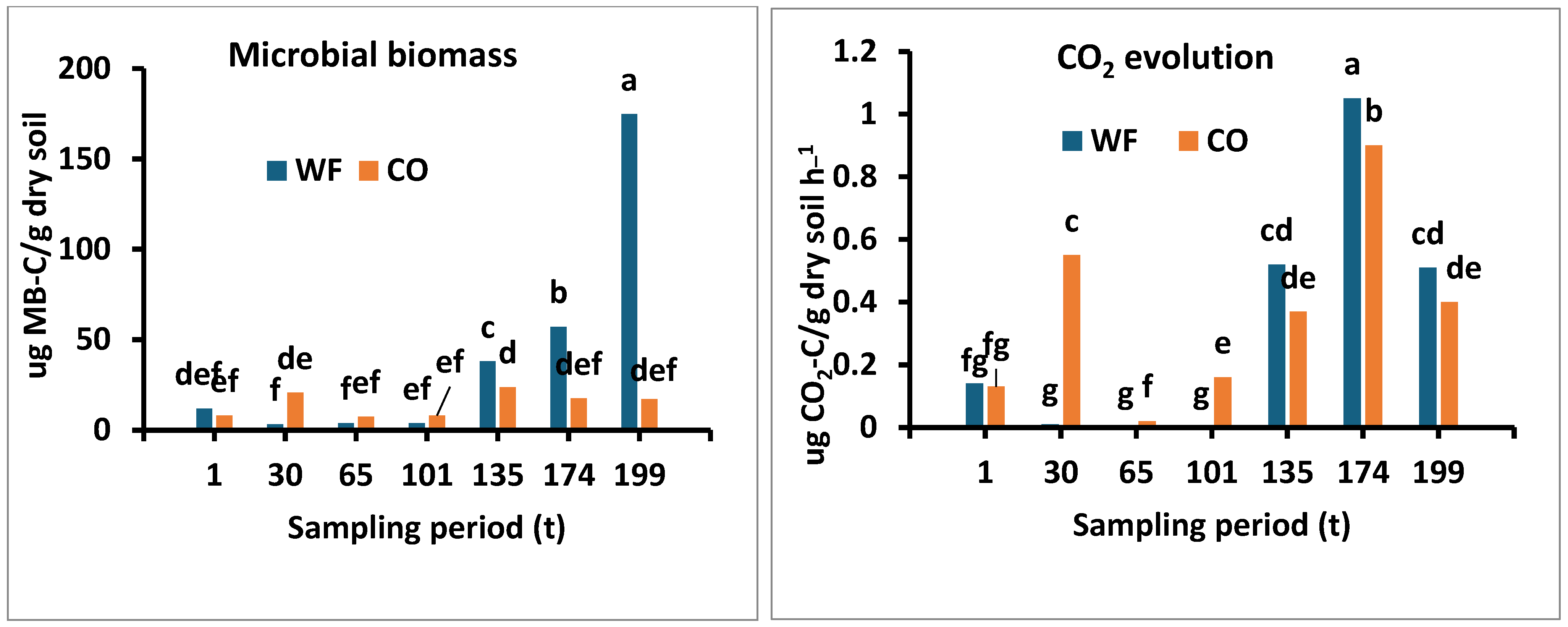
3.2.1. Microbial Biomass
3.2.2. Microbial CO2 Evolution
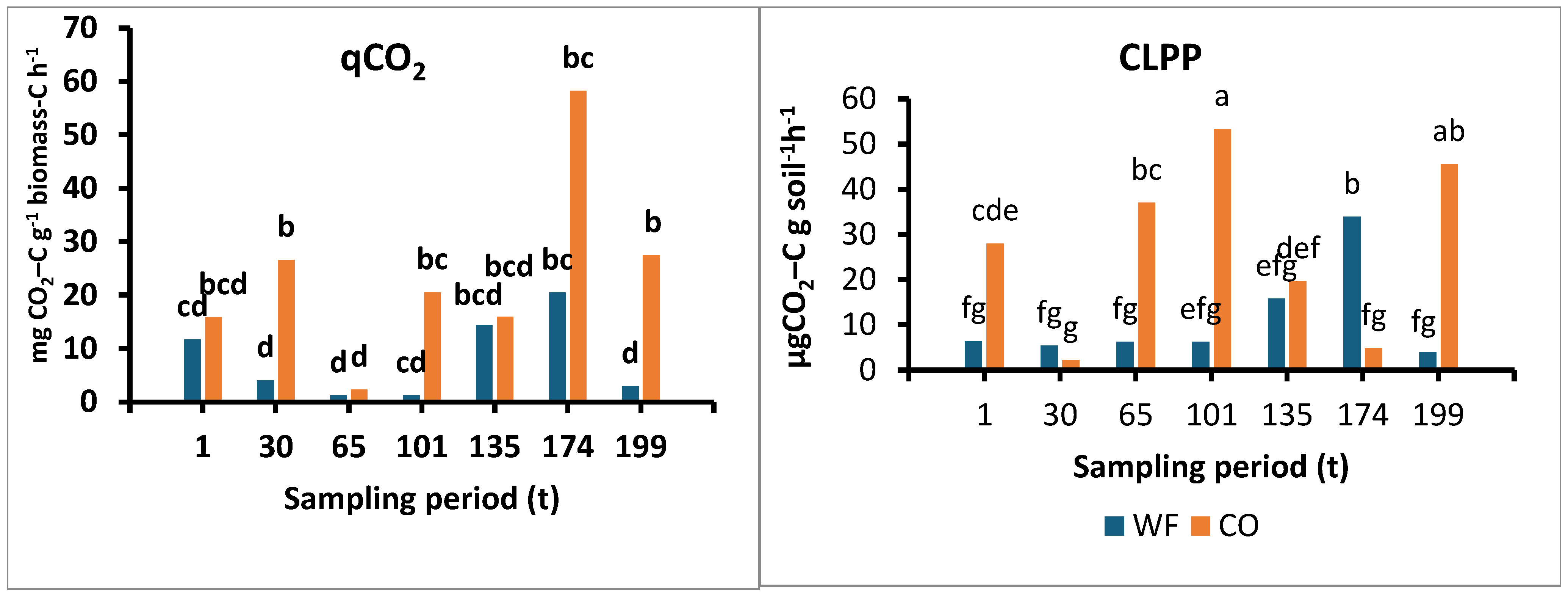
3.2.3. Metabolic Quotient (qCO2)
3.2.4. CLPP
3.2.5. Cmic/Corg Ratio
3.2.6. Correlation Between Abiotic Variables and Biotic Components
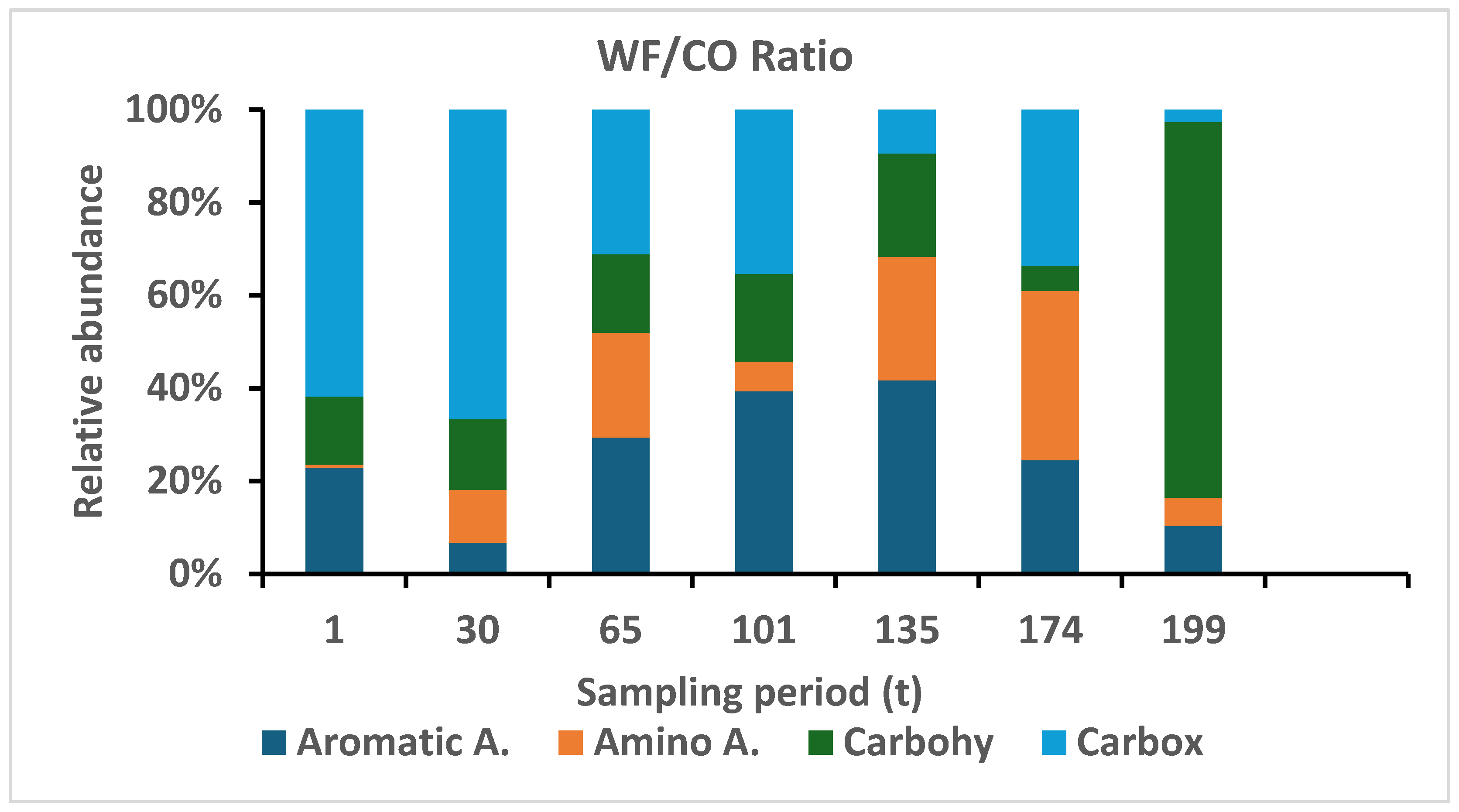
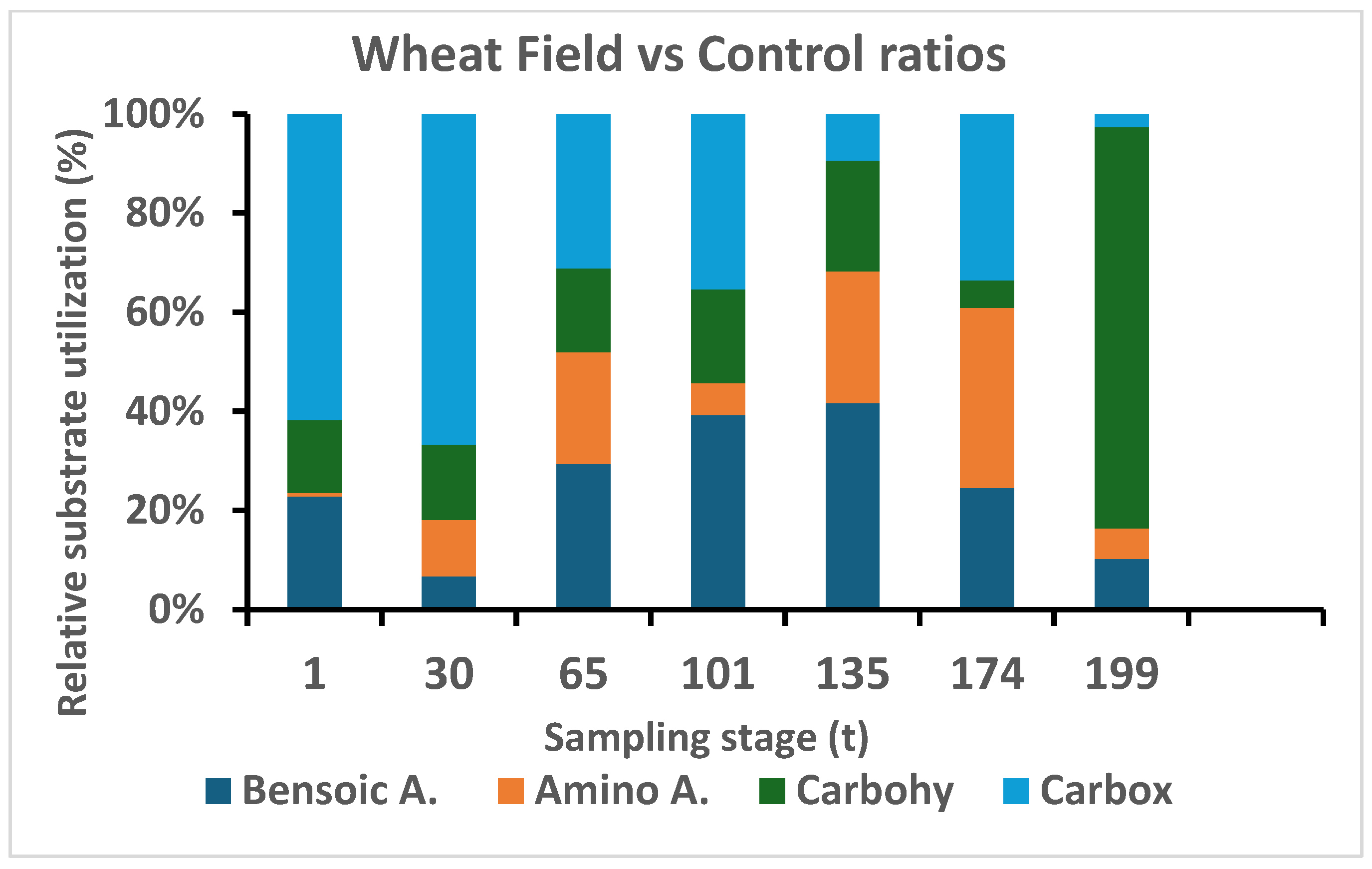
3.2.7. Substrate Utilization (SIR)
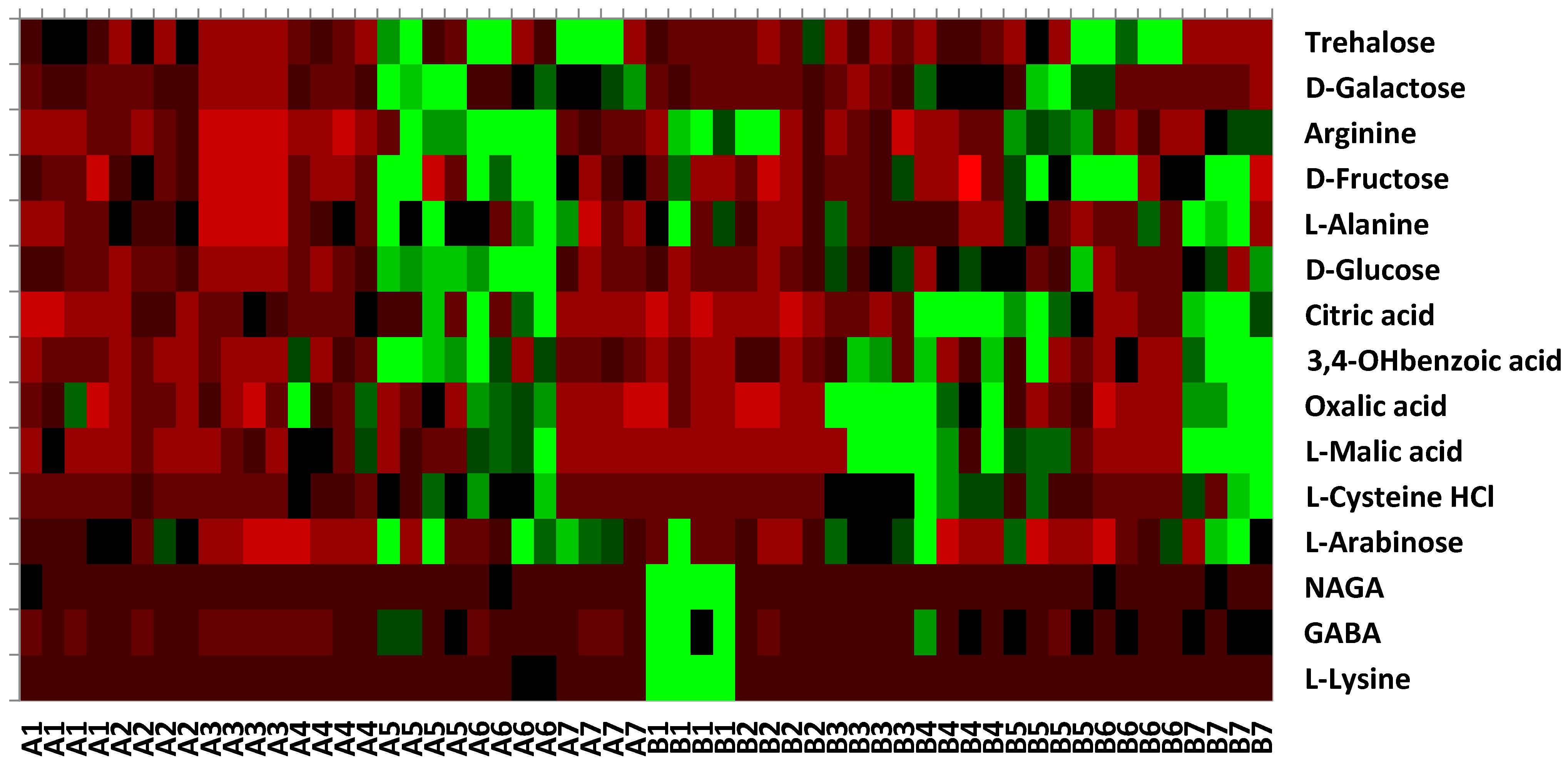
3.2.8. Physiological Profile (CLPP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar]
- van der Heijden, M.G.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar]
- Brady, N.C. The Nature and Properties of Soils, 8th ed.; Collier Macmillan Publishers: London, UK, 1974; pp. 14–20, 111–128. [Google Scholar]
- Belnap, J.; Lange, O.L. (Eds.) Biological Soil Crusts: Structure, Function, and Management; Springer Science and Business Media: Berlin, Germany, 2013; pp. 150, 167–169. [Google Scholar]
- Weisskopf, L.; Tomasi, N.; Santelia, D.; Martinoia, E.; Langlade, N.B.; Tabacchi, R.; Abou-Mansour, E. Isoflavonoid exudation from white lupin roots is influenced by phosphate supply, root type and cluster-root stage. New Phytol. 2006, 171, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [PubMed]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Soil Microbial Community and Their Relationship with Soil Properties Across Various Landscapes in the Mu Us Desert. Forests 2023, 14, 2152. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Ebblewhite, N.; Dobson, G. Soil microbial community structure: Effects of substrate loading rates. Soil Biol. Biochem. 1998, 31, 145–153. [Google Scholar] [CrossRef]
- Landesman, W.J.; Treonis, A.M.; Dighton, J. Effects of a one-year rainfall manipulation on soil nematode abundances and community composition. Pedobiologia 2011, 54, 87–91. [Google Scholar]
- Marziliano, P.A.; Lafortezza, R.; Medicamento, U.; Lorusso, L.; Giannico, V.; Colangelo, G.; Sanesi, G. Estimating belowground biomass and root/shoot ratio of Phillyrea latifolia L. in the Mediterranean forest landscapes. Ann. For. Sci. 2015, 72, 585–593. [Google Scholar] [CrossRef]
- Chialva, M.; Lanfranco, L.; Bonfante, P. The plant microbiota: Composition, functions, and engineering. Curr. Opin. Biotechnol. 2022, 73, 135–142. [Google Scholar]
- Mueller, C.W.; Baumert, V.; Carminati, A.; Germon, A.; Holz, M.; Kögel-Knabner, I.; Vidal, A. From rhizosphere to detritusphere–Soil structure formation driven by plant roots and the interactions with soil biota. Soil Biol. Biochem. 2024, 193, 109396. [Google Scholar] [CrossRef]
- Liu, G.; Sonobe, R.; Wang, Q. Spatial variations of soil respiration in arid ecosystems. Open J. Ecol. 2016, 6, 192. [Google Scholar] [CrossRef][Green Version]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Hanna, K. The soils of Israel and their distribution. Eur. J. Soil Sci. 1963, 14, 1365–2389. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Dinauer, R.C. Methods of soil analysis Madison. WI Am. Soc. Agron. 1965, 9, 653–708. [Google Scholar]
- Ben-Dor, E.; Banin, A. Determination of organic matter content in arid-zone soils using a simple “loss-on-ignition” method. Commun. Soil Sci. Plant Anal. 1989, 20, 1675–1695. [Google Scholar] [CrossRef]
- Corwin, D.L.; Rhoades, J.D. An improved technique for determining soil electrical conductivity-depth relations from above-ground electromagnetic measurements. Soil Sci. Soc. Am. J. 1982, 46, 517–520. [Google Scholar] [CrossRef]
- Oren, A.; Steinberger, Y. Coping with artifacts induced by CaCO3–CO2–H2O equilibria in substrate utilization profiling of calcareous soils. Soil Biol. Biochem. 2008, 40, 2569–2577. [Google Scholar] [CrossRef]
- Sherman, C.; Steinberger, Y. Microbial functional diversity associated with plant litter decomposition along a climatic gradient. Microb. Ecol. 2012, 64, 399–415. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar]
- Fließbach, A.; MaÈder, P. Microbial biomass and size-density fractions differ between soils of organic and conventional agricultural systems. Soil Biol. Biochem. 2000, 32, 757–768. [Google Scholar]
- Anderson, T.H.; Domsch, A.K. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Odum, E.P. Trends expected in stressed ecosystems. Bioscience 1985, 35, 419–422. [Google Scholar] [CrossRef]
- Insam, H.; Parkinson, D.; Domsch, K.H. Influence of macroclimate on soil microbial biomass. Soil Biol. Biochem. 1989, 21, 211–221. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Weng, B.; Su, J.; Nie, S.; Gilbert, J.A.; Zhu, Y.G. The phenological stage of rice growth determines anaerobic ammonium oxidation activity in rhizosphere soil. Soil Biol. Biochem. 2016, 100, 59–65. [Google Scholar] [CrossRef]
- Igwe, A.N.; Quasem, B.; Liu, N.; Vannette, R.L. Plant phenology influences rhizosphere microbial community and is accelerated by serpentine microorganisms in Plantago erecta. FEMS Microbiol. Ecol. 2021, 97, 85. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, F.; Zhang, J.; Chen, Y.; Zhang, C.; Liu, G.; Zhang, H.; Ma, C.; Zhang, J. The Variation in the Rhizosphere Microbiome of Cotton with Soil Type, Genotype and Developmental Stage. Sci. Rep. 2017, 7, 3940. [Google Scholar] [CrossRef]
- Yin, C.; Mueth, N.; Hulbert, S.; Schlatter, D.; Paulitz, T.C.; Schroeder, K.; Dhingra, A. Bacterial communities on wheat grown under long-term conventional tillage and no-till in the Pacific Northwest of the United States. Phytobiomes 2017, 1, 83–90. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Razavi, B.S.; Sanaullah, M.; Kuzyakov, Y. Phenological stage, plant biomass, and drought stress affect microbial biomass and enzyme activities in the rhizosphere of Enteropogon macrostachyus. Pedosphere 2019, 29, 259–265. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Seasonal changes in soil microbial biomass and mineralizable C and N in wheat management systems. Soil Biol. Biochem. 1994, 26, 1469–1475. [Google Scholar] [CrossRef]
- Diosma, G.; Aulicino, M.; Chidichimo, H.; Balatti, P.A. Effect of tillage and N fertilization on microbial physiological profile of soils cultivated with wheat. Soil Tillage Res. 2006, 91, 236–243. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chakrabarti, K.; Chakraborty, A.; Ghosh, S. Effect of long-term fertilizers and manure application on microbial biomass and microbial activity of a tropical agricultural soil. Biol. Fertil. Soils 2011, 47, 227–233. [Google Scholar]
- Bera, T.; Sharma, S.; Thind, H.S.; Yadvinder, S.; Sidhu, H.S.; Jat, M.L. Changes in soil biochemical indicators at different wheat growth stages under conservation-based sustainable intensification of rice-wheat system. J. Integr. Agric. 2018, 17, 1871–1880. [Google Scholar]
- Wang, J.; Xue, C.; Song, Y.; Wang, L.; Huang, Q.; Shen, Q. Wheat and Rice Growth Stages and Fertilization Regimes Alter Soil Bacterial Community Structure, But Not Diversity. Front. Microbiol. 2016, 7, 1207. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Stefan, S.; Frank, E. Intensity of heat stress in winter wheat phenology compensates for the adverse effects of global warming. Environ. Res. Lett. 2015, 10, 24012. [Google Scholar]
- Fei, M.; Sun, M.; Liu, X.Y.; Wang, J.Y.; Zhang, X.C.; Ma, B.L.; Xiong, Y.C. Phenological responses of spring wheat and maize to changes in crop management and rising temperatures from 1992 to 2013 across the Loess Plateau. Field Crops Res. 2016, 196, 337–347. [Google Scholar]

| Sampling Time (t) | Soil Moisture | Organic Matter | pH | Electric Cond. | Cmic/Corg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| WF | CO | WF | CO | WF | CO | WF | CO | WF | CO | |
| 0 | 3.89 d | 3.87 d | 2.7 a | 2.0 bc | 8.1 a | 7.9 ab | 99.9 ab | 98.95 ab | 5.07 d | 6.37 c |
| 30 | 20.18 a | 20.17 a | 2.0 bc | 1.8 bcd | 7.8 bc | 7.8 bc | 68.1 c | 78.8 ab | 0.5 e | 29.73 b |
| 65 | 12.46 c | 10.01 d | 1.1 f | 0.5 g | 7.9 ab | 7.9 ab | 67.05 c | 80.9 ab | 0.01 e | 4.35 c |
| 101 | 12.46 ab | 17.99 b | 1.1 b | 1.9 bcd | 7.9 ab | 7.9 ab | 67.0 bc | 81.2 ab | 0.01 e | 8.42 c |
| 135 | 13.19 c | 12.75 c | 1.4 def | 1.4 ef | 7.8 bc | 7.6 c | 79.3 bc | 84.0 ab | 35.37 b | 26.43 b |
| 174 | 3.34 d | 3.39 d | 1.5 def | 1.2 ef | 7.8 bc | 7.6 c | 76.1 bc | 101.2 ab | 66.88 a | 69.77 a |
| 199 | 3.18 d | 2.88 d | 2.9 a | 1.7 def | 8.0 ab | 7.9 ab | 112.5 a | 116.43 a | 17.59 c | 23.26 b |
| Wheat Site (WF) | |||||||
|---|---|---|---|---|---|---|---|
| Variables | SM (%) | OM (%) | pH | EC (µS cm−1) | MB | CO2 | qCO2 |
| SM (%) | |||||||
| OM (%) | −0.27 | ||||||
| pH | −0.31 | 0.44 * | |||||
| EC (µS cm−1) | −0.39 * | 0.64 *** | 0.26 | ||||
| MB | −0.58 ** | 0.44 * | 0.1 | 0.53 ** | |||
| CO2 | −0.60 *** | −0.07 | −0.37 | 0.13 | 0.51 ** | ||
| qCO2 | −0.27 | −0.1 | −0.33 | −0.1 | −0.15 | 0.64 *** | |
| CLPP | −0.17 | −0.31 | −0.48 ** | −0.12 | −0.01 | 0.71 **** | 0.62 *** |
| Control site (CO) | |||||||
| Variables | SM (%) | OM (%) | pH | EC (µS cm−1) | MB | CO2 | qCO2 |
| SM (%) | |||||||
| OM (%) | 0.1 | ||||||
| pH | 0.12 | 0.02 | |||||
| EC (µS cm−1) | −0.69 **** | 0.28 | −0.01 | ||||
| MB | 0.09 | 0 | −0.25 | −0.05 | |||
| CO2 | −0.16 | 0.14 | −0.43 * | 0.24 | 0.57 ** | ||
| qCO2 | −0.26 | 0.32 | −0.45 * | 0.39 * | 0.08 | 0.8 2 **** | |
| CLPP | −0.06 | 0.04 | 0.50 ** | 0.14 | −0.44 * | −0.57 ** | −0.32 |
| Wheat Site (WF) | |||||||
|---|---|---|---|---|---|---|---|
| Variables, WF | SM (%) | OM (%) | pH | EC (µS cm−1) | Aromatic | Amino Acids | Carbohydrates |
| SM (%) | |||||||
| OM (%) | −0.27 | ||||||
| pH | −0.31 | 0.44 * | |||||
| EC (µS cm−1) | −0.39 * | 0.64 *** | 0.26 | ||||
| Aromatic | −0.16 | −0.26 | −0.49 ** | −0.19 | |||
| Amino acids | −0.25 | −0.31 | −0.49 ** | −0.09 | 0.63 *** | ||
| Carbohydrates | −0.39 * | −0.17 | −0.44 * | 0.07 | 0.43 * | 0.80 **** | |
| Carboxylic acids | −0.09 | −0.28 | −0.40 * | −0.13 | 0.54 ** | 0.77 **** | 0.48 ** |
| Control site (CO) | |||||||
| SM (%) | |||||||
| OM (%) | 0.1 | ||||||
| pH | 0.12 | 0.02 | |||||
| EC (µS cm−1) | −0.69 **** | 0.28 | −0.01 | ||||
| Aromatic | −0.11 | −0.14 | 0.36 | 0.23 | |||
| Amino acids | −0.21 | 0.42 * | 0.39 * | 0.18 | 0.05 | ||
| Carbohydrates | −0.13 | −0.13 | −0.39 * | 0.11 | −0.04 | −0.06 | |
| Carboxylic acids | 0.07 | −0.2 | 0.39 * | 0.05 | 0.67 **** | 0.09 | 0.07 |
| Pre-Sowing (t0)—1 | Germination (t30)—2 | Tillering (t65)—3 | Heading and Flowering (t101)—4 | Grain Filling (t135)—5 | Maturity (t174)—6 | Stubble Field (t199)—7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF | CO | WF | CO | WF | CO | WF | CO | WF | CO | WF | CO | WF | CO | |
| 3,4-OHbenzoic acid | xx | xx | YY | xx | YY | xx | ||||||||
| Citric acid | xx | YY | xx | YY | xx | |||||||||
| L-Malic acid | xx | xx | YY | xx | ||||||||||
| Oxalic acid | xx | YY | xx | YY | xx | |||||||||
| D-Fructose | YY | xx | YY | xx | xx | |||||||||
| D-Galactose | YY | xx | ||||||||||||
| D-Glucose | YY | xx | YY | xx | ||||||||||
| L-Arabinose | xx | xx | YY | YY | YY | xx | ||||||||
| Trehalose | YY | xx | YY | xx | YY | |||||||||
| GABA | xx | |||||||||||||
| L-Alanine | xx | YY | YY | xx | ||||||||||
| L-Cysteine HCl | xx | YY | YY | xx | ||||||||||
| L-Lysine | xx | |||||||||||||
| NAGA | xx | |||||||||||||
| Arginine | xx | xx | YY | xx | YY | |||||||||
| Total number of substrate used | 0 | 6 | 0 | 1 | 0 | 3 | 1 | 6 | 10 | 7 | 11 | 3 | 2 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinberger, Y.; Levi, M.; Applebaum, I.; Sherman, C.; Doniger, T.; Unc, A. The Impact of Wheat Growth Stages on Soil Microbial Communities in a Rain-Fed Agroecosystem. Microorganisms 2025, 13, 838. https://doi.org/10.3390/microorganisms13040838
Steinberger Y, Levi M, Applebaum I, Sherman C, Doniger T, Unc A. The Impact of Wheat Growth Stages on Soil Microbial Communities in a Rain-Fed Agroecosystem. Microorganisms. 2025; 13(4):838. https://doi.org/10.3390/microorganisms13040838
Chicago/Turabian StyleSteinberger, Yosef, May Levi, Itaii Applebaum, Chen Sherman, Tirza Doniger, and Adrian Unc. 2025. "The Impact of Wheat Growth Stages on Soil Microbial Communities in a Rain-Fed Agroecosystem" Microorganisms 13, no. 4: 838. https://doi.org/10.3390/microorganisms13040838
APA StyleSteinberger, Y., Levi, M., Applebaum, I., Sherman, C., Doniger, T., & Unc, A. (2025). The Impact of Wheat Growth Stages on Soil Microbial Communities in a Rain-Fed Agroecosystem. Microorganisms, 13(4), 838. https://doi.org/10.3390/microorganisms13040838






